Introduction
Lacewings of the genus Chrysoperla (Neuroptera: Chrysopidae) are some of the most important and frequently used natural enemies in biological control of agricultural pests (Chang et al. 2000; Miller et al. 2004). Chrysoperla externa (Hagen, 1861) is a predator with a high degree of adaptability to different climates; it allows this species to have a wide geographical distribution (Gitirana et al. 2001). Its wide prey range, including soft-bodied insects such as aphids, whiteflies, and thrips, allows for its use in many biological control programs (Guarín 2003). The predatory potential of the Chrysopidae family increases with their size during the larval stage (Velázquez-Grisales 2004; Loera et al. 2001). Adults feed on nectar, pollen or honey produced by insects (Loera et al. 2001). They are easy to breed in laboratories (Cardoso and Lazzari 2003).
In order to work with a natural enemy, either a predator or a parasitoid, in a biological control program, it is important to know about its biology. This information is basic and necessary in order to implement it in the integrated pest management programs (Salamanca Bastidas et al. 2010).
Therefore, considering the potential of C. externa for integrated pest management programs, this study was performed in order to learn about the biological aspects of C. externa fed on Melanaphis sacchari (Zehntner, 1897) (Hemiptera: Aphididae), an invasive pest considered one of the aphid species of greatest economic importance on the world (ElSayed 2013; Peña-Martínez et al. 2015) and recently introduced to Mexico (SENASICA 2014).
Materials and methods
All experiments were performed in the Entomophagous Insect laboratory of the Centro Nacional de Referencia de Control Biológico (IE-CNRCB) (18º55̓’3452”N 103º53̓01.95”W) under conditions of 25 ± 2 °C, 60-70 % R. H. and 14:10 h L:D cycle.
Insect sources
C. externa and M. sacchari were collected in sorghum fields in the village of Tecuanillo in the municipality of Tecomán, Colima, México (18°51̓30.37”N 103°52̓52.03”W; 15 masl). Prior to the biological study, chrysopids were identified based on morphological characteristics (Brooks 1994). Aphids were identified using molecular analysis through the extraction of genomic DNA using the DNeasy® Blood and Tissue (QIAGEN® 2006). Specimens were kept at the Colección de Insectos Entomófagos del Centro Nacional de Referencia de Control Biológico, Tecomán Colima, México.
A colony of C. externa, feeding on M. sacchari was established in the department of IE-CNRCB. The second generation was used to carry out the subsequent experiments.
The aphids were reared on Sorghum vulgare L. plants in 5 L pots and placed in the CNRCB greenhouses.
Development and survival of Chrysoperla externa
The study started with a cohort of 135 C. externa eggs under 24 h old. They were placed in groups of 10 in 5 cm diameter Petri dishes and covered them with damp filter paper in order to prevent drying and mortality. The incubation period from egg laying to the emergence of larva 1 was recorded.
In order to monitor the larval stage, the recently emerged larvae were transferred individually to Petri dishes with a daily average of 50 M. sacchari nymphs as food. As a feeding substrate for the aphids, at the bottom of the dish, it was placed 5 cm diameter of a S. vulgare leaf. Every 24 h the density of the nymphs was adjusted, and the leaf of sorgo was changed to a fresh one. The duration of each larval stage was registered according to the size of the larva and the presence of exuviae.
The beginning of the pupa stage was established when the cocoon was observed, and it culminated in the emergence of the adult.
Once the adults emerge, their sex was determined, and they were transferred with entomological tweezers individually to vessels 350 mL. They were monitored until death. In order to facilitate ventilation and keep the adults from escaping, a 4 cm diameter opening covered with organza cloth was made. On the bottom side of the cup, a 1.5 cm diameter perforation was made and covered with cotton and moistened daily to provide water to the adults. Inside the cup, a 2 x 10 cm impregnated paper piece with an artificial diet composed of honey, beer yeast, pollen, ascorbic acid and spirulina (Palomares-Pérez et al. 2017).
The duration of the biological cycle is presented as mean and standard deviation using the Excel program (Ott 1993). To compare longevity between females and males, a Student’s t-test was conducted using the statistical program SAS 9.2 (SAS INSTITUTE 2008).
Reproduction of Chrysoperla externa
50 pupae were selected and placed them in an 18 cm diameter, 7 cm tall dish. It was monitored 24 h periods, once the adults emerged; they were collected with entomological tweezers and using a stereoscopic microscope to determine sex, according to size and apical form of abdomen. Thirteen both females and males were selected and placed in pairs in 350 mL styrofoam cups until his death. When a male died, another replaced it. Observations were made every 24 h to register the start of egg lying, the number of eggs laid per day and mortality of females.
