Introduction
Among insects, mating has a huge influence on the reproductive physiology of females. Some of the processes that can be affected by mating are, for instance, vitellogenesis, oviposition, fecundity, and fertility; however, the effect on such processes varies depending on the group of insects (Engelmann 1970; Raabe 1986).
Females of the insect Dactylopius coccus Costa, 1829 are used to extract carminic acid, a natural red dye currently employed in several industries (Méndez-Gallegos et al. 2003). Their ovaries are made up of more than 400 ovarioles of the telotrophic type, where the germarium, located apically and where nutritive cells are located, is connected by the trophic cord to a single oocyte contained in the vitellarium (Ramírez-Cruz et al. 2008). From the molt to adult, the female of D. coccus has ovarioles with oocytes completely immature since it is a sinovigenic species, and from that moment onwards, maturation starts; this maturation is characterized by the progressive increase of size of the germarium and the vitellarium over several days. When the female reaches 13 days of age, its ovarioles show the first mature oocytes where the chorion is formed (choriogenesis); at this age, the germarium has already entered into a degenerative stage and tends to be resorbed. Only one gonadic cycle takes place (Ramírez-Cruz 2014).
It is known that in D. coccus mating is necessary for the oviposition to take place (Marín and Cisneros 1977; Pérez-Guerra and Kosztarab 1992), and it also increases the concentration of carminic acid in females (Briseño-Garzón and Llanderal 2008; Lagowska and Golan 2009). However, there is no information about the effect of mating on ovarian maturation in this species. Due to that, in order to contribute to the better understanding of the reproductive physiology of D. coccus, the main objective of this work was to determine how mating affects maturation of the ovary, considering the oocytes of each ovariole.
Materials and methods
Insects were reared on cladodes of Opuntia ficus indica (L.) Mill (Cactaceae) using the method of inverted hanging cladode proposed by Aldama-Aguilera and Llanderal-Cázares (2003). From April to July 2013, a greenhouse for rearing cochineal was used, with an average temperature of 21 °C and an average relative humidity of 50 %, at the Colegio de Postgraduados in Montecillo, Mexico, located at 19º29’N and 98º54’W, at an altitude of 2,250 mals. Females from this colony were isolated and grouped into two experimental groups: mated females, and virgin females. Although mating was not experimentally verified in the group of mated females, it was assumed that it took place because they were in the presence of males. To obtain virgin females, some of the infested cladodes were kept in isolation inside wooden cages with glass in two of their walls and organza fabric in the other two; to ensure the virginity of females, it was taken into account that the pupal stage of males lasts about 12 days (Llanderal-Cázares and Nieto-Hernández 2001), so cocoons were carefully removed as soon as they were observed, and the cages were inspected for the emergence of adult males.
To evaluate the effect of mating on maturation, from each of the experimental groups mentioned above, 20 mated females that had not yet oviposited and 20 virgin females were randomly selected; all females were three months of age, counted from the time that cladodes were infested with crawlers. To evaluate the degree of maturation of the ovarioles in both groups of females, they were fixed in Carnoy’s solution for 24 hours, and they were rinsed with 70 % ethanol (Martínez 2002); to clear ovarioles, whole females were placed in a mixture of pure glycerin - 70 % ethanol (1:2) (for three days at least) until they were dissected. Finally, under the stereomicroscope, both ovaries of each female were extracted, and all their ovarioles were counted and classified according to their degree of maturation, and some morphophysiological characteristics described for ovariole maturation of D. coccus (Ramírez-Cruz and Llanderal-Cázares 2013; Ramírez-Cruz 2014).
Results
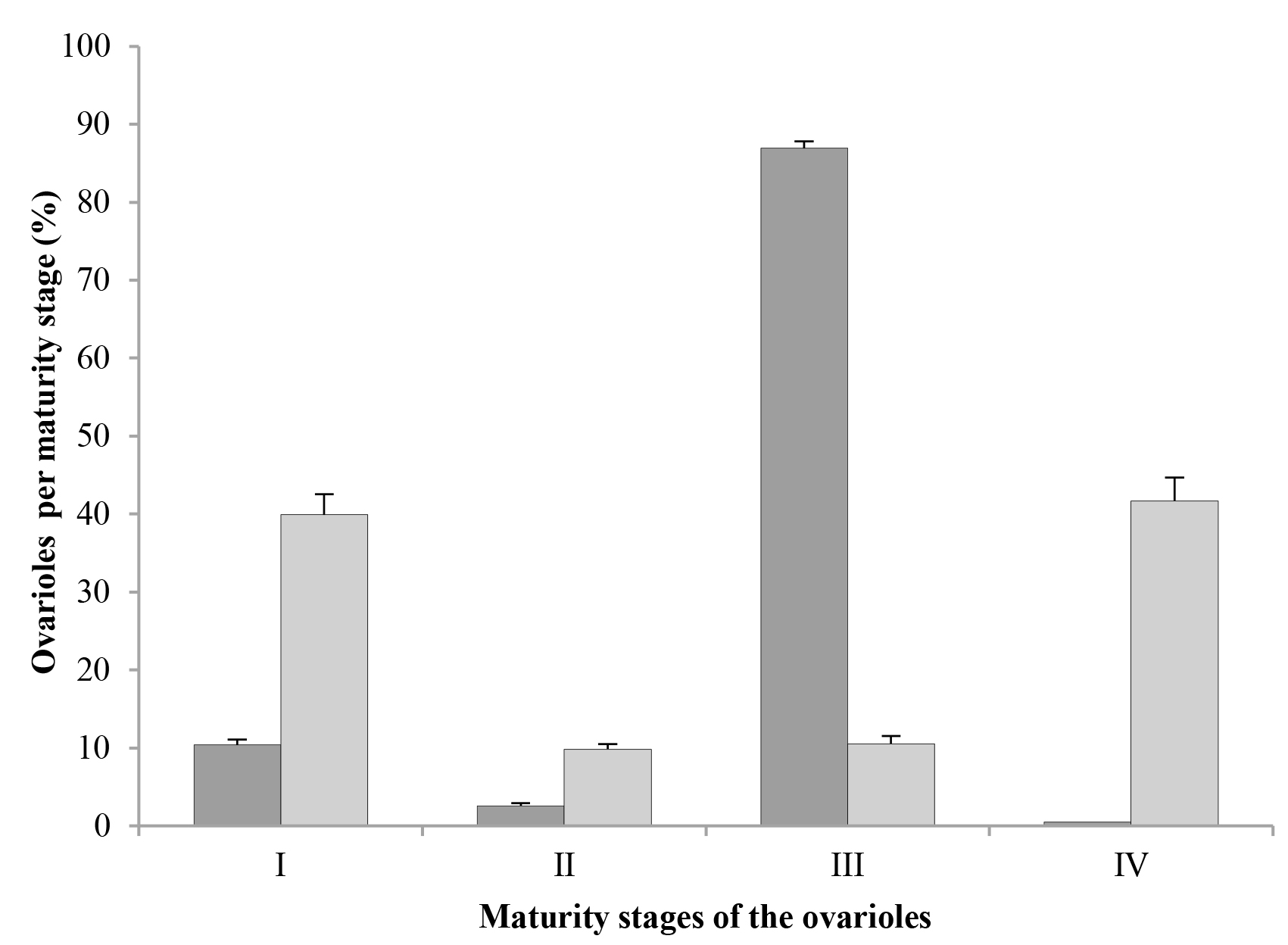
The ovarioles of both mated and virgin females were morphophysiologically characterized and grouped in at least four maturation stages (Fig. 1): Stage I, which included ovarioles with oocytes in previtellogenesis or vitellogenesis. Stage II, which included ovarioles with the germarium in the middle of the process of being resorbed, and thus, it was no longer functional. Stage III, which showed ovarioles with the germarium completely or almost completely resorbed. Stage IV, comprised degenerating ovarioles or ovarioles being resorbed, with a high degree of resorption of the germarium and its oocyte in the vitellarium.
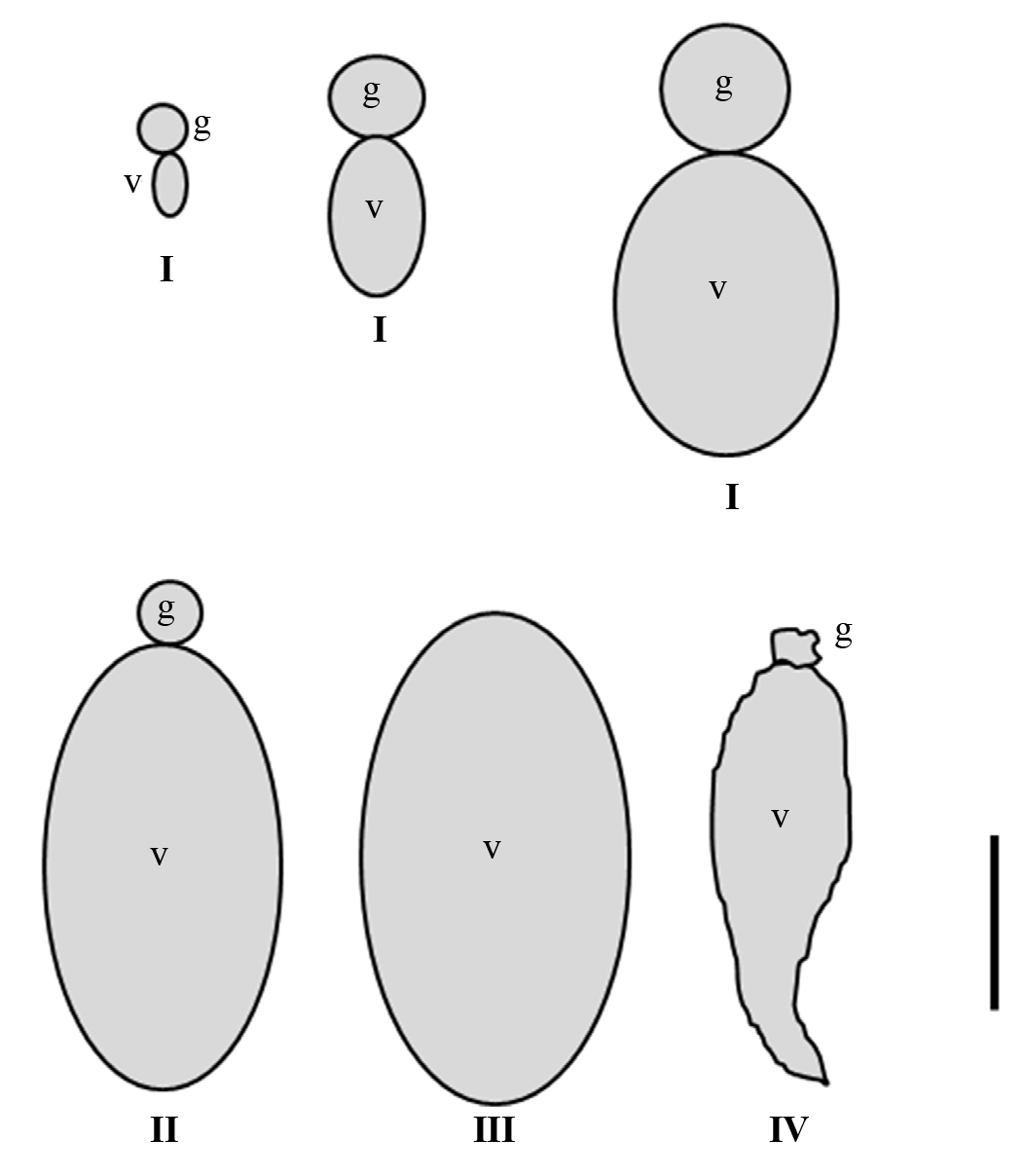
Figure 1 Schematic representation of the ovarioles morphology of the stages of maturation I - IV taken into account, in three-month-old adult females of Dactylopius coccus. The diagrams represent the average sizes of the ovarioles from each stage. g, germarium; v, vitellarium. Scale bar: 200 µm.
Stage I
In both mated and virgin females, ovarioles in stage I (oocytes in previtellogenesis or vitellogenesis) were found (Figs. 2A, 2B, respectively); however, in mated females, only about 10 % of their ovarioles (52.1 on average) were in stage I, while in virgin females, the percentage of ovarioles in this stage was almost 40 % (167.2 ovarioles on average) (Fig. 3).
