Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Latinoamericana de Psicología
Print version ISSN 0120-0534
rev.latinoam.psicol. vol.41 no.1 Bogotá Jan./Apr. 2009
CIRCADIAN REGULATION OF DAILY RHYTHMS IN OREXINERGIC NEURONS IN DIURNAL AND NOCTURNAL RODENTS
REGULACIÓN CIRCADIANA DE LOS RITMOS DIARIOS EN NEURONAS OREXINÉRGICAS EN ROEDORES DIURNOS Y NOCTURNOS.
Gladys S. Martínez
Fundación Universitaria Konrad Lorenz Bogotá - Colombia
Michael D. Schwartz
University of Washington, Seattle, WA 98195 USA
Laura Smale
Michigan State University, East Lansing, MI 48824-1117, USA
Antonio A. Nuñez
Michigan State University, East Lansing, MI 48824-1117, USA
Corresponding author. Facultad de Psicología, Fundación Universitaria Konrad Lorenz. gmartinez@fukl.edu
Abstract
The work presented here focuses on the differential regulation of circadian rhythmicity by the central nervous systems of the diurnal Arvicanthis niloticus (or g rass rat) and the nocturnal Rattus norvegicus (or lab rat). In grass rats, neurons expressing orexin (ORX) showed a significant daily endogenous rhythm in the expression of Fos that correlated with the rhythm in sleep and wakefulness, and was reversed when compared to that seen in lab rats. In grass rats ORX-positive neurons received substantial projections from vasoactive intestinal polypeptide (VIP) neurons of the suprachiasmatic nucleus (SCN). In contrast few VIP positive fibers were seen adjacent to ORX- positive neurons in lab rats. This species difference sug gests a direct control by the SCN on neurons expressing ORX in grass rats and a more indirect regulation in lab rats. These results are consistent with the hypothesis that differences between diurnal and nocturnal species are due to differences in the functions of targets of the SCN such as the ORX neurons and the dorsomedial hypothalamus (DMH).
Key words: Orexin, grass rat; sleep wake cycle, animal circadian rhythms, species differences
Resumen
El trabajo que se presenta aquí se centra en la regulación diferencial que ejerce el sistema nervioso central sobre ritmos circadianos en una especie diurna, Arvicanthis niloticus, o rata Nile grass y una especie nocturna, Rattus norvegicus, o rata de laboratorio. En Nile grass, las neuronas que expresan orexina (ORX) mostraron un ritmo endógeno diario en la expresión de Fos, ritmo que correlaciona con el ciclo de sueño y vigilia de esta especie y que es opuesto en comparación con el ritmo visto en ratas de laboratorio. En Nile grass las neuronas de ORX reciben proyecciones sustanciales desde neuronas del núcleo supraquiasmático (SCN) que expresan el péptido vasoactivo intestinal (VIP). En contraste, en ratas de laboratorio se encontraron muy pocas fibras positivas para VIP adyacentes a neuronas de ORX. Esta diferencia entre especies sugiere un control directo por parte del SCN sobre neuronas que expresan ORX en Nile grass y una regulación más indirecta en ratas de laboratorio. Estos resultados son consistentes con la hipótesis según la cual las diferencias entre especies diurnas y nocturnas se deben a diferencias en las funciones de regiones que reciben eferencias del SCN tales como las neuronas de ORX y el hipotálamos dorsomedial (DMH).
Palabras clave: Orexina, rata Nile grass, ciclo sueño-vigilia, ritmo circadiano animal, diferencias entre especies.
Introducción
Most organisms, including humans, exhibit 24-hourcircadian rhythms in physiology, hor monal function, and behavior. In mammals, these rhythms are controlled by an endogenous circadian pacemaker located in the suprachiasmatic nucleus (SCN) of the hypothalamus (Moore & Eichler, 1972; Stephan & Zucker, 1972). Several lines of research with different animal models suggest that the SCN may function in a similar way across species (Vansteensel, Michel, & Meijer, 2008) and that the phase of its rhythms, with respect to the day-night cycle, is the same in diurnal and nocturnal mammals. For example, rates of glucose utilization and electrical activity in the SCN peak during the light phase in both nocturnal and diurnal species (Ruby & Heller, 1996; Schwartz, Reppert, Eagan, & Moore-Ede, 1983). The same is true for the expression of the immediate early gene product Fos; it is high during the light phase independently of whether the species is diurnal or nocturnal (Katona, Rose, & Smale, 1998; Kononen, Koistinaho, & Alho, 1990). The expression of clock genes is also similar in the SCN of diurnal and nocturnal species (Smale, Nunez, & Schwartz, 2008).
Despite these similarities, diurnal and nocturnal species differ in some SCN features: in both the nocturnal hamster and the diurnal chipmunk, photic stimulation induces Fos expression in the SCN if applied during the subjective night, whereas light pulses applied during the subjective day induce Fos expression in the chipmunk SCN but not in the hamster s SCN (Abe, Honma, Shinohara, & Honma, 1995). Differences in Fos expression in SCN neurons containing vasopressin (VP) have also been reported between the diurnal grass rat (Arvicanthis niloticus) and the nocturnal lab rat; whereas little or no colocalization is found in the SCN of the labrat, high colocalization of Fos and VP during the light period is found in the grass rat (Rose, Novak, Mahoney, Nunez, & Smale, 1999). It must be noted, however, that the species differences in the SCN do not segregate in a clear fashion with respect to the diurnal or nocturnal habits of the species studied so far.
