Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Colombiana de Ciencias Pecuarias
Print version ISSN 0120-0690On-line version ISSN 2256-2958
Rev Colom Cienc Pecua vol.20 no.4 Medellín Oct./Dec. 2007
Evaluation of propofol as an anesthetic in swine tracheal transplant surgery¶
Evaluación del propofol como anestésico para cerdos sometidos a trasplante de tráquea
Elmer J Gaviria1, MD; Juan G Restrepo1,2*, MV, SSc; Juan D Marín1, MD; Gonzalo Arango1, MD; Diego Aramburo2, MV; Felipe Franco2, MV; Luis F Tintinago1, MD.
1 Grupo Vía Aérea, Hospital Universitario "San Vicente de Paúl", Facultad de Medicina, Universidad de Antioquia, Medellín, Colombia.
2 Grupo de Investigación CENTAURO, Escuela de Medicina Veterinaria, Facultad de Ciencias Agrarias, Universidad de Antioquia. Medellín, Colombia.
(Recibido: 18 octubre, 2006; aceptado: 20 septiembre, 2007)
Summary
Because the swine have been used as an ideal animal model for different medical investigations, it has been useful to the advance in vital organs transplant field. The trachea transplant is a surgical procedure which requires special conditions in anesthetic depth and muscular relax, for a long period, and in addition, an excellent intra and post-operatory analgesic. The aim of this study was to use a combination of xylacine and ketamine, as premedication and evaluate propofol as a general anesthetic in trachea transplant donor or recipient pigs. All the methodology was under the approval of the Committee of Ethics for the Experimentation with Animals of the University of Antioquia. Ten donors and 10 recipients female Yorkshire pigs having a body weight of about 30 kg were used. Trachea extraction from a donor and its transplantation to a recipient in the same surgical procedure was performed. The average body weight (PP) was 30 ± 2.92 kg for both the groups, the average value were as follows: time of recumbency (TR) 8.25 ± 2:85 min; latency period (PL) 6.05 ± 1.73 min, (for both groups); surgical time (TQ) for donors and recipients was 80 ± 0.02, and 247 ± 0.02 min, respectively; heartbeat rate (FC) 90.34 ± 8.14 bpm, O2 saturation (SO2) 95.47 ± 1.79 %; exhaled PCO2 31.13 ± 1.89 mmHg; temperature (T) for both groups was 37.51 ± 0.74oC. The mean arterial pressure average (PAM) for both group was 65.47 ± 5.94 mmHg; the average time of esternal recumbecency (TRE) for donor female pigs was 16.50 ± 4.09 min, and the average time to stand up (TP) for swine recipients was 30.70 + 3.27 min. These results indicate that Propofol can be considered as a safe anesthetic for use in continuous perfusion. Since it has not an analgesic effect it is strongly recommended to combine it with opioids during anesthetic-surgical procedures; it can be also used with neuromuscular preanesthetics or inhaled anesthetics.
Key words: anesthetic in swine, propofol, experimental surgery, female swine, tracheal transplant.
Resumen
Los cerdos se han utilizado como modelo animal ideal para diversas investigaciones médicas; han sido útiles para el avance en el trasplante de órganos. El trasplante de tráquea es un procedimiento quirúrgico que requiere condiciones especiales en profundidad anestésica y relajación muscular por un período largo, y además, una analgesia intra y del postoperatoria excelente. Nuestra investigación utiliza una combinación de xylacine y ketamina, como premedicación y evaluar el propofol como anestésico general en cerdos donantes y receptores en quienes el trasplante de la tráquea sería hecho. Toda la metodología contó con la aprobación del Comité de Ética para la Experimentación con los Animales de la Universidad de Antioquia. Utilizamos 10 donantes y 10 cerdos raza Yorkshire hembras con un peso corporal de cerca de 30 kilogramos. Se realizó la extracción de la tráquea de un donante y el trasplante a un receptor en el mismo procedimiento quirúrgico. El peso corporal (PP) fue de 30 ± 2.92 kg para todo el grupo, el tiempo de recumbencia (TR) para ambos grupos fue de 8.25 ± 2.85 min, el período de latencia (PL) para ambos grupos fue de 6.05 ± 1.73 min, el promedio de tiempo quirúrgico (TQ) para los donantes fue de 80 min ± 0.02, el TQ de los receptores fue de 247m ± 0.02. La presión arterial media (PAM) para todo el grupo fue de 65.47 ± 5.94 mmHg, el promedio de frecuencia cardiaca (FC) para ambos grupos fue de 90.34 ± 8.14 ppm, el promedio de saturación de oxigeno (SO2) fue de 95.47 ± el 1.79% y el CO2 espirado fue de 31.13 ± 1.89 mmHg y el promedio de la temperatura (t) para ambos grupos fue de 37.51 ± 0.74oC. La tiempo de recumbencia esternal (TRE) para las cerdas donantes fue de 16.50 ± 4.09 min y el tiempo para pararse (TP) para los receptores fue de 30.70 ± 3.27 min. El propofol se puede considerar como anestésico seguro para el uso en la perfusión continua durante la anestesia. Puesto que no tiene un efecto analgésico se recomienda combinarlo con opioides durante procedimientos anestésico-quirúrgicos; puede también ser utilizado con preanestésicos, relajantes musculares o anestésicos inhalados.
Palabras clave: anestesia en cerdos, cirugía experimental, hembras suinas, propofol, trasplante de traquea.
Introduction
Researches on surgical interventions in animal experimental models have been carried out in several species of small mammals as rodents, lagomorphs, feline, canine and swine. Rodents and lagomorphs exhibit anatomical structures and physiologic mechanisms that greatly differ from that of humans. Canines and felines are companion animals, so due to its historical-cultural relationship with humankind a barrier exists for animal testing. Porcine is a specie used for profit purposes, but they have the advantage of having a lot of similarities with human’s anatomy and physiology, with regard to form, size and function mainly of cardiovascular, digestive and breathing systems. So, the swine have been used as an ideal animal model for different medical investigations. Moreover, it has been useful to the advance in vital organs transplant field, such as hearth, kidney, liver and lung; as well as in non vital transplants of B-pancreatic cells and cornea (9, 10).
The trachea transplant is a surgical procedure which requires special conditions in anesthetic depth and for some long periods a good muscular relaxation, and in addition, an excellent intra and post-operatory analgesia (7, 12). The most common drug combinations used for premedication and anesthesia are acepromazine-ketamine, diazepam-ketamine-xylacine, xylacine-ketamine-thiopental and xylacine-ketamine-thiopental and halogen anesthesics (21, 26).
Ketamine hydrochloride is a very versatile drug since it can be injected intramuscular or intravenous way, without causing tissue injuries. It exhibits a wide distribution to the corporal tissues, hepatic metabolism by desmethyilation and hydroxