Introduction
Ants are one of the most important groups of insects in the world, with an elevated presence and influence on the structure and dynamics of terrestrial ecosystems, especially in the tropics and their low-altitude areas. Due to their abundance and varied biology, they are useful tools for conservation studies and for monitoring threatened areas. Knowledge about the evolutionary history, phylogenetic relationships, and the classification and identification of the species of Neotropical ants, provides biologists and naturalists updated information for the recognition of the genera and species of the region.
Ants have long been considered a natural group within the Hymenoptera. The possession of the metapleural gland is one of the unique traits in ants, considered the morphological synapomorphy par excellence, even visible in some fossils of Cretaceous amber of almost 100 million years old (Wilson et al. 1967; Agosti et al. 1998; Barden and Grimaldi 2013, 2014; LaPolla et al. 2013; Barden 2017; Barden et al. 2017; Lattke and Melo 2020). The presence of a petiole, geniculated antennae, infrabucal sac, deciduous wings in females, and perennial acellular colonies with workers (always apterous females), are additional and unique features within the Hymenoptera (Bolton 2003; Taylor 2007).
Boudinot (2015) offers more unique characters in ants, with the inclusion of new and comparative information on males. Boudinot et al. (2020) provide a diagnosis of the Formicoidea superfamily based especially on Mesozoic specimens. These authors propose the name Formicapoidina for Formicoidea plus Apoidea (sphecid wasps and bees), and they list 10 traits to define Formicoidea, among those: the elongation of the postgenal bridge; the mandibular condyle and the procoxae; “petiolization” of the first metasomal segment; prora (anteroventral process of the second external metasoma); and angled junction of Rs and M in the forewing. They recognize two clades, the basal Camelomecia clade and the total clade Formicidae, which in turn is divided into the clades Sphecomyrmines (with three Cretaceous subfamilies: Haidomyrmecinae, Zigrasimeciinae, and Sphecomyrminae) and Antennoclypeata (with Cretaeous Brownimeciinae and Crown clade Formicicidae). Borysenko (2017) compares plesiomorphic and autoapomorphic characters to separate “basal” ants (stem groups) from modern ants (crown groups) and performs a first statistical analysis of the scape lengths and flagellomeres of worker antennae to separate the basal (and extinct) groups of modern ants. This author uses criteria from the Phylum Code (PhyloCode) to define the “crown” group of ants such as Formicidae Stephens, 1829; the clade originated with the most recent common ancestor of Martialis heurekaRabeling and Verhaagh, 2008, and Formica rufa Linnaeus, 1761. This clade then corresponds to the family Formicidae Latreille, 1809, excluding the basal groups. Pan-Formicidae Borysenko, 2017, is defined as “the total clade composed of the crown clade FormicidaeP and all extinct species that share a more recent common ancestor with FormicidaeP rather than with any living species that is not a member of FormicidaeP”. Similarly, Borysenko (2017) defines all the subfamilies of Formicidae under the same criteria. One of the various consequences of the Borysenko (2017) study is that clades such as Armaniidae / Armaniinae could be included within Sphecomyrminae, and Cretaceous genera such as Brownimecia would remain in the main group of ants.
With the internal phylogeny of Aculeata using four nuclear genes, Pilgrim et al. (2008) confirm the monophyly of Aculeata, Chrysidoidea, and Apoidea, but they establish the paraphyly of Vespoidea, proposing several superfamilies and suggesting a proximity between apoids and ants, a new and unexpected relationship. The same relationship was confirmed in the analysis using genomes (“phylogenomics”) by Johnson et al. (2013) where the relationship between apoids and ants is confirmed (Fig. 1).
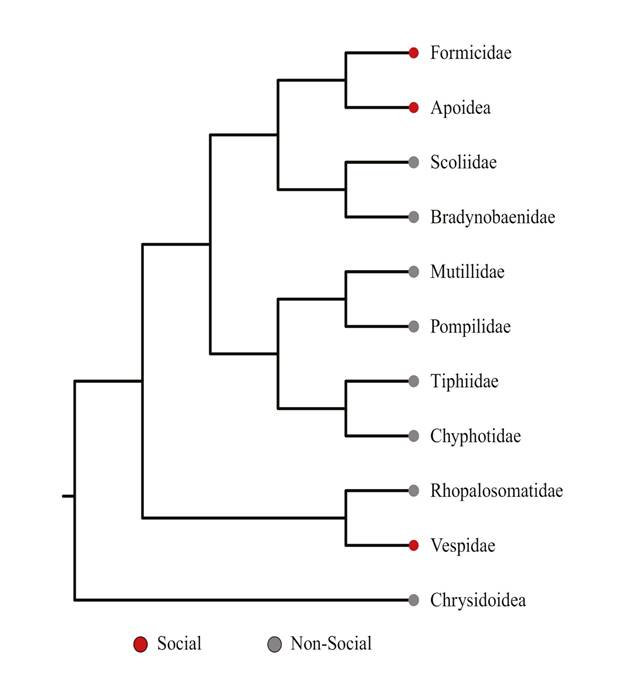
Figure 1 Phylogenetic relationship within Hymenoptera Aculeata, after Johnson et al. (2013). Social lineages marked with red points at tips.
These relationships, if stable, are very important not only when looking for suitable outside groups, but also when establishing suitable scenarios in the first steps of the evolution of ants. An ancestor between ants and neighboring groups no longer consists of an ectoparasitoid wasp (according to the phylogeny of Brothers 1999), but of a wasp that digs a nest in the ground that it supplies at once or gradually with prey, as occurs in Ampulicidae and Sphecidae, which are basal apoids. This scenario may make the recent proposal of the first soil-dwelling ants more plausible, subsequently originating lineages that would colonize the litter and trees (Lucky et al. 2013).
The recent publication of the phylogeny and history of Hymenoptera by Peters et al. (2017) offers a more stable panorama since several clades proposed by morphology and genes are confirmed. This includes the paraphyly of Aculeata and Chrysidoidea, and Vespoidea as defined by Brothers (1999) and the sister group relationships between ants and Apoidea.
This position implies that the ants must comprise the superfamily Formicoidea with a family (Formicidae) or two (depending on how the nature of the extinct taxon Armaniidae / Armaniinae is interpreted). On the other hand, the ancestor of this Jurassic clade suggests the origin of the ants towards the Upper Jurassic or the Jurassic-Cretaceous transition (Moreau and Bell 2013).