Research about how the SCN communicates circadian rhythmicity to brain regions that regulate specific behaviors in diurnal species has shown some similarities between diurnal and nocturnal species. It is known for example, that the SCN projects to about the same regions in rats (Leak & Moore, 2001; Watts, 1991), hamsters (Kriegsfeld, Leak, Yackulic, LeSauter, & Silver, 2004; Morin, Goodless- Sanchez, Smale, & Moore, 1994), humans (Dai, Swaab, Van der Vliet, & Buijs, 1998; Dai, Van Der Vliet, Swaab, & Buijs, 1998), and more recently in the grass rat (Schwartz & Smale, 2004). It is also known that both VP and VIP are present in the SCN of almost all mammals (as reviewed in Smale & Boverhof, 1999). Despite these similarities, some studies suggest that differences in behavioral rhythms in diurnal and nocturnal species may result from: a) differences in the responsiveness of brain regions receiving signals from the SCN, b) differences in activity in brain areas adjacent to the SCN that may modify SCN signals or c) differences in connectivity between SCN subpopulations (Nunez, Bult, McElhinny, & Smale, 1999; Smale, Lee, & Nunez, 2003).
Neurons expressing the peptide orexin are found exclusively in the lateral hypothalamic area (LHA). They send axonal projections to all the components of the ascending arousal system (Peyron et al., 1998), and are predominantly active during wakefulness (Estabrooke et al., 2001; Torterolo, Yamuy, Sampogna, Morales, & Chase, 2001). Daily rhythms in the activity of ORX-A neurons were reported in a study with lab rats showing that Fos expression in these neurons peak at night (Estabrooke et al., 2001). In addition, prepro-ORX mRNA and ORXA in the pons and in the preoptic/anterior hypothalamic area increase during the dark hours and decrease during the day in lab rats, suggesting a role of ORX in the regulation of the sleep/wake cycle (Taheri et al., 2000). In lab rats, the rhythm of neural activity (i.e., expression of Fos) in ORX neurons persists in constant darkness (Estabrooke et al., 2001), which suggests that the rhythm is controlled by the circadian clock of the SCN. Additional evidence for circadian regulation of ORX in nocturnal species has been provided by recent experiments in which lesions to the SCN of lab rats abolished circadian rhythms of ORX levels in the cerebrospinal fluid (CSF) (Zhang et al., 2004). Experiments with the diurnal grass rat have also shown a daily rhythm in the expression of Fos in a pattern that corresponds to the activity rhythms of this species, but it is still unknown if such a rhythm persists in constant darkness (Martinez, Smale, & Nunez, 2002).
The aim of the experiments presented here was to investigate if the rhythms in neural activity in areas of the brain containing ORX persist under constant conditions in a diurnal rodent, and also to determine if ORX neurons might receive projections from the SCN in the diurnal grass rat and in the nocturnal lab rat.
General Method
Subjects
All of the grass rats (Arvicanthis niloticus) used in the experiments came from the breeding colony established in this laboratory and derived from the original group caught in Kenya in 1993 (Katona & Smale, 1997). Sexually mature Long.Evans lab rats (Rattus norvegicus) were purchased from Harlan Sprague Dawley, Indiana. Animals were allowed free access to food (PMI Nutrition Prolab RMH 2000, Brentwood, MO, USA for grass rats; standard rat chow, Harlan Teklad 22/5 rodent diet 8640 for lab rats) and tap water and were housed in standard plastic Plexiglas cages (34X28X17 cm for grass rats and 57Ã25Ã20 cm for lab rats). Animals were kept in constant darkness (DD) or under a 12:12 light-dark cycle (LD); for both conditions, a dim red light (<5 lux) was on at all time. Ambient temperature was kept at 22 °C and humidity at 48%.
All experiments were performed in compliance with the Michigan State University All-University Committee on Animal Use and in accordance with the standard in the National Research Council Guide for Care and Use of Laboratory Animals (National Academy of Science). All efforts were made to minimize the suffering and number of animals used in these experiments.
Experiment 1: Patterns of Fos expression in neurons expressing ORX in constant darkness
Method
Grass rats were first put in a 12:12 LD cycle with infrared motion detectors over the cage to monitor rhythms in general activity. Data for activity rhythms were collected with the Dataquest 3 program (Data Sciences International, St Paul, MN, USA). After a 1-2 weeks period in LD, lights were turned off at 18:00 hr and left off for 21-22days. After this period of free running, actograms for each animal were independently examined by two investigators to determine activity onset. Actograms for each animal were used to determine activity onset and time of perfusions. Animals were perfused at circadian time (CT) 5 (n=6), CT 13 (n=8), and CT 22 (n=7). These times were chosen because actograms showed that grass rats are active at CTs 5 and 13, and less active (and apparently sleeping) at CT 22 (Figure 1 ).
Animals were given an overdose of Nembutal (0.5cc) and were transcardially perfused with 0.01M phosphate buffered saline (PBS), pH 7.2, followed by 4% paraformaldehyde. Brains were removed and postfixed for about 2 hours in paraformaldehyde before being transferred to a 20% sucrose solution in 0.1M PBS overnight. Brains were sectioned at 30µm thickness (coronal plane) using a freezing, sliding microtome. They were divided in three alternate sets and stored into cryoprotectant in a -70ºC freezer until ready to use.
One set of this tissue was processed for dual immunocytochemistry (ICC) for Fos and ORX-B. Incubations were done in 0.1M PBS with 3% Triton-X- 100 (PBS-TX), at room temperature on a shaker. Free floating sections were rinsed in 0.1 M PBS (6X10) and preincubated in 5% normal donkey serum (NDS, Jackson Immunoresearch Labs, CA) for 1 hr. Sections were then rinsed (1X10 min) and incubated in rabbit anti-cFos (Santa Cruz Biochemistry, Santa Cruz, CA, 1:25,000) with 3% NDS on a rotator for about 24 hr at 4°C. Tissue was then washed in 0.1M PBS (3X10 min), placed in biotinylated donkey anti-rabbit (Jackson, 1:200) with 3% NDS for 1.5 hr, rinsed again in 0.1M PBS (3X10 min), and incubated in avidin-biotin complex solution (ABC Vectastain Elite Kit, Vector Laboratories, Burlingame, CA) for 1.5 hr. After 3X10 min rinses in 0.1M PBS, tissue was pre-incubated in diaminobenzidine (DAB) in acetate buffer with 2.5% nickel sulfate, reacted with 3% H2O2, rinsed in 0.1 M PBS-TX buffer (5X10 min) and then in 0.1M PBS (3X10 min). Tissue was then processed for ORX by incubating first in normal horse serum (NHS, Vector) and then in the primary antibody goat anti-ORX B (Santa Cruz, 1:60,000) and the biotinylated secondary horse anti-goat (Vector, 1:200), with 3X10 min rinses between incubations. ORX was visualized by reacting the tissue with DAB in Tris buffer and 30% H2O2. To verify specificity of each primary antibody, control sections were processed in the same way as described above, except that tissue was incubated without primary antibody. These treatments eliminated staining. Tissue was then rinsed, mounted onto gelatin-coated slides, dried, dehydrated, and coverslipped with DPX mounting medium.
Data Analysis
Counts were made on images of one section obtained at 10X magnification using a Zeiss AxioScop 2 plus microscope. A rectangle with the same characteristics as that used on animals kept in LD (Martinez et al., 2002) was placed over the pictures using the same landmarks as in our earlier study (Martinez et al., 2002). Labeling was confirmed by examining the tissue under 40X magnification. For each section, the number of cells containing ORX (both single and double labeled), double labeled cells, and cells expressing Fos but not labeled for ORX were unilaterally drawn and counted in each of the three areas (medial, central, and lateral). All counts were made without knowledge of the CT at which the animals were perfused.
A two-way ANOVA was used to evaluate the effects of CT and area on the number of cells expressing ORX. A square root transformation was used prior to this analysis. In the case of the percentage of ORX cells expressing Fos a two-way ANOVA was not performed because of lack of homogeneity of the variance even after standard transformations were used. Instead, non parametric one-way ANOVAs (Kruskal.Wallis test) were used. The effects of CT and area on the number of cells expressing only Fos were evaluated by using a two-way ANOVA; a log transformation was performed on these data. Tukey.s adjustment for multiple comparisons was used after the two-way ANOVAs to evaluate differences between means, and nonparametric multiple comparisons were used following the Kruskal.Wallis tests. Differences for all tests were considered significant when p<0.05. A correlational analysis was used to determine the relationship between Fos expression in and outside ORX neurons.
Results
Representative examples of single and double-labeled ORX-ir cells in grass rats are shown in Figure 3 . No labeled cells were seen in the control sections.
Distribution of ORX-ir cells
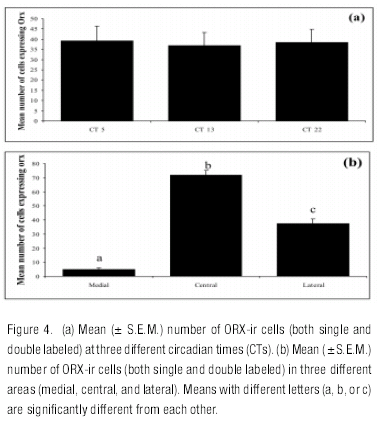
The distribution of ORX-ir cells was similar to what has been previously reported in grass rats (Martinez et al., 2002; Nixon & Smale, 2004; Novak, Mintz, Smale, & Albers, 2000). A two-way ANOVA showed significant effects of area (F=197,44, df =2, 54, p<0.001) and no effect of CT (p= 0.79) or any interaction (p= 0.67; see Figures 4a and b). Pairwise comparisons showed significant differences between the three areas, with the number of ORX cells higher in the central area than in the lateral and medial areas and with significantly fewer ORX cells in the medial area compared to the other two.
Fos Expression in ORX Neurons in Constant Darkness
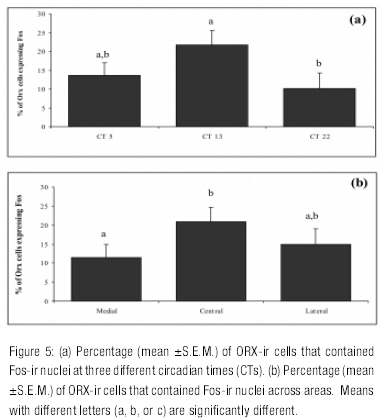
The one-way ANOVA on the percentage of ORX cells expressing Fos showed a significant effect of CT, with CT 13 being higher than CT 22 (H=6.69, df = 2, p<0.03) (Figure 5a ). The same non parametric analysis showed a significant effect of area (H=8.56, df = 2, p<0.01), with the percentage of ORX cells expressing Fos being higher in the central area than in the medial area (Figure 5b ).
Fos expression in non-ORX neurons
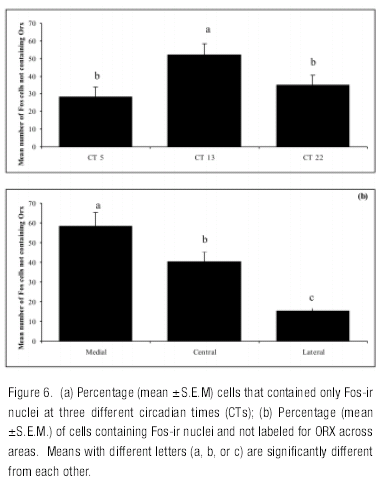
A similar rhythm in Fos expression to that found for ORX neurons was found for cells that did not contain ORX as indicated by a significant main effect of CT (F=11,72, df=2, 62, p<0.001). The number of Fos cells not containing ORX was higher at CT 13 than at CTs 22 and 5. In these neurons there was also a significant effect of area (F=35.24, df =2, 62, p<0.001) and no interaction (F=0.19, df =4, 62, p<0.94). Pair comparisons showed significant differences between all three areas, with the number of Fos cells not containing ORX being higher in the medial than in the central and lateral regions (Figure 6 ). Further analysis revealed a significant positive correlation (r =.30, df=61, p=0.01) between cells expressing Fos, but not ORX and double labeled cells.
Experiment 2: Distribution of VIP and VP fibers in the lateral hypothalamus in grass and lab rats
Method
Animals were perfused as described above, except that brains were postfixed for about 8 hours in nparaformaldehyde before being transferred to a 20% sucrose solution in 0.1M PBS overnight. Tissue was then processed for dual ICC using the procedure described for Fox and ORX ICC. Reagents used for each reaction were as follows:
VIP/ORX
VIP: NDS (Jackson), guinea pig anti-VIP (Peninsula Laboratories, Belmont, CA; 1:1500 for lab rats, 1:1000 for grass rats), and biotinylated donkey anti-guinea pig (Jackson, 1:200). ORX: NHS (Vector), goat anti-ORX B (Santa Cruz, 1:60,000), and biotinylated horse anti-goat (Vector, 1:200).
VP/ORX
VP: NGS (Vector), guinea pig anti-VP (Peninsula, 1: 40,000), and biotinylated goat anti-guinea pig (Jackson, 1:200). ORX: NHS (Vector), goat anti-ORX B (Santa Cruz, 1:60,000), and biotinylated horse anti-goat (Vector, 1:200).
High resolution photomicrographs of areas known to receive SCN projections in rats and mouse (Abrahamson et al., 2001) were taken using a digital camera (AxioCam MRc, Car Zeiss, Gottingen, Germany) attached to a Zeiss light microscope (AxioScop 2 plus) to confirm labeling for VIP or VP in both lab and grass rats. Image, contrast, and balance were adjusted using Zeiss Axiovision Software (Carl Zeiss Vision).
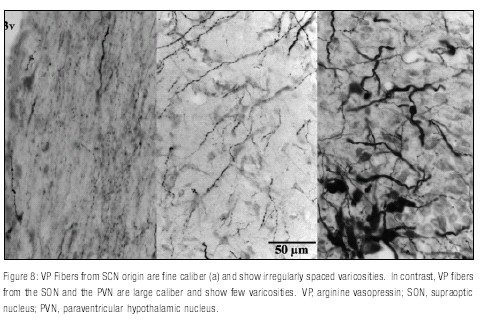
To examine the relationship between VIP or VP projections from the SCN and ORX-ir cells a picture of every section containing ORX neurons (average of 16 sections per animal) was taken at lower magnification (10X) to visualize the whole region of the hypothalamus with ORX+ cells. Counts of the total number of these neurons were made by evaluating the tissue at 40X magnification. After this, every neuron stained for ORX was evaluated at high magnification (100X, oil immersion lens) to determine if it was contacted by a VIP or VP labeled fiber; only axosomatic contacts of VIP or VP fibers on ORX neurons were considered. As proposed by Kriegsfeld et al. (2002) only those cases in which a VIP or VP bouton-like structure was observed in apposition with ORX neurons, the bouton and the fiber were on the same focal plane, and the labeled bouton was a clear continuation of axon were scored as contacts (Figure 2 ). By following these criteria, those cases in which the bouton looked more like a granule than a fiber were not counted. The origin of VP fibers was determined by contrasting them with previous descriptions of these fibers in mice; in contrast to fine caliber fibers from the SCN, fibers from the SON and PVN are large caliber and show few varicosities (Abrahamson, Leak, & Moore, 2001). With these data, the percentage of ORX neurons contacted by VIP or VP fibers per animal was determined. The non parametric Mann-Whitney U Test was used to test the differences in the number of fibers contacting Orx neurons between the two species.
Results
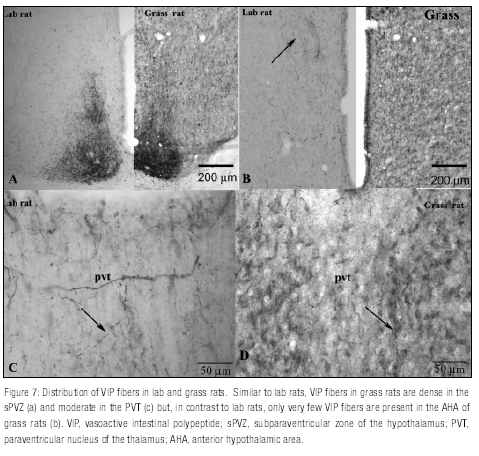
As it has been reported previously in lab rats, VIP fibers are dense in the subparaventricular zone of the hypothalamus (sPVZ, Figure 7a ), moderate in the paraventricular nucleus of the thalamus (PVT, Figure 7c), and less dense in the anterior hypothalamus (Figure 7b ). A similar distribution was seen in grass rats but a clear difference was found at the level of the anterior hypothalamus where only a few VIP fibers were seen in grass rats (Figure 7b ).In lab rats, VP fibers are abundant in the anteroventral periventricular nucleus (AVPe) and the anterior hypothalamic area (AHA), moderate in the DMH and even less dense in the ventromedial hypothalamus (VMH).
Analysis of VP contacts showed very few VP/ORX contacts in both grass rats and lab rats, and in both cases those contacts seemed to come from neurons of the supraoptic nucleus (SON) and the paraventricular nucleus of the hypothalamus (PVN) rather than from the SCN (Figure 8 ).
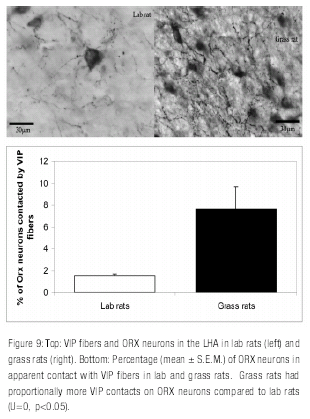
Inspection of each animal.s tissue under high magnification showed clear species differences in the abundance of VIP fibers approaching ORX neurons. Grass rats had proportionally more VIP contacts on ORX neurons compared to lab rats (7.9% and 1.6% respectively; U=0, p=0.05, one-tailed). See Figure 9 .
General Discussion
In Experiment One neural activity in a cell population regulating arousal in a diurnal species showed a daily rhythm in the expression of Fos in constant conditions as expected of a true circadian rhythm. Although only 3 CTs were sampled, the temporal pattern of Fos expression in ORX neurons after 3 weeks or more in constant darkness (DD) is almost identical to what is seen under a LD cycle (Martinez et al., 2002). Thus, the rhythm of Fos expression in ORX cells is endogenous and therefore likely to be under the control of the SCN in both lab rats and grass rats (Estabrooke et al., 2001), even though the phase of the rhythm is completely reversed when the two species are compared.
Differences in the phase of the rhythm of Fos expression in the ORX system of diurnal and nocturnal species may result from differences along the pathways that communicate circadian information from SCN to ORX neurons. In lab rats, the densest projection from the SCN is to the sPVZ, including the lower subparaventricular area (LSPV) (Watts, 1991). The SCN also provides inputs to the dorsomedial hypothalamus (DMH) (Watts, 1991). Both the sPVZ and the DMH have been postulated to serve as important relay sites for SCN signals that control vigilance (Aston-Jones, Chen, Zhu, & Oshinsky, 2001; Chou et al., 2003), and in lab rats, lesions in the sPVZ or in the DMH disrupt circadian rhythms of sleep (Chou et al., 2003; Lu et al., 2001). The DMH of lab rats receives direct inputs from the SCN and also indirect inputs via the zPVZ (reviewed by Chou et al., 2003), and it sends glutamatergic, and thus excitatory, projections to ORX neurons (Chou et al., 2003). Thus, in the lab rat, the circadian regulation of activity in ORX neurons may involve an indirect route with the DMH as the primary relay nucleus of SCN signals to the ORX system.
The anatomical distribution of ORX cells, doublelabeled cells and cells expressing Fos but not ORX seen in DD was identical to those seen in LD (Martinez et al., 2002). Also, the number of ORX cells was not affected by sampling time in both DD and LD (present data and Martinez et al., 2002). But one remarkable difference between DD and LD was seen in the population of cells that shows Fos expression but no ORX labeling. Whereas in LD Fos expression outside ORX neurons was not rhythmic and/or did not match the activity patterns of grass rats (Martinez et al., 2002), in DD a rhythm was present with a pattern that not only resembled the pattern of activity of grass rats but also that of the rhythm of Fos expression in ORX neurons. However, the distribution of cells that express Fos but not ORX was identical in LD and DD and different from that seen for ORX neurons. If expression of Fos outside ORX neurons in DD was due to Fos expression in .missed. ORX cells, then one would expect a preferential increase of cells expressing only Fos in the central area, where ORX neurons are abundant. Thus, the increase in Fos expression at CT 13 seen in DD but not in LD may represent an endogenous rhythm of neural activity in the non-ORX cells of the LHA that is masked by the light-dark cycle and only expressed in DD. It is unusual to encounter a circadian rhythm that is absent under LD but present in DD, but examples exist in the literature: in mice, the expression of mRNA for gastrin-releasing peptide (GRP) in the SCN is not rhythmic in LD but shows a low amplitude circadian rhythm in DD (Dardente et al., 2004).
As in LD (Martinez et al., 2002), there was only a modest positive correlation between the expression of Fos in ORX neurons and in cells with no ORX labeling, thus the two populations appear to be relatively independent of each other under both LD and DD. The results of Experiments in LD (Martinez et al., 2002) and DD (present experiment) suggest the presence of a group of non-ORX cells in the LHA that is under circadian control, is affected by light, and exhibits a similar pattern of expression of Fos to that seen in ORX neurons, but only in DD. The specific phenotype of these cells remains unknown but may include neurons that express melaning concentrating hormone (MCH). Neurons that express ORX or MCH are differentially regulated by adrenergic inputs to the LHA (Modirrousta, Mainville, & Jones, 2004).