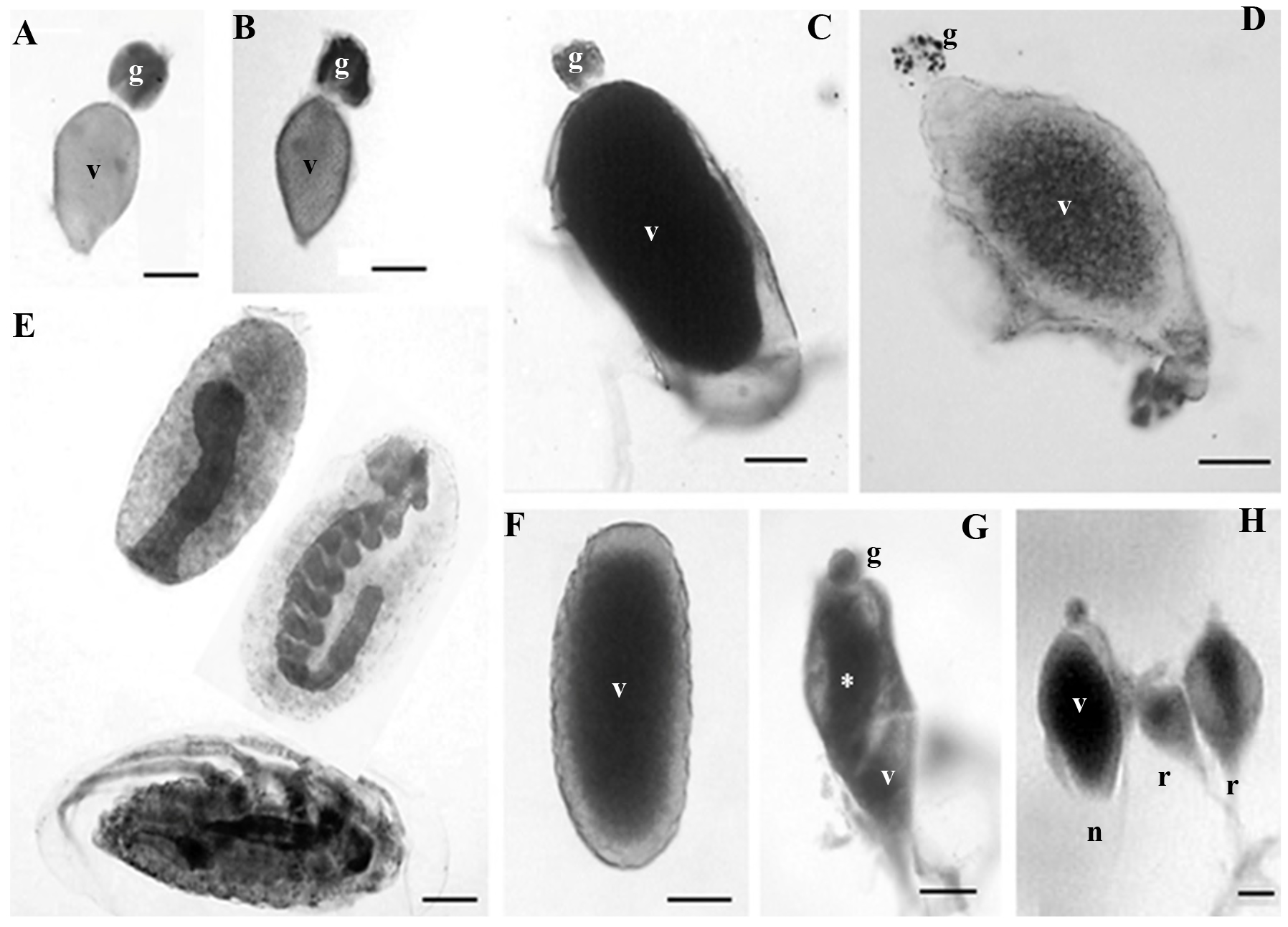
Figure 2 Ovarioles in different maturation stages, in three-month-old mated and virgin females of Dactylopius coccus. A. Stage I, mated; in vitellogenesis. B. Stage I, virgin; in vitellogenesis. C. Stage II, mated; the vitellarium (v) contains a mature oocyte. D. Stage II, virgin; the vitellarium (v) contains an oocyte without chorion. E. Stage III, mated; two early embryos can be observed (above) and a completely developed nymph (below). F. Stage III, virgin; mature oocyte. G. Stage IV, virgin; the germarium (g) and traces of yolk (asterisk) can still be observed in the oocyte. H. Three ovarioles from a virgin female where an ovariole (n) can be observed next to two ovarioles in different stages of resorption (r); in the latter, traces of yolk can still be seen in the center of both oocytes. g, germarium; v, vitellarium. Scale bar: 100 µm.