Branstetter et al. (2017) use ultra-conserved elements (UCE) to explore the internal relationships of Aculeata with an emphasis on ants and Apoidea. This study offers strong support for the relationship between ants and Apoidea and an Upper Jurassic or Lower Cretaceous antiquity, as in some previous studies. With this series of data, the position of ants within Aculeata appears to be clear. However, Camacho et al. (2018) draw attention to the incongruities in the trees that show internal relationships in Aculeta, especially between ants and apoids. These authors suggest the need for more data and different analytic dynamics, in addition to the standard ones, to improve our understanding of the relationships between ants and other aculeates.
Bolton (2003) provides a list of ant synapomorphies based on morphological traits; Boudinot (2015), in the first synthesis of ants from the point of view of male morphology, recapitulates and expands these synapomorphies. Naturally, several of these undergo changes such as secondary loss or modifications, or they do not apply (see Bolton (2003), and Boudinot (2015) for exceptions, and Taylor (2007)). Boudinot (2015), offers other attributes that help to characterize ants, even if they are not synapomorphies: antenna with 4 to 12 flagellomeres in the female or 5 to 13 in the male; bulbous neck (radicle) and scape with a common axis; epicnemius very small, not visible in situ; abdominal segment II with sternum and tergus equally sclerotized; variable wing venation, with extreme reductions, without closed cells; jugal lobe present or absent; abdominal sternum IX can be complex and have apical modifications (such as projections, teeth, and lobes).
Currently, 16 subfamilies of living ants are recognized (12 in the Neotropics: Heteroponerinae is included within the Ectatomminae: Feitosa and Prada-Achiardi 2019), all of them are monophyletic (Ward 2010, 2014; Borowiec et al. 2019). Table 1 lists the world’s living and fossil ant subfamilies.
Table 1 Fossil (*) and living taxa of the world, with distribution and habits. Subfamilies present in the Neotropics in bold. Boudinot et al.(2020) relate several Mesozoic genera as uncertain.
| Taxon | Distribution | Notes |
|---|---|---|
| FORMICOIDEA | ||
| Armaniidae* | Cretaceous, Northern Hemisphere. | Unknown biology. |
| FORMICIDAE | ||
| Brownimeciinae* | Cretaceous, USA. | Unknown biology. Specialized mandibles. |
| Formiciinae* | Eocene, Europa. | |
| Haidomyrmecinae* | Cretaceous, Burma, northern Myanmar (Asia). | Unknown biology. Specialized mandibles. |
| Sphecomyrminae* | Cretaceous, Northern Hemisphere. | Unknown biology. |
| Zigracimeciinae* | Cretaceous, Burma, Canada. | Unknown biology |
| Martialinae | Brazil. | Underground, unknown biology; probably specialized predator. |
| Leptanillinae | Old World. | Underground, specialized predator. |
| Agroecomyrmecinae | Neotropics. | Soil; unknown biology; probably specialized predator. |
| Amblyoponinae | Worldwide. | Soil. Mostly predators. |
| Apomyrminae | Afrotropical. | Soil. Predators. |
| Paraponerinae | Neotropical. | Mainly on the ground, nest under tree stumps; omnivorous. |
| Ponerinae | Worldwide, mostly tropical. | Mainly on the ground, with not very large colonies; great diversity of social biology (with some species without queens) and feeding habits. |
| Proceratiinae | Worldwide. | Mainly on the ground, general to specialized predators. |
| Aneuretinae | Sri Lanka. | Wood. Predator. |
| Dolichoderinae | Worldwide, mainly temperate. | Wide range of nesting habits, behavior and food sources. Includes relationships with other insects or plants. |
| Dorylinae | Worldwide, mainly tropical. | Ground or ground surface. Include army and driver ants with apterous queens. Predators. Includes Cerapachyinae, Ecitoninae, Leptanilloidinae, Aenictinae, Aenictogitoninae as junior synonyms. |
| Formicinae | Worldwide, mainly temperate. | Wide range of nesting habits, behavior and food sources. Includes parasitisms and relationships with other insects or plants. |
| Ectatomminae | Australia, Asia, New World. | Mainly on the ground, some arboreal. Nesting biology and food sources varied. Includes Heteroponerinae. |
| Myrmicinae | Worldwide. | Wide range of nesting habits, behavior and food sources. Includes parasitisms and relationships with other insects or plants. |
| Myrmeciinae | Australia. | Ground. Predators. |
| Pseudomyrmecinae | Worldwide, mainly tropical. | Mainly arboreal, omnivorous, some associated with plants. |
Phylogenies based on morphology
Keller (2011) performs the only modern study on the phylogeny of ants using external morphology of females with an emphasis on poneromorphs. This study is based on 139 characters (many of them new) and 105 terminals (taxa), covering most subfamilies and genera (except Martialinae). The main result is that the poneromorph ants form a paraphyletic group, as they include dorylomorphs, leptanilomorphs, and myrmicomorphs. Although this research agrees with previous studies questioning the monophyly of poneromorphs (Moreau et al. 2006; Brady et al. 2006), it does not agree for other groupings as suggested by gene-based phylogenies. Cantone and von Zuben (2019) use hindwing venation to explore the phylogeny of ants. These authors propose three types of wing venation morphology; the first one (I) is the most primitive; and II and III are derived by reduction. Part of the hypotheses of these authors suggest a high degree of convergence between subfamilies and unrelated genera.
Gene-based phylogenies
Recent molecular analyses (Moreau et al. 2006; Brady et al.2006; Rabeling et al. 2008; Schmidt 2013; Moreau and Bell 2013) coincide on several important points, such as the monophyly of the Formicidae, most subfamilies, and some suprafamilial clades. If we take as reference the phylogeny of Moreau and Bell (2013), Leptanillinae is the sister group of the rest of the ants (and not Martialinae as in Rabeling et al. 2008), forming a trichotomy between Martialinae, the poneroid subfamilies (Amblyoponinae, Proceratiinae, Paraponerinae, Agroecomyrmecinae, and Ponerinae), and the formicoid group (the rest of the subfamilies). In addition to the support of the formicoid group, the study supports the dorylomorph subclade (sensu Bolton 2003), and the subclades Dolichoderinae + Aneuretinae, and Formicinae + (Ectatomminae s.l. + Myrmicinae). Another important difference in the proposal of Moreau and Bell (2013) and the previous ones is that the sister group of Myrmicinae is Ectatomminae (and not Formicinae), a topic already suggested by William Brown earlier in Brown (1954). Since support for the group made up of the poneroid subfamilies is low, Ward (2014) suggests these subfamilies are an unresolved polytomy.