In grass rats, the circadian control of the ORX system may depend upon direct projections of the SCN to ORX neurons. Studies of SCN efferent projections in grass rats have documented the presence of fibers of SCN origin in the region of the LHA where ORX neurons reside (Schwartz & Smale, 2004), whereas BDA injections in the LSPV and the SCN have confirmed cell soma and fiber-fiber appositions to ORX neurons from both regions (Schwartz & Smale, 2006), which suggests that in grass rats both direct and indirect projection from the SCN are likely to contribute to phase preference.
The direct inputs of the SCN to areas that control arousal in grass rats and lab rats were the focus of Experiment Two. In grass rats there was evidence for a substantial contribution of VIP neurons of the SCN to the inputs received by ORX neurons. In contrast, very few contacts of this type were seen in lab rats. Differences in VIP projections from the SCN to ORX neurons may contribute to species differences in the regulation of the sleep wake cycle: whereas in grass rats regulation may be in part mediated by direct projections from the SCN to areas involved in arousal, in the nocturnal lab rat this regulation may involve an indirect pathway that connects the SCN to neurons in the lateral and posterior hypothalamus.
In a previous study with lab rats, biotinylated dextran amine (BDA) injections in the SCN resulted in a high number of labeled fibers in close proximity to ORX neurons, and injections of choleratoxin b subunit (CTb) in the posterior hypothalamus showed retrograde labeling of cells that contained VP or VIP in the SCN (Abrahamson et al., 2001). Although it was reported in that study that immunocytochemical analysis revealed frequent contacts between VP and VIP fibers with ORX neurons, no data supporting that statement were presented (Abrahamson et al., 2001). The present data show very few contacts between VIP fibers and ORX neurons and no contact between VP fibers from SCN ORX neurons in lab rats. Since no data were presented in the study by Abrahamson et al. (2001), it is difficult to account for the apparent discrepancies. It is possible that they are related to differences in the type of contacts counted in each study. Whereas in the previous study both axosomatic and axodendritic putative contacts were considered, in the present study only axosomatic contacts were counted. Proximity to the trigger zone in axosomatic synapses allows for a faster and larger effect compared to the more distal axodendritic synapses. More recent studies confirm the sparse direct input from the SCN to orexin neurons in lab rats (and also in mice) (Ohno & Sakurai, 2008; Sakurai et al., 2005; Yoshida, McCormack, Espana, Crocker, & Scammell, 2006).
The results of the two experiments document at a functional- anatomical level differences and similarities in the neural systems that regulate arousal or wakefulness in diurnal and nocturnal rodents. Although there are differences in the timing of numerous behaviors between diurnal and nocturnal species, the neural basis for those differences has only recently become a topic for research. The work of others (reviewed by Smale et al., 2003) illustrates how the SCN, which regulates circadian behavior, shows many common functional and anatomical features when diurnal and nocturnal mammals are compared. Differences in the temporal control of behavior may be the result of differences either within the SCN (in clock cells, in output cells, or in the interactions between them) or outside the SCN, either in cells that receive direct input from the SCN or in intermediate areas that modulate SCN signals in a different way in diurnal and nocturnal species and then send this altered signal to brain targets. More recent data suggests that circadian control may depend on differences in the responsiveness of target areas of the SCN (Kalsbeek et al., 2008).
There is evidence that SCN axonal projections reach areas that control wakefulness and that are reversed with respect to neural activity in diurnal and nocturnal species (Novak, Smale, & Nunez, 2000). However, until the work presented here in Experiment 2, there was no evidence of direct contacts between SCN axons and neurons that produce ORX. The data from Experiment 2 indicate a monosynaptic pathway from the SCN to the ORX neuronal group of the LH. Since the SCN seems to function in a similar way across species, differences in rhythms of sleep and wakefulness may result from functional differences in these direct outputs from the SCN. Data from Experiment 2 also serve to identify differences in the contribution of neurons of the SCN to the inputs received by these ORX neurons in grass and lab rats. Whereas grass rats receive substantial inputs from VIP neurons of the SCN to neurons expressing ORX, lab rats receive very few VIP projections. Differences in VIP projections from the SCN to ORX neurons may contribute to species differences in the regulation of the sleep wake cycle: whereas in grass rats regulation may be in part mediated by direct projections from the SCN to areas involved in arousal, in the nocturnal lab rat this regulation may involve an indirect pathway that connects the SCN to neurons in the lateral and posterior hypothalamus. The DMH and the sPVZ have recently been proposed as relay centers between the SCN and areas regulating sleep and wakefulness in lab rats (Aston- Jones et al., 2001; Deurveilher & Semba, 2005).
The data for the ORX system reported in here and in Martinez et al. (2002) are consistent with the hypothesis that species differences are due to functional neural differences downstream from the SCN. Rhythms of neural activity in ORX neurons are reversed in diurnal and nocturnal species, and in both lab rats (Estabrooke et al., 2001) and grass rats the rhythm in ORX neurons is truly circadian, since it persists in constant darkness. The ORX system can be added to a list of areas that regulate sleep and wakefulness and that show a reversal or salient species differences in neural activity when diurnal and nocturnal animals are compared. This list includes them VLPO and the histaminergic system (Novak & Nunez, 1998; Novak, Smale et al., 2000).