Stage II
Both mated and virgin females had fewer ovarioles in stage II (with a degenerating germarium) than in stage I; in mated females, just a little more than 2 % of their ovarioles (12.75 ovarioles on average) were in this stage, while in virgin females this number was closer to 10 % (38.2 ovarioles on average) (Fig. 3). However, the most important difference between these two groups was the maturation stage of the oocytes, since in mated females, ovarioles in stage II showed from oocytes that were almost mature until eggs with embryos in midblastula stage (Fig. 2C), while virgin females had ovarioles in stage II with either oocytes close to maturity or mature oocytes where choriogenesis had already concluded (Fig. 2D).
Stage III
In mated females, almost 87 % of their ovarioles (441.2 ovarioles on average) were in stage III (germarium completely or almost completely resorbed) (Fig. 3), and they had eggs with embryos from the stage of late blastula until pre-nymphs or completely developed nymphs (Fig. 2E). On the other hand, the percentage of ovarioles in this stage in virgin females was only slightly above 10 % (45.15 ovarioles on average) (Fig. 3); however, these ovarioles contained only mature oocytes and they never had embryos (Fig. 2F). Thus, it is important to notice that the ovarioles of virgin females can adequately complete their maturation and develop oocytes with chorion, although in low numbers.
Stage IV
In mated females, the number of ovarioles in stage IV (degenerating or being resorbed) was insignificant, about 0.16 % (two ovarioles on average), whereas in female virgins, 41.68 % of their ovarioles (193.4 ovarioles on average) were in the stage of degeneration (Fig. 3). In both cases, ovariole resorption occurred only in those that contained oocytes at the end of vitellogenesis or close to maturity, but that had not started choriogenesis yet. In most of them it was possible to observe the germarium, although some only presented the vitellarium; both structures showed evident signs of degeneration and resorption, followed by shrinking of the ovariole (Fig. 2G). In the vitellarium, oocytes also showed different degrees of yolk degradation (Fig. 2H).
Discussion
In this study, the fact that virgin females of D. coccus presented ovarioles with oocytes in vitellogenesis shows that this process is independent of mating; however, in these females the process seems to be delayed or even inhibited, because at three months after crawler emergence, about 40 % of their ovarioles were still in stage I of maturation; this contrasts with mated females, where only about 10 % of their ovarioles were in that stage, and about 87 % were in stage III. This indicates that, although mating in D. coccus is not necessary for their oocytes to start vitellogenesis, it is necessary to ensure the adequate conclusion of the process.
In the same way, the fact that in virgin females we found ovarioles in stages II and III with oocytes with chorion indicates that these females are able to complete maturation normally, albeit in lower proportions than mated females. This demonstrates that, although mating in D. coccus is not necessary for the complete maturation of its oocytes, it is necessary to increase the number of mature oocytes that will be produced.