Borowiec et al. (2019) explore the phylogeny of ant subfamilies based on the sequences of 11 nuclear genes and exploring the heterogeneity in the composition of sequences between taxa (emphasis on non-formicomorphs), as well as the divergence times of the clades. These authors conclude that the ants arose towards the Albian / Aptian [Lower Cretaceous, about 124/104 million years ago] and that Martialinae + Leptanillinae are the sister group of the rest of the ants. A third important conclusion is the monophyly of the poneroid group with the relationships ((Apomyrminae + Am-blyoponinae) + Proceratiinae) + ((Agroecomyrmecinae + Paraponerinae) + Ponerinae) (Fig. 2).
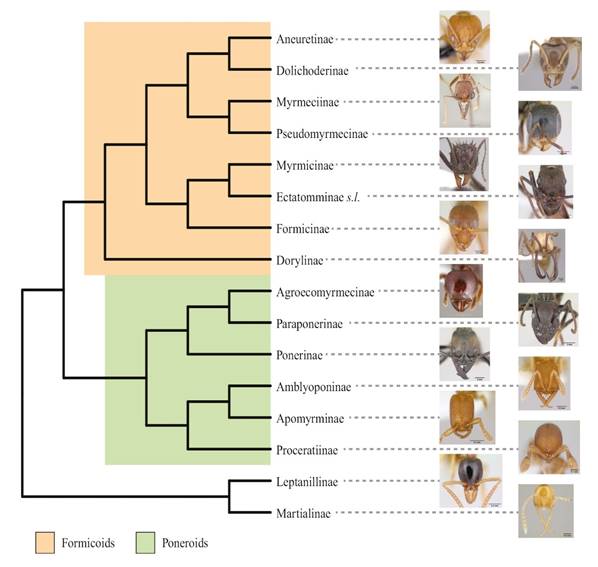
Figure 2 Phylogenetic relationship of the ant subfamilies, based on the 11 nuclear genes, modified from Borowiec et al. (2019). Photographs taken from AntWeb (2021), full credits in the acknowledgements section.
In addition to their study of ant phylogeny, Moreau and Bell (2013) explore the evolution of ants under the hypothesis of the tropics as a “museum” (lineage reservoir site) or “cradle” (site of origin of many groups) of the diversification of these insects. These authors establish an age of between 158 and 139 million years ago and 10 periods of significant changes in the diversification of ants. The authors conclude that the Neotropical region has been both a “cradle and a museum”.
Internal explorations in the subfamilies have been carried out in Amblyoponinae (Ward and Fisher 2016), Dolichoderinae (Ward et al. 2010; Boudinot et al. 2016), Dorylinae (Brady et al. 2014; Borowiec 2019), Formicinae (Blaimer et al. 2015), Myrmicinae (Ward et al. 2015), Ponerinae (Schmidt 2013), and Pseudomyrmecinae (Ward and Downie 2005).
Classification
Table 1 lists the subfamilies of fossil and living ants according to the most recent proposals. Currently, the Neotropical region comprises 12 subfamilies, 137 genera, and about 3,100 described species (Table 2). Figure 3 shows the diversity of ant genera and species in the world by subfamily, and for the Neotropical region to January of 2021. Figure 4 shows the comparison of ant genus and species counts for the Neotropical region, discriminated by country.
Table 2 Synopsis of the subfamilies, tribes and genera of extant Neotropical ants. Number of species refer only to Neotropics. In front of each subfamily the number of genera is provided.
| Agroecomyrmecinae (1) | Subfamily Ectatomminae (5) |
|---|---|
| Tatuidris Brown and Kempf, 1968 - 1, Central America to Brazil | Tribe Ectatommini |
| Subfamily Amblyoponinae (2) | Ectatomma F. Smith, 1858 - 14, Neotropical |
| Fulakora Mann, 1919 - 12, Neotropical | Gnamptogenys Roger, 1863 - 90, Neotropical |
| Prionopelta Mayr, 1866 - 5, Neotropical | Typhlomyrmex Mayr, 1862 - 7, Neotropical |
| Subfamily Dolichoderinae (12) | Tribe Heteroponerini |
| Tribe Bothryomyrmecini | Acanthoponera Mayr, 1882 - 4, Neotropical |
| Bothriomyrmex Emery, 1869 - 1, Honduras, Costa Rica | Heteroponera Mayr, 1877 - 13, Neotropical |
| Tribe Dolichoderini | Subfamily Formicinae (14) |
| Dolichoderus Lund, 1831 - 54, Neotropical | Tribe Camponotini |
| Tribe Leptomyrmecini | Camponotus Mayr, 1861 - 450, Neotropical |
| Anillidris Santschi, 1936 - 1, Argentina, Brazil. Azteca Forel, 1878 - 70, Neotropical | Colobopsis Mayr, 1861 - 8, Mexico and Bahamas to Costa Rica |
| Dorymyrmex Mayr, 1866 - 41, neotropical | Tribe Gigantiopini |
| Forelius Emery, 1888 -18, neotropical | Gigantiops Roger, 1863 - 1, South America |
| Gracilidris Wild and Cuezzo, 2006 - 1, Colombia, Brazil, Paraguay | Tribe Formicini |
| Leptomyrmex Mayr, 1862 - 1, Brazil | Formica L., 1758 - 1, Mexico to Guatemala |
| Linepithema Mayr, 1866 - 19, Neotropical | Tribe Lasiini |
| Tribe Tapinomini | Nylanderia Emery, 1906 - 23, Neotropical |
| Liometopum Mayr, 1861 - 1, Mexico to Guatemala | Paratrechina Motschulsky, 1893 - 1, Neotropical |
| Tapinoma Foerster, 1850 - 20, Neotropical | Zatania LaPolla, Kallal and Brady, 2012 - 5, Central America and West Indies |
| Technomyrmex Mayr, 1870 - 2, Costa Rica, Panama, Colombia | Tribe Melophorini |
| Subfamily Dorylinae (12) | Lasiophanes Emery, 1895 - 5, Chile, Argentina |
| Acanthostichus Mayr, 1887 - 19, Neotropical | Tribe Myrmelachistini |