References
Abe, H., Honma, S., Shinohara, K., & Honma, K. I. (1995). Circadian modulation in photic induction of Foslike immunoreactivity in the suprachiasmatic nucleus cells of diurnal chipmunk, Eutamias asiaticus. Journal of comparative physiology. A, Sensory, neural, and behavioral physiology., 176(2), 159-167. [ Links ]
Abrahamson, E. E., Leak, R. K., & Moore, R. Y. (2001). The suprachiasmatic nucleus projects to posterior hypothalamic arousal systems. Neuroreport, 12(2),435-440. [ Links ]
Aston-Jones, G., Chen, S., Zhu, Y., & Oshinsky, M. L.(2001). A neural circuit for circadian regulation of arousal. Nature Neuroscience, 4(7), 732-738. [ Links ]
Chou, T. C., Scammell, T. E., Gooley, J. J., Gaus, S. E.,Saper, C. B., & Lu, J. (2003). Critical role of dorsomedial hypothalamic nucleus in a wide range of behavioral circadian rhythms. The Journal of Neuroscience, 23(33), 10691-10702. [ Links ]
Dai, J., Swaab, D. F., Van der Vliet, J., & Buijs, R. M. (1998). Postmortem tracing reveals the organization of hypothalamic projections of the suprachiasmatic nucleus in the human brain. Journal of Comparative Neurology, 400(1), 87-102. [ Links ]
Dai, J., Van Der Vliet, J., Swaab, D. F., & Buijs, R. M. (1998). Postmortem anterograde tracing of intrahypothalamic projections of the human dorsomedial nucleus of the hypothalamus. Journal of Comparative Neurology, 401(1), 16-33. [ Links ]
Dardente, H., Menet, J. S., Challet, E., Tournier, B. B., Pevet, P., & Masson-Pevet, M. (2004). Daily and circadian expression of neuropeptides in the suprachiasmatic nuclei of nocturnal and diurnal rodents. Molecular Brain Research, 124(2), 143-151. [ Links ]
Deurveilher, S., & Semba, K. (2005). Indirect projections from the suprachiasmatic nucleus to major arousalpromoting cell groups in rat: Implications for the circadian control of behavioural state. Neuroscience, 130(1), 165-183. [ Links ]
Estabrooke, I., McCarthy, M. T., Ko, E., Chou, T. C., Chemelli, R. M., Yanagisawa, M. (2001). Fos Expression in Orexin Neurons Varies with Behavioral State. The Journal of Neuroscience, 21(5), 1656-1662. [ Links ]
Kalsbeek, A., Verhagen, L. A., Schalij, I., Foppen, E., Saboureau, M., Bothorel, B. (2008). Opposite actions of hypothalamic vasopressin on circadian corticosterone rhythm in nocturnal versus diurnal species. European Journal of Neuroscience, 27(4), 818-827. [ Links ]
Katona, C., Rose, S., & Smale, L. (1998). The expression of Fos within the suprachiasmatic nucleus of the diurnal rodent Arvicanthis niloticus. Brain Research, 791(1-2), 27-34. [ Links ]
Katona, C., & Smale, L. (1997). Wheel-running rhythms in Arvicanthis niloticus. Physiology & behavior, 61(3), 365-372. [ Links ]
Kononen, J., Koistinaho, J., & Alho, H. (1990). Circadian rhythm in c-fos-like immunoreactivity in the rat brain. Neuroscience Letters, 120(1), 105-108. [ Links ]
Kriegsfeld, L. J., Leak, R. K., Yackulic, C. B., LeSauter, J., & Silver, R. (2004). Organization of suprachiasmatic nucleus projections in Syrian hamsters (Mesocricetus auratus): an anterograde and retrograde analysis. Journal of Comparative Neurology, 468(3), 361-379. [ Links ]
Leak, R. K., & Moore, R. Y. (2001). Topographic organization of suprachiasmatic nucleus projection neurons. Journal of Comparative Neurology, 433(3), 312-334. [ Links ]
Lu, J., Zhang, Y. H., Chou, T. C., Gaus, S. E., Elmquist, J. K., Shiromani, P., (2001). Contrasting effects of ibotenate lesions of the paraventricular nucleus and subparaventricular zone on sleep-wake cycle and temperature regulation. The Journal of Neuroscience, 21(13), 4864-4874. [ Links ] [ Links ]
Modirrousta, M., Mainville, L., & Jones, B. E. (2004, October 23-27). Orexin and MCH neurons express c- Fos under different conditions of sleep deprivation vs recovery and bear different adrenergic receptors. Paper presented at the Society for Neuroscience.s 34th Annual Meeting, San Diego, CA. [ Links ]
Moore, R. Y., & Eichler, V. B. (1972). Loss of a circadian adrenal corticosterone rhythm following suprachiasmatic lesions in the rat. Brain Research, 42(1), 201-206. [ Links ]
Morin, L. P., Goodless-Sanchez, N., Smale, L., & Moore, R. Y. (1994). Projections of the suprachiasmatic nuclei, subparaventricular zone and retrochiasmatic area in the golden hamster. Neuroscience, 61(2), 391-410. [ Links ]
Nixon, J. P., & Smale, L. (2004). Individual differences in wheel-running rhythms are related to temporal and spatial patterns of activation of orexin A and B cells in a diurnal rodent (arvicanthis niloticus). Neuroscience, 127(1), 25-34. [ Links ]