Similarly to what was found in this study with D. coccus, there are other species in Hemiptera where normal maturation of oocytes is independent of mating, since virgin females produce mature oocytes, although in lower numbers than in mated females; some examples are found in the Miridae Adelphocoris lineolatus (Goeze, 1778) (Masner 1966) and Helopeltis antonii Signoret, 1858 (Siswanto et al. 2009), in the Triatominae Triatoma infestans (Klug, 1834) (Asin and Crocco de Ayerbe 1992) and Triatoma protracta (Uhler, 1894) (Mundall 1978), and in Pentatomidae Perillus bioculatus (Fabricius, 1775) (Adams 2000a, b), Podisus maculiventris (Say, 1832) (De Clercq and Degheele 1997), Podisus nigrispinus (Dallas, 1851) (Soares et al. 2011) and Nezara viridula (L., 1758) (Fortes et al. 2011). However, in contrast with the latter, there are other Hemiptera where females are incapable of developing any mature oocyte unless mating takes place; such is the case of Dindymus versicolor (Herrich-Schaeffer, 1853) (Pyrrhocoridae) (Friedel 1974), Diaphorina citri Kuwayama, 1908 (Psyllidae) (Dossi and Consoli 2010) and Anthocoris tomentosus Péricart, 1971 (Anthocoridae) (Horton et al. 2005). About that, it is known that in Hemiptera, mating affects oocyte maturation by activating the corpora allata or maintaining their activity for longer, in order to synthesize juvenile hormone (Davey 1997). Although the hormonal mechanism that regulates oocyte maturation in D. coccus is unknown, the fact that only a small percentage of the oocytes of virgin females completed vitellogenesis and choriogenesis normally (mature oocytes in stages II and III) in this study, could suggest that the corpora allata of such females are active only for a short period of time (compared with mated females) before being deactivated; therefore, the ovarioles whose oocytes had started but not concluded vitellogenesis would start degenerating or would be resorbed (stage of maturation IV). Something similar has been observed in T. protracta (Mundall and Engelmann 1977); however, profound studies are required to really know the endocrine mechanism involved in oocyte maturation in D. coccus.
On the other hand, oocyte resorption in insects is a phenomenon produced by several factors, both internal and external that occurs mainly under adverse conditions (Bell and Bohm 1975). In this study, the absence of mating in D. coccus induced high levels of ovariole and oocyte resorption (stage of maturation IV), and affected mainly vitellogenesis, since three months after emergence, little more than 40 % of the ovarioles of virgin females started to be resorbed before their oocytes completed that phase and began synthesizing the chorion. In contrast, in mated females, only 0.16 % of the ovarioles showed that phenomenon. Ovariole resorption in D. coccus was morphologically characterized by the degeneration of the germarium, vitellarium and its respective oocyte, with the consequent degradation of the yolk, and although the mechanism of yolk degradation in D. coccus is unknown, it is probably degraded and released into the hemolymph, similarly to what happens in the bug Plautia crossota stali Scott, 1874 (Hemiptera: Pentatomidae); during oocyte resorption in virgin females of this species, vitellins are quickly degraded by proteases and released into the hemolymph as peptides or amino acids (Kotaki 2003).
It is also known that in many species of insects, virgin females do not oviposit mature oocytes, but they are retained in the ovaries or oviducts, where they can be resorbed, and the released material during that resorption can serve as nutritive reserves, used either to mature more oocytes or to increase the life cycle of the females (Engelman 1970). This could suggest that the constituents of the yolk of the oocytes that degenerate during the resorption of the ovarioles of D. coccus are employed as nutritive reserves, especially by virgin females, to sustain their metabolic activity or even to increase their longevity, since this could explain why virgin females of D. coccus live longer than mated females, because according to Marín and Cisneros (1977) and Pérez-Guerra and Kosztarab (1992), virgin females of D. coccus do not oviposit and live longer.
In this study, the fact that no embryos were found in the ovarioles of virgin females of D. coccus confirms that mating is necessary for the species to produce fertile eggs, as previously established by Flores-Flores and Tekelenburg (1995). Besides, the finding of completely developed nymphs inside the ovarioles of mated females of D. coccus proves that all their embryonic development is intraovarian, and that places it as a clearly ovoviviparous species. However, in Dactylopius tomentosus (Lamarch, 1801), egg incubation after oviposition takes from 15 to 20 days (Mathenge et al. 2009), which would indicate that, unlike D. coccus, D. tomentosus is an oviparous species and not ovoviviparous.
Conclusions
Although mating in D. coccus is not necessary for its oocytes to initiate and adequately complete the vitellogenesis and choriogenesis, it does promote both processes, as it greatly increases the proportion of mature oocytes to produce in mated females. The lack of mating induced the resorption of the ovarioles and their oocytes, and the vitellogenesis was the affected phase.
It is necessary to deep more into the effect of mating in D. coccus to elucidate, among other aspects, the hormonal mechanism involved in oocyte maturation, as well as to determine which other factors, besides mating, condition the resorption of ovarioles and oocytes, and the mechanism involved in this process. Besides, it is important to know the final destination of the components of the yolk released by oocytes during their resorption and establish if these components contribute to the longer life of virgin females.