| Cheliomyrmex Mayr, 1870 - 4, Neotropical | Brachymyrmex Mayr, 1868 - 53, Neotropical |
| Cylindromyrmex Mayr, 1870 - 10, Neotropical | Myrmelachista Roger, 1863 - 47, Neotropical |
| Eciton Latreille, 1804 - 12, Neotropical | Tribe Plagiolepidini |
| Labidus Jurine, 1807 - 7, Neotropical | Acropyga Roger, 1862 - 27, Neotropical |
| Leptanilloides Mann, 1923 - 18, Neotropical | Anoplolepis Santschi, 1914* - 1, Introduced in Mexico and Chile |
| Neocerapachys Borowiec, 2016 - 2, Panama and South America | Plagiolepis Mayr, 1861* - 1, Introduced in Mexico, Bahamas and West Indies |
| Nomamyrmex Borgmeier, 1936 - 2, Neotropical | Prenolepis Mayr, 1861 - 1, Mexico, Jamaica, Panama |
| Neivamyrmex Borgmeier, 1955 - 103, Neotropical | Subfamily Martialinae (1) |
| Ooceraea Roger, 1862* - 1, introduced in West Indies | Martialis Rabeling and Verhaag, 2008 - 1, Brazil |
| Sphinctomyrmex Mayr, 1866 - 3, Brazil | Protalaridris Brown, 1980 - 7, Colombia, Venezuela, Ecuador |
| Syscia Roger, 1861 - 34, Central America, Colombia, Brazil | Pseudoatta Gallardo, 1916 - 1, Argentina |
| Subfamily Myrmicinae (67) | Rhopalothrix Mayr, 1870 - 8, Neotropical |
| Tribe Pogonomyrmecini (3) | Sericomyrmex Mayr, 1865 - 19, Neotropical |
| Hylomyrma Forel, 1912 - 14, Neotropical | Strumigenys F. Smith, 1860 -186, Neotropical |
| Patagonomyrmex Johnson and Moreau, 2016 - 3, Argentina and Chile | Talaridris Weber, 1941 - 1, Colombia, Trinidad, Brazil, Guiana |
| Pogonomyrmex Mayr, 1868 - 31, Neotropical | Trachymyrmex Forel, 1893 - 6, Mexico to Guatemala |
| Tribe Stenammini (2) | Tranopelta Mayr, 1866 - 2, Neotropical |
| Aphaenogaster Mayr, 1863 - 4, Central America and Colombia | Wasmannia Forel, 1893 - 10, Neotropica |
| Stenamma Westwood, 1839 - 40, Central America to Ecuador | Xerolitor Sosa-Calvo, Schultz, Ješovnik, Dahan and Rabeling, 2018 - 1, Bolivia, Brazil |
| Tribe Crematogastrini (9) | Tribe Solenopsidini (12) |
| Cardiocondyla Emery, 1869 - 2, Neotropical | Adelomyrmex Emery, 1897 - 16, Neotropical |
| Carebara Westwood, 1840 - 30, Neotropical | Bariamyrma Lattke, 1990 - 1, Costa Rica, Venezuela |
| Crematogaster Lund, 1831- 80, Neotropical | Cryptomyrmex Fernández, 2003 - 2, Guiana, Brazil, Paraguay |
| Nesomyrmex Wheeler, 1910 - 86, Neotropical | Kempfidris Fernández, Lattke and Feitosa, 2014 - 1, South America |
| Perissomyrmex M.R. Smith, 1947 - 1, Central America | Megalomyrmex Forel, 1885 - 38, Neotropical |
| Temnothorax Mayr, 1861 - 55, Central America, West Indies, Northern South America | Monomorium Mayr, 1855 - 22, Neotropical |
| Tetramorium Mayr, 1855 - 4, Neotropical | Oxyepoecus Santschi, 1926 -16, South America |
| Trichomyrmex Mayr, 1865 - 1, Neotropical | Rogeria Emery, 1915 - 21, Neotropical |
| Xenomyrmex Forel, 1884 - 4, Neotropical | Solenopsis Westwood, 1840 - 115, Neotropical |
| Tribe Attini (41) | Stegomyrmex Emery, 1912 - 2, Panama and South America |
| Acanthognathus Mayr, 1887 - 6, Neotropical | Syllophopsis Santschi, 1915* - 1, introduced in Puerto Rico, Lesser Antilles |
| Acromyrmex Mayr, 1865 - 25, Neotropical | Tropidomyrmex Silva, Feitosa, Brandão and Diniz, 2009 - 1, Brazil |
| Allomerus Mayr, 1878 - 8, Neotropical | Subfamily Paraponerinae (1) |
| Amoimyrmex Cristiano Cristiano, Cardoso and Sandoval-Gómez, 2020 - 3, southern South America | Paraponera F. Smith, 1858 - 1, Neotropical |
| Apterostigma Mayr, 1865 - 40, Neotropical | Subfamily Ponerinae (17) |
| Atta Fabricius, 1804 -14, Neotropical | Tribe Ponerini |
| Basiceros Schulz, 1906 - 7, Neotropical | Anochetus Mayr, 1861- 25, Neotropical |
| Blepharidatta Wheeler, 1915 - 5, South America | Belonopelta Mayr, 1870 - 2, Mexico to Colombia |
| Cephalotes Latreille, 1802 -160, Neotropical | Centromyrmex Mayr, 1866 - 3, Neotropical |
| Cyatta Sosa-Calvo, Schultz, Brandão, Klingenberg, | Cryptopone Emery, 1893 - 5, Neotropical |
| Feitosa, Rabeling, Bacci, Lopes and Vasconcelos, 2013 - 1, Bolivia, Brazil | Dinoponera Roger, 1861 - 9, South America |
| Cyphomyrmex Mayr, 1862 - 21, Neotropical | Hypoponera Santschi, 1938 - 35, Neotropical |
| Daceton Perty, 1833 - 2, Neotropical | Leptogenys Roger, 1861- 80, Neotropical |
| Diaphoromyrma Fernández, Delabie and Nascimento, 2009 - 1, Brazil | Mayaponera Schmidt and Shattuck, 2014 - 6, Neotropical |
| Eurhopalothrix Brown and Kempf, 1961 - 11, Neotropical | Neoponera Emery, 1901 - 76, Neotropical |