Novak, C. M., Mintz, E. M., Smale, L., & Albers, H. E. (2000, November 4-9). Localization of hypocretinimmunoreactivity in the diurnal rodent, arvicanthis niloticus. Paper presented at the Society for Neuroscience.s 30th Annual Meeting, New Orleans, L.A. [ Links ]
Novak, C. M., & Nunez, A. A. (1998). Daily rhythms in Fos activity in the rat ventrolateral preoptic area and midline thalamic nuclei. American Journal of Physiology-Regulatory Integrative and Comparative Physiology, 275(5 Pt 2), R1620-1626. [ Links ]
Novak, C. M., Smale, L., &Nuñez, A. A. (2000). Rhythms in Fos expression in brain areas related to the sleep-wake cycle in the diurnal Arvicanthis niloticus. American Journal of Physiology-Regulatory Integrative and Comparative Physiology, 278(5), R1267-1274. [ Links ]
Nuñez, A. A., Bult, A., McElhinny, T. L., &Smale, L. (1999). Daily rhythms of Fos expression in hypothalamic targets of the suprachiasmatic nucleus in diurnal and nocturnal rodents. Journal of Biological Rhythms, 14(4), 300-306. [ Links ]
Ohno, K., & Sakurai, T. (2008). Orexin neuronal circuitry: role in the regulation of sleep and wakefulness. Frontiers in Neuroendocrinology, 29(1), 70-87. [ Links ]
Peyron, C., Tighe, D. K., van den Pol, A. N., de Lecea, L., Heller, H. C., Sutcliffe, J. G. (1998). Neurons containing hypocretin (orexin) project to multiple neuronal systems. The Journal of Neuroscience, 18(23), 9996-10015. [ Links ] [ Links ]
Ruby, N. F., & Heller, H. C. (1996). Temperature sensitivity of the suprachiasmatic nucleus of ground squirrels and rats in vitro. Journal of Biological Rhythms, 11(2), 126-136. [ Links ]
Sakurai, T., Nagata, R., Yamanaka, A., Kawamura, H., Tsujino, N., Muraki, Y. (2005). Input of orexin/ hypocretin neurons revealed by a genetically encoded tracer in mice. Neuron, 46(2), 297-308. [ Links ]
Schwartz, M. D., & Smale, L. (2004, October 23-27). Efferent Projections Of The Lower Subparaventricular Zone In The Nile Grass Rat. Paper presented at the Society for Neuroscience.s 34th Annual Meeting,San Diego, CA. [ Links ]
Schwartz, M. D., & Smale, L. (2006, October 14-18). The suprachiasmatic nucleus and the lower subparaventricular zone project onto orexin-A neurons in a diurnal rodent. Paper presented at the Society for Neuroscience.s 36th Annual Meeting, Atlanta, GA. [ Links ]
Schwartz, W. J., Reppert, S. M., Eagan, S. M., & Moore-Ede, M. C. (1983). In vivo metabolic activity of the suprachiasmatic nuclei: a comparative study. Brain Research, 274(1), 184-187. [ Links ]
Smale, L., & Boverhof, J. (1999). The suprachiasmatic nucleus and intergeniculate leaflet of Arvicanthis niloticus, a diurnal murid rodent from East Africa. Journal of Comparative Neurology, 403(2), 190-208. [ Links ]
Smale, L., Lee, T., & Nunez, A. A. (2003). Mammalian diurnality: some facts and gaps. Journal of Biological Rhythms, 18(5), 356-366. [ Links ]
Smale, L., Nunez, A. A., & Schwartz, M. D. (2008). Rhythms in a diurnal brain. Biological Rhythm Research, 39(3), 305-318. [ Links ]
Stephan, F. K., & Zucker, I. (1972). Circadian rhythms in drinking behavior and locomotor activity of rats are eliminated by hypothalamic lesions. Proceedings of the National Academy of Sciences of the United States of America, 69(6), 1583-1586. [ Links ]
Taheri, S., Sunter, D., Dakin, C., Moyes, S., Seal, L., Gardiner, J. (2000). Diurnal variation in orexin A immunoreactivity and prepro-orexin mRNA in the rat central nervous system. Neuroscience Letters, 279(2), 109-112. [ Links ]
Torterolo, P., Yamuy, J., Sampogna, S., Morales, F. R., & Chase, M. H. (2001). Hypothalamic Neurons that Contain Hypocretin (Orexin) Express c-fos During Active Wakefulness and Carbacholinduced Active Sleep. Sleep Research Online, 4(1), 25-32. [ Links ]
Vansteensel, M. J., Michel, S., & Meijer, J. H. (2008). Organization of cell and tissue circadian pacemakers: a comparison among species. Brain Research Reviews, 58(1), 18-47. [ Links ]
Watts, A. G. (1991). The efferent projections of the suprachiasmatic nucleus: Anatomical insights into the control of circadian rhythms. In D. C. Klein, R. Y. Moore & S. M. Reppert (Eds.), Suprachiasmatic Nucleus: The Mind.s Clock (pp. 77-106). New York: Oxford University Press. [ Links ]
Yoshida, K., McCormack, S., Espana, R. A., Crocker, A., & Scammell, T. E. (2006). Afferents to the orexin neurons of the rat brain. Journal of Comparative Neurology, 494(5), 845-861. [ Links ]
Zhang, S., Zeitzer, J. M., Yoshida, Y., Wisor, J. P., Nishino, S., Edgar, D. M. (2004). Lesions of the suprachiasmatic nucleus eliminate the daily rhythm of hypocretin-1 release. Sleep, 27(4), 619-627. [ Links ]
Recibido: febrero de 2009.
Aceptado: marzo de 2009