Life table analysis
The life table was studied through groups of individuals (cohorts) born in the same space of time, from birth until death of the last adult (Rabinovich 1980; Begon et al. 1999). A cohort of 163 eggs was analyzed with the methodology described in the biological cycle, specifying for each age interval the following parameters: X = age (days), Nx = total number of individuals observed at the beginning of each stage or state, lx = proportion of survivors at the beginning of the age (Nx/N0), dx = number of deaths between ages lx y lx + 1, qx = death rate (dx/lx), K = death force (K = log10Nx-log10Nx+1) and ex = life expectancy (Tx/lx).
Results and discussion
Development and survival
The biological cycle of C. externa presented an average duration of 29.5 ± 6.2 d from the egg until adult emergence, with a range of 18 to 45 d. Biological cycle may vary by feed type and temperature; Auad et al. (2001) and Giffoni et al. (2007) who have observed the developmental time of C. externa tend to be faster at 26 °C fed Bemisia tabaci (Gennadius, 1889) (Hemiptera: Aleyrodidae) (26.1 ± 0.10 d), which is attributed to their generalist feeding behavior.
The eggs hatched on average at 5.1 ± 0.3 d, data fits with the range of 5 to 6 d reported by Ribeiro (1988) when using different prey. Similarly, Fonseca et al. (2015), using Rhopalosiphum maidis (Fitch, 1856) (Hemiptera: Aphididae) as food, reported an embryonic period of 3 to 15.1 d. According to the above, it is concluded that depending on the prey and the temperature, the eggs of C. externa presents different periods of incubation. The temperature and the nutritional quality of prey are decisive factors in the biological performance of predator insects, altering for instance the developmental time of pre-imaginal stages and reproductive performances of adults (e.g. fecundity, fertility) (Michaud 2005; Cabral et al. 2006; Jalali et al. 2010).
The larval period presented durations of 13.8 ± 4.5 d, superior to the data from Lima (2004) , who reported a period of 10.8 d fed on Brevicoryne brassicae (L., 1758) (Hemiptera: Aphididae). This difference can be linked to food quality in which a shorter life cycle is the result of good nutrition and faster adaptation by the predator (Soffiantini Lira and De Luna Batista 2006).
The pupa state was completed in 10.6 ± 1.4 d. In the study by Giffoni et al. (2007) , average duration of 7.5 d was found when they were fed on Aphis craccivora (Koch, 1854) (Hemiptera: Aphididae).
Adult longevity was 63.5 ± 26.7 d (Table 1). These results are within the average life range of these predators reported by Soffiantini Lira and De Luna Batista (2006).
Table 1 Biological cycle (days) of Chrysoperla externa fed on Melanaphis sacchari at 25 ± 2 °C, 60-70 % RH, 14 light h.
| State and/or Stage | Total | Females (n = 50) | Males (n = 36) | ||||
|---|---|---|---|---|---|---|---|
| n1 | Mean ± S.D.² | Range | Mean ± S.D.² | Range | Mean ± S.D.² | Range | |
| Egg | 135 | 5.1 ± 0.3 | 5-6 | 5.1 ± 0.2 | 5-6 | 5.1 ± 0.2 | 5-6 |
| Larva 1 | 135 | 4.3 ± 1.4 | 1-9 | 4.5. ± 1.2 | 4-9 | 4.3 ± 1.0 | 3-8 |
| Larva 2 | 111 | 3.2 ± 1.1 | 1-7 | 3.3 ± 1.1 | 1-7 | 3.1 ± 0.9 | 3-4 |
| Larva 3 | 104 | 6.3 ± 2.0 | 1-11 | 6.2 ± 1.9 | 3-10 | 6.4 ± 2.0 | 3-10 |
| Pupa | 100 | 10.6 ± 1.4 | 10-12 | 10.7 ± 0.9 | 9-13 | 10.5 ± 1.0 | 8-12 |
| Adult | 86 | 63.5 ± 26.7 | 6-104 | 65.5 ± 28.6 | 6-104 | 60.8 ± 24.0 | 9-97 |
1Number of individuals = n. 2 Standard deviation = S.D.
Out of a total of 86 adults, 50 were females and 36 males; this represents a 1.4:1 sex ratio. The developmental time was slightly longer for females, although the difference was not statistically significant (F = 1.42; Pr = 0.2785) (Table 1). Duration of the larval stage with respect to sex did not show a significant difference (F = 1.09; Pr = 0.6153) (Table 1).
The survival of the different biological stages indicates full development of C. externa fed on M. sacchari, the opposite is reported by Giffoni et al. (2007) and Salamanca Bastidas et al. (2010); they mention that the biological cycle of C. externa is incomplete, and it only reaches the pupal stage when they feed on Aphis nerii (Boyer, 1841) (Hemiptera: Aphididae) and immature states of Neohydatothrips signifer (Priesner, 1932) (Thysanoptera: Thripidae). Giffoni et al. (2007) also refer a wide variation in the biological cycle of C. externa feeding on different species of aphids; when it consumes A. craccivora and R. maidis its biological cycle from egg to adult is 20.7 and 36.8 d, respectively. It is important to remember that insect development, in general, is also affected by laboratory conditions, mainly temperature, relative humidity and exposure to light (McEwen et al. 2001).