| Kalathomyrmex Klingenberg and Brandão, 2009 -1, South America | Odontomachus Latreille, 1804 - 24, Neotropical |
| Lachnomyrmex Wheeler, 1910 - 16, Neotropical | Ponera Latreille, 1804 - 2 Mexico to Guatemala |
| Lenomyrmex Fernández and Palacio, 1999 - 6, Costa Rica to Ecuador | Pachycondyla F. Smith, 1858 - 17, Neotropical |
| Mycetagroicus Brandão and Mayhé-Nunes, 2001 - 4, Brazil, Bolivia | Pseudoponera Emery, 1920 - 4, Neotropical |
| Mycetarotes Emery, 1913 - 4, Colombia, Venezuela, Ecuador, Brazil, Paraguay | Rasopone Schmidt and Shattuck, 2014 - 25, Neotropical |
| Mycetophylax Emery, 1913 - 22, Neotropical | Simopelta Mann, 1922 - 21, Neotropical |
| Mycetosoritis Wheeler, 1907 - 6, Mexico to Nicaragua | Thaumatomyrmex Mayr, 1887 - 12, Neotropical |
| Mycocepurus Forel, 1893 - 4, Neotropical | Tribe Platythyreini |
| Mycetomoellerius Solomon, Rabeling, Sosa-Calvo and Schultz, 2019 - 29, Neotropical | Platythyrea Roger, 1863 - 8, Neotropical |
| Myrmicocrypta F. Smith, 1860 - 24, Neotropical | Subfamily Proceratiinae (3) |
| Ochetomyrmex Mayr, 1877 - 2, South America | Tribe Proceratiini |
| Octostruma Forel, 1912 - 34, Neotropical | Discothyrea Roger, 1863 - 10, Neotropical |
| Paramycetophylax Kusnezov, 1956 - 1, Brazil and Argentina | Proceratium Roger, 1863 - 16, Neotropical |
| Paratrachymyrmex Solomon, Rabeling, Sosa-Calvo and Schultz, 2019 - 9, neotropical | Tribe Probolomyrmecini |
| Phalacromyrmex Kempf, 1960 - 1, Brazil | Probolomyrmex Mayr, 1901 - 8, Neotropical |
| Pheidole Westwood, 1839 - 463, Neotropical | Subfamily Pseudomyrmecinae (2) |
| Procryptocerus Emery, 1887 - 40, Neotropical | Myrcidris Ward, 1990 - 2, Colombia, Guiana, Brazil |
| Pseudomyrmex Lund, 1831 - 150, Neotropical |

Figure 3 A. Number of ant genera in Neotropical region compared to the rest of the world. B. Number of ant species in Neotropical region compared to the rest of the world. Data updated to January 2021 (Bolton 2021).
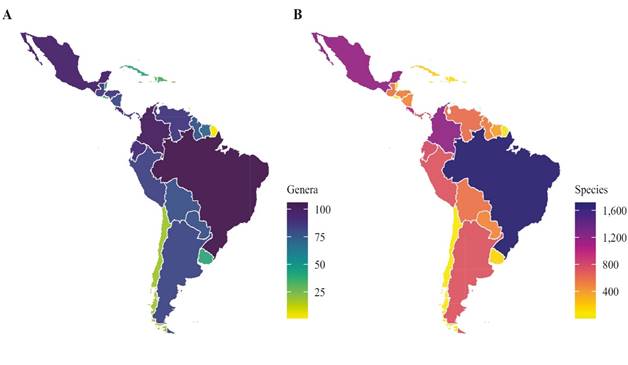
Figure 4 Comparison of ant genera (A) and species (B) count in the Neotropical region (discriminated by country). Brazil has the highest diversity with ~80 % of the genera and ~40 % of the species present in the Neotropical region, followed by Colombia (~73 % genera and ~29 % species) and Mexico (~70 % genera and ~28 % species).
Ant diversity and hyperdiverse insect families
Insects are considered the most diverse group of eukaryotes on the planet, with more than a million species described in some 1,200 families and 28 living orders. Certainly, this diversity is concentrated in the holometabolous orders, and within these in several subgroups, such as Parasitoida in Hymenoptera, Polyphaga in Coleoptera, Pupipara in Diptera, or Macrolepidoptera in Lepidoptera. Furthermore, of the more than 1,000 living insect families, only 19 can be considered hyperdiverse with an arbitrary estimate of 10,000 or more species (Fig. 5).
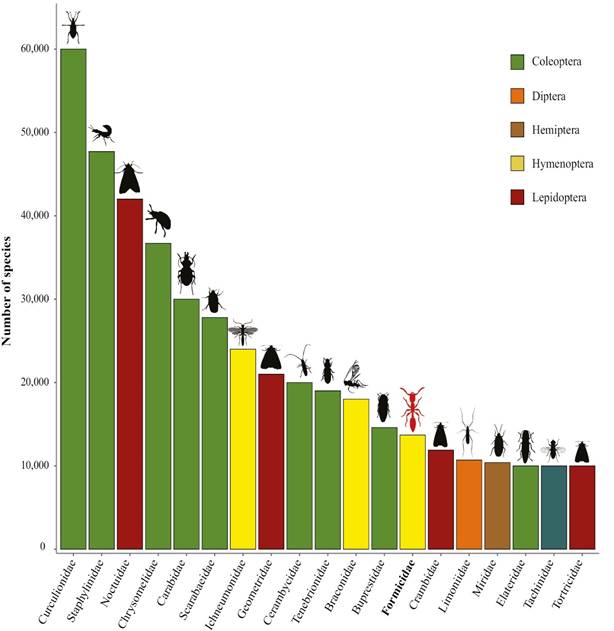
Figure 5 The most hyperdiverse insect families in the world places ants (red silhouette) occupying the 13th position. Free silhouettes images obtained from http://phylopic.org/.
Except for Miridae (Hemiptera), the rest belong to the four hyperdiverse insects orders, namely, Hymenoptera, Coleoptera, Diptera, and Lepidoptera. Formicidae occupies the 13th position with its almost 15,000 described species (Fig. 5). It probably does not rise much more on this scale, because in tremendously diverse groups such as moths there are not as many taxonomists working on their descriptions as there are in ants. If some estimates suggest more than 20,000 species of ants in the world, surely these must be more numerous in groups than the poorly known as Geometridae, Noctuidae, Chrysomelidae, or Staphylinidae. Another issue is reasonable identification capacity at least to the genus level. In this sense and at least for America, only for the families Formicidae and Braconidae are there tools (such as identification manuals, keys, and catalogs) that allow the user to be secure (or at least acceptable) in identifications of subfamilies and genera. In the case of ants, the sources of information for arriving at a species names are greater, with more than 70 % of the known species being named with some security.
Systematics
The Neotropical region is probably the richest realm for ants (Fisher 2010). To date, it comprises 137 genera and around 3,100 species, 38 % of the genera, and 39 % of the world’s species (Fig. 3). It is also the region with the highest number of endemics (Fernández 2003a). Part of the explanation is that the Neotropics has been the “cradle and museum” of the myrmecofauna (Moreau and Bell 2013); it is region that generates clades and preserves them. Within the Neotropical region, South America has had a complex geological and climatic history throughout the ages, especially since the Cenozoic; the subcontinent is a sum of cratons, each of them with a history that speaks of diversity of ecosystems and landscapes. On the other hand, episodes of connection and isolation with other continental masses have enriched the groups of ants that dominate South American landscapes, especially since the mid-Cenozoic (LaPolla et al. 2013). If we add to this the existence of about nine genera yet to be described (R. Feitosa and J Sosa-Calvo pers. comm.; FF personal observation), along with many species, in the near future the Neotropics will reaffirm itself as the metropolis of the ants.
The panorama for the taxonomy of ants is relatively acceptable, especially when compared to other megadiverse groups such as the Scarabaeidae, Staphylinidae, Tachinidae, Noctuidae, or Chrysomelidae. Virtually all genera of ants can be identified from workers, and this scenario is valid even for males (Boudinot 2019). Likewise, there are keys, monographs, or synopses of most genera, leaving only a few orphaned groups (Table 3). This vision can be summarized with the key groups like this:
Hypoponera. Small and homogeneous ants, very difficult to delimit species. Shawn Dash’s unpublished doctoral thesis suggests (Dash 2011), however, that the genus is workable with good use of morphometry and other tools. Currently, Amanda Dias and Rodrigo Feitosa (Federal University of Paraná) are developing a project on the systematics and phylogeny of the Neotropical Hypoponera.
Camponotus. After Pheidole this is the most diverse genus in the world and in the Neotropics, with about 450 species in this region. As with Pheidole, the taxonomy of Camponotusis based on major workers, making it difficult to put a species name on the many smaller workers commonly collected in the field. It is a genus that has occupied about 30 years of work of William P. Mackay (University of Texas at El Paso), who recently published a review of the fauna of Colombia (Mackay 2019b). Mackay (2019a) provides a synopsis of the subgenera of Camponotus and review the subgenus Camponotus.
Pheidole. Probably the largest and most complex genus of ants in the world has more than 700 species in the Neotropics. Wilsonʼs (2003) review and Longino (2009a) update are two great steps for clarifying the group’s taxonomy. However, secure identification is highly dependent on major workers (as in Camponotus), which creates a problem with the numerous museum exhibits that only have minor, with no direct link to major workers. Longino (2009b) presents an interactive key that can allow the identification of species with minor workers. Regional synopses are currently underway for Central America (Longino, J. T.), Colombia (Guerrero, R. J. and colleagues), and Brazil (Feitosa, R. and colleagues).
Crematogaster. Very common ants and difficult to identify due to the problems separating intra- from interspecific variation. The internal phylogeny of the group recently explored (Pedraza, L. and colleagues) maintains some species groups, divides others, and creates some new groups. These groups of species can promote regional or local reviews that can clarify the taxonomy of the group. A review of the groups associated with limata is underway.
Solenopsis. Reviews by Trager (1991), Pacheco and Mackay (2013), and Pitts et al. (2018) collectively cover the entire Solenopsis fauna of the New World. However, most species belong to this bothersome group and species identification without females is very difficult. The differences between workers are very subtle with the drawback of intraspecific or even intranidal variation.
Carebara. This is a complex genus, comprising monomorphic, dimorphic, polymorphic, and intercaste species. Monomorphic species (Carebara in the old sense) are probably dimorphic since the soldiers have not been able to be associated with field collections. The identification of species with only minor workers (Carebara lignata group) is practically impossible due to traditional morphology. One reason is that these workers are very small and many external morphological attributes have been removed; C. minuta could be the smallest ant in the world, at just 0.96 mm total length.
Azteca. This is perhaps the greatest of all challenges since identification with only workers is practically impossible. Except for specific groups (such as those associated with Cecropia) it is necessary to have a series of nests with the queen included.
Some genera orphaned in the past will soon have their taxonomy resolved; for example, for Acromyrmex (Christian Rabeling and colleagues, Arizona State University), Anochetus (Itanna Fernandes and colleagues, INPA, Brazil), Aphaenogaster (Jack Longino, The University of Utah), Atta (Corina Barrera, Universidad Estadual Paulista - UNE-SP), Basiceros (Beto Brandão and Rodrigo Feitosa, Brazil), Hypoponera (Amanda Dias and Rodrigo Feitosa, Federal University of Paraná), Myrmelachista (Rodolfo Probst, The University of Utah), Myrmicocrypta (Jeffrey Sossa-Calvo and Ted Schulz, Smithsonian), Nylanderia (John LaPolla, Towson University), Syscia (Jack Longino, The University of Utah) and Tapinoma (Roberto Guerrero, Universidad del Magdalena).
A synopsis of subfamilies, tribes and genera of extant Neotropical ants is presented in Table 2, and the list of the Neotropical ant genera that have been reviewed in the last 25 years, including keys for the Neotropical region or at least for some countries in the region, is presented in Table 3.
Table 3 Ant genera with synopsis or keys for Neotropics, or Neotropical countries, in the last 25 years.
Species delimitation
In groups of ants with an elevated number of species (and a generally wide distribution), morphology alone is insufficient to know how many species there are, how to separate them, and how to provide identification keys for non-specialist users. This implies that researchers must use various tools and multiple evidence to find out how diverse the group under consideration is. A clear advantage for this strategy is to have a rich knowledge of evolution, speciation and population dynamics. This information, together with biology and distributional data, offers an overview of the evolutionary biogeography of the group of ants studied. As happens in other taxa, one of these approaches involves the use of genetic information to establish the boundaries between species. This approach to species delimitation tries to determine how species and their populations are grouped in a broader sense than its classification (Carstens et al. 2013).
With the development of better analytical methods, increased computational capacity and the proliferation of genomic information, these genetic tools have become increasingly useful. It is important to keep in mind that the primary goal of these methodologies, based on molecular markers, is to identify independent evolutionary lineages and validate them as biodiversity units (Queiroz 2007; Carstens et al. 2013). It is from these units that species are discriminated using independent contrasts based on ecological, geographic, physiological, or morphological data (Leavitt et al. 2015; Prebus 2020). The main difficulty with this approach is the variability in the resulting delimitations, which, if not contrasted with other types of evidence (or if not available), it is not clear where the boundary should be placed between different species. For example, a Solenopsis study in Ecuador (Delsinne et al. 2012) shows high variability in some of the species but the marker used, COI (Cytochrome Oxidase Subunit I), was insufficient to resolve the limits of these ants on its own. Furthermore, fine molecular techniques and the abuse of the cryptic species concept can skyrocket the number of species in any group of insects. An additional problem of a practical nature is the near impossibility of identifying species whose delimitation is purely based on genetic markers. A user would have to repeat the same laboratory and analytic protocols to differentiate species, without the guarantee of obtaining the same results, precisely because of the genetic variation between the populations.