Without a doubt, the results of this study show that C. externa is capable of completing its biological cycle by feeding only on M. sacchari nymphs. This is important to consider if one seeks to introduce and establish this predator in an area where it is not found naturally.
Reproduction of Chrysoperla externa
The pre-oviposition period to egg laying ranged from 2 to 11 d with an average of 6.5 ± 3.1 d, time similar to the reported by Saini and Salto (1999), but longer to the reported by Elkarmi et al. (1987) for other species of the same genus, Chrysoperla rufilabris (Burmeister, 1839) and Chrysoperla carnea (Stephens, 1836) they presented a period of 3 d. In nature, a lengthy period prior to egg laying is a disadvantage for the survival of the species, since adults who have not reproduced yet are exposed longer to their natural enemies (Canard 1981).
The average number of eggs laid per female was 228.3 ± 139.1, with a range from 39 to 542. The daily number of eggs laid was 2.8 ± 0.2. The longest-lived female lived for 105 d, while the shortest-lived lasted 12 d.
The fertility curve indicates cyclical behavior with a descending trend and maximum production peaks at 15, 45 and 53 d. Subsequently, egg laying decreases constantly until the death of the female (Fig. 1).
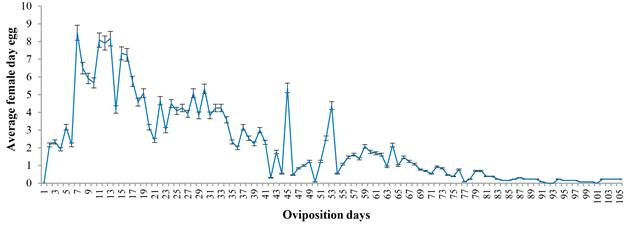
Figure 1 Eggs oviposited per day by females of Chrysoperla externa fed on Melanaphis sacchari at 25 ± 2 C; 60- 70 % RH; 14 light h.
In population terms, the fecundity was mx = 168.9. The net reproduction rate Ro registered a value of 113.2, meaning that population will increase approximately 113 % in generation time (T) of 28.9 d. The intrinsic growth rate (rm) was 0.163, this means the population would multiply 16.3 % from one unit of time to the next. Finally, the finite reproductive rate (λ) of 1.17 indicates that for each chrysopid at any given time, there will be 1.17 individuals in the next time unit. Without a doubt, these results show the reproductive and colony establishment potential of C. externa is possible when it feeds on M. sacchari.
Life table analysis
The population parameters of cohort table showed that specific death rate (qx) maintains high values in the egg stage (0.17). This is reflected in mortality intensity (K) (0.08) (Table 2).
Table 2 Horizontal life table (days) of Chrysoperla externa fed on Melanaphis sacchari at 25 ± 2 °C, 60-70 % RH, 14 light h.
| X | Nx | lx | dx | qx | ex | K |
|---|---|---|---|---|---|---|
| Egg | 163 | 1.00 | 28 | 0.17 | 4.00 | 0.08 |
| Larva 1 | 135 | 0.82 | 24 | 0.17 | 3.64 | 0.08 |
| Larva 2 | 111 | 0.68 | 7 | 0.06 | 3.08 | 0.01 |
| Larva 3 | 104 | 0.63 | 4 | 0.03 | 2.20 | 0.01 |
| Pupa | 100 | 0.61 | 14 | 0.14 | 1.86 | 0.07 |
| Adult | 86 | 0.52 | 86 | 1 | 1 | 0.00 |
X = biological state; Nx = number of individuals; lx = proportion of survivors; dx = number of deaths; qx = death rate; K = mortality force; ex = life expectancy.
Both the egg stage and the first larval stages showed a higher mortality (qx) (Table 2). This data suggests a type III survival curve (Southwood 1978; Rabinovich 1980), which indicates a high mortality rate in the early stage of C. externa.
In the evaluated population, 86 individuals reached the adult stage (Table 2), it means that 63.7 % of insects handled complete their development, more than the reported by Salamanca Bastidas et al. (2010), who indicate that only 30 % of C. externa individuals complete their cycle when they feed on R. maidis.
The greatest viability was registered in larva 3 (96.2 %), this is important when considering that this stage is the most voracious and therefore the most predatory. Carvalho et al. (1998) and Figueira et al. (2000) report inferior viability in larva 3 when they are fed on eggs of Alabama argillacea (Hübner, 1823) (Lepidoptera: Noctuidae) (90 %) and nymphs of Schizaphis graminum (Rondani, 1852) (Hemiptera: Aphididae) (70 %).
Conclusions
The 63.7 % of insects completed their biological cycle; therefore, it is concluded that M. sacchari satisfies the necessary nutritional requirements to complete the four biological stages of C. externa. The net reproduction rate Ro, the intrinsic growth rate (rm) and the finite reproduction rate (λ) show the reproductive potential of C. externa when is fed on M. sacchari.