At the very least, the examples shown above indicate that in some cases, the separation of species by morphology is conservative, and at least the species richness of a group is not being artificially maximized (see also Andersen et al. 2016).
Of course, the opposite can be expected, accepting broad species criteria where morphological and genetic diversity is considered as simply being variants of a polymorphic species. After all, in insects, it has long been accepted that there is great phenotypic variation due to historical and ecological conditions of the various lineages or populations of many widely distributed species.
The ant landscape in the Neotropics is mixed. For 70 % - 75 % of the genera there are reviews, monographs, synopses, or taxonomic notes that allow reasonable sorting and identification of species. The AntWeb (2021) digital portal also offers high-quality photographs of most types of collections usually inaccessible to Latin American biologists. This digital page as well as that of the AntCat (Bolton 2021) also includes the Barry Bolton catalog and access to many digitized documents with original descriptions of species from Linnaeus. If the monotypic genera and those currently being reviewed (Hypoponera, Nylanderia, Crematogaster, Myrmicocrypta, and Tapinoma) are added to this information, the panorama is encouraging.
The nature or complexity of each group is what defines what types of tools should be used to delimit ant species. In genera as diverse as Strumigenys or Pheidole, morphology and/or morphometry may suffice to separate and name specimens. It is more a matter of precision and patience to name a specimen or establish if it is a new taxon. In genera such as Solenopsis or Azteca it is clear that many data sources must be used: biochemical (as cuticle chemistry), genetic (several markers), and natural history (ecology, behavior).
It is important to remember that the definition of a species represents the conclusion of a scientific investigation based on the knowledge and tools available at the time of the study. This definition may change with the development of new techniques, even in groups that are currently considered well- known. The promises of barcoding lagged far behind in the promise of replacing traditional taxonomy. Only the use of different criteria and tools can give a reasonable idea for the separation of evolutionary lineages and consequently the proposition and recognition of species. The integration of various information resources together with progress in data analysis will allow a better approach to the diversity of ants.
New tools and techniques useful for delimiting species continue to appear. For example, Ješovnik et al. (2017) used genomic data based on ultra-conserved elements (UCE) to reconstruct evolutionary histories and infer species limits in Sericomyrmex. Near infrared spectroscopy allows the analysis of hydrocarbons and other chemical compounds present on the surface of ants, facilitating the correct identification of cryptic species (Kinzner et al. 2015). The recent use of X-ray microtomography in the taxonomy of ants (Sarnat et al. 2016; Fischer et al. 2016; Hita Garcia et al. 2017) is very promising, although some challenges remain to be achieved (Hita Garcia et al. 2017). This technique allows detailed evaluations of the external and internal morphology of the specimen without causing damage. Volumetric models of the specimen are obtained that can be visualized and manipulated in three dimensions with resolution from micrometer to nanometer. Virtual reconstructions can be rotated, sectioned, and measured to gain a comprehensive understanding of the anatomy and morphology of the studied specimen (Hita Garcia et al. 2017).
Recently, Prebus (2020) used an integrated approach for the delimitation of species within the salvini group of the genus Temnothorax; this approach included molecular data of the UCE type, sequences of the mitochondrial genome, morphometric data, and geographical distributions analyzed under novel strategies that allow discrimination between possible population structure and reproductive isolation in the possible resulting species (species delimitation hypothesis). The Prebus (2020) results are based on a methodological scheme very rich in procedures and extremely useful for those genera with difficulties in the delimitation of the species (e.g., Hypoponera). But again, it is crucial to keep in mind that these innovative methods do not replace, but rather complement, other traditional methods and procedures (Schlick-Steiner et al. 2010). Let us remember that “the taxonomist’s nightmare is the evolutionary biologist’s delight” (Dejaco et al. 2016).
Perspectives and challenges
Whitfield and Kjer (2008) point out that it is possible that, due to ancient rapid radiation from many insect lineages, the morphological and/or molecular information has been erased or misrepresented in such a way that we lose clues for reconstructing the phylogenies of basal groups (stem groups). If ants originated long ago (perhaps towards the Jurassic-Cretaceous transition) in a short period of explosive diversification, clarifying the relationships of their basal groups will be difficult. If so, this would explain the difficulty in finding good support for the group closest to the root and sister group of the rest of the ants (Martialinae? Leptanilinae? The clade formed by both subfamilies? See Borowiec et al. 2019). However, the recent development of phylogenomics has in-creased group separation in the Insecta (Misof et al. 2014) and Hymenoptera (Johnson et al. 2013). Certainly, this new resource for information, together with computational advances, will make it possible to resolve various current uncertainties in the Formicidae.
One of the obstacles to the knowledge and protection of biodiversity is precisely knowing what we have and where. The enormous diversity of insects, which even in the most conservative estimates approximates 3 million species, shows that there are a large number of species to be described. Added to this problem is the progressive disappearance of qualified taxonomists who cover known groups. Many groups of insects remain virtually unknown or were reviewed decades ago. Even in economic interest groups (such as parasitoid moths or wasps), there is an urgent need for specialists. This has led some biologists to propose faster ways to describe the world’s entomofauna. Some proposals are controversial, such as describing species without control specimens (i.e., no types; for example, see Marshall and Evenhuis 2015). Others are very recent and will surely generate many controversies. An example of this is the description of species with gene characters that accompany focus stacking photographs, biology, and distribution data [Hoenle et al. (2020) for Odontomachus davidsoni]. In this sense, the morphological information is reduced to one image or a few images. Can this work for other ants? As in ants, there are many modern studies in DNA sequencing; in principle, it is not a problem to characterize species by specific sequences of chosen genes. However, many ants form or appear to form complexes in which one image is not sufficient to delimit or separate species. The presence of castes, the reproductive male or female may also be required. In other cases, there is morphological variation to such a degree that many images would be required, some in scanning electron microscopy (SEM), which in the long run would go against quick and simple descriptions. On the other hand, in ants like Solenopsis, genetic variation is high, so much so that there are conflicts between morphology and genes in defining a supposed species. Integrative taxonomy is a strategy that has the strength to accomplish a revision of a given taxon, although it requires time and resources that justify, in part, the so-called “taxonomic impediment”.
The impediment for specimen exportation
A final problem in ant systematics, as well as that of any other group of organisms, is more one of government regulation than of biology. The majority of countries in the Neotropical region have created and promoted laws and regulations of such complexity that it is practically impossible to collect and exchange specimens between countries and, therefore, between colleagues (Fernández 2011; Acosta and Pérez-González 2019). When cooperation between institutions and countries is the best strategy to face the “taxonomic impediment” and the gradual lack of resources in biodiversity studies, the governments themselves have created a tangle of regulations that discourages even the most persevering. This has forced some biologists to move between relative “illegality” and civil disobedience, and others to carry out local studies where the need to resort to sources of specimens outside the country is minimized.
Challenges and tasks
Although recent years have witnessed great advances in ant phylogeny and systematics, there are still several challenging tasks to achieve a better understanding of the diversity of Neotropical ants.
What is the sister group of ants? Recently, it has been proposed that the sphecid wasps (broad sense) and bees (Anthophila), that is the Apoidea superfamily, is the sister clade of Formicidae. Boudinot et al. (2020) propose the clade Formicapoidina for this association. This has several consequences in terms of visualizing the ancestry of both groups that probably lived towards the end of the Jurassic, and exploring the morphological changes has led to apoids and ants since the Cretaceous. It is interesting that Cariridris bipetiolata has been considered an Ampulicidae and not as an ant as proposed since this hymenopteran is from the Lower Cretaceous of Brazil and this family is at the base of Apoidea, not far from the supposed ancestor of Apoidea + Formicidae. On the other hand, if Apoidea is a superfamily, ants must be upgraded to a superfamily, like Formicoidea, with only one family, depending on the status of the fossil taxon Armaniidae * / Armaniinae *. Boudinot et al. (2020) propose new names, clades, and diagnosis within the Formicoidea.
Where is the root of the ant tree? The latest proposals have varied between Leptanillinae and Martialinae. The most recent phylogeny by Borowiec et al. (2019) propose both subfamilies as the sister group of the rest of ants (Poneroid plus Formicoid clades). It is important to highlight the observation of Borowiec et al. (2019) in the sense that it is not about increasing samples and genes, but about performing analyses by changing external groups and analytical methods. For example, several of the outer groups and Leptanillinae are richer in DNA nucleotide adenine (A) and thymine T pairs, while Martialinae and the rest of ants are richer in DNA nucleotide guanine (G) and cytosine (C) pairs.
Although the Poneroid clade appears to be monophyletic, some of its internal relationships are not entirely clear, especially the position of Proceratiinae. Within the Proceratiinae the genus Proceratium appears to be paraphyletic, and within the Ponerinae the genera Mesoponera, Euponera, and Cryptopone are not monophyletic (Borowiec et al. 2019). Likewise, the status of Mayaponera and Rasopone merits further study (Longino, pers. comm.).
In Dorylinae, the phylogenetic relationships and delimitation in the genera around the old concept of “Cerapachyinae” still needs to be explored in greater depth (Borowiec 2016, 2019). For the Neotropical region, the genus Eciton, one of the most studied in natural history needs a thorough revision to resolve the status of several of its subspecies.
The subfamily Myrmicinae is the most diverse and therefore it is where most problems have arisen as barriers to a clear picture of its phylogeny and diversity. The phylogeny of Ward et al. (2015) is a huge step forward, but some groups inevitably require further study. One of them is the internal phylogeny of the Solenopsidini tribe, especially the genera that have been described around Monomorium. The resurrections of Epelysidris and Trichomyrmex in Ward et al. (2015) and Chelaner (Sparks et al. 2019) clearly show the artificiality of Monomorium and the status of Nothidris, Antichthonidris (both from southern South America); and Monomorium delabieiFernández, 2007 (Northeast of Brazil) should be studied, which surely corresponds to more than one genus.
The hyperdiverse genera: Under this category are genera such as Pheidole (Wilson 2003) and Camponotus in the Neotropical region. Pheidole can have between 600 - 700 species or more in the Neotropical region, taking into account the large number of new species awaiting study and description. In addition to being diverse, both genera are very common at the regional level (Camponotus even approaches the level of the páramo in the Andes cordillera); therefore, they represent a great challenge in species identification. Taxonomic keys in Wilson (2003) are based on major workers, but since many field samples and museum collections contains mainly specimens with only minor workers, we need other tools to identify such material. John Longino’s Pheidole interactive taxonomic keys (Longino 2009b) are a great help, although they require a lot of detailed measurements and time, which a non-specialist user might consider a handicap. A similar situation occurs in Camponotus, where the published reviews and/or keys (e.g., Mackay and Mackay 2019) are mainly based on major workers. For Pheidole and Camponotus, new and varied identification tools are needed, without forgetting the elaboration of traditional and illustrated keys based on minor workers, at least on the local scale of the country (as Pheidole Project in Colombia).
Atta genus group. This is a group of great importance at the level of ecology and agricultural economics, but especially for its long and complex evolutionary history with mutualistic fungi, parasites, and bacteria. These ants comprised the Atta genus group in Attini tribe (sensuWard et al. 2015). Many studies have been conducted on coevolution among the group, in its associated fungi, and between both. Also, and as a consequence of these studies, some genera have been redefined and described, especially Mycetosoritis, Cyphomyrmex and Trachymyrmex. A consequent challenge with these changes is to offer keys or tools that allow the general user to correctly identify the genera of these ants, especially the leaf-cutter ants (Acromyrmex, Amoimyrmex and Atta).
Genera in need of revision. In the systematics section, genera were mentioned in need of revision but with ongoing projects in phylogeny and systematics. Many others are equally important but we do not know of global or local revision plans. Among these, we highlight Discothyrea, Odontomachus, Platythyrea, Neivamyrmex, Rogeria, Nesomyrmex and Xenomyrmex.
Critical areas. Ants in the Neotropical region has been unevenly studied, with well-inventoried countries like Costa Rica in contrast to others like Bolivia with modern studies practically non-existent. In addition to the importance of knowing the biodiversity of the entire Neotropics for biogeographic and conservation studies, the gradual destruction of forests forces an acceleration of research to increase our knowledge of critical and threatened areas, such as the Central American and inter-Andean valleys, the Chocó-Darién moist forests, the foothills of the Andean mountain range, large areas of Bolivia, the Yungas rainforest, northwestern Brazil, portions of the Cerrado savanna, and the Chaco region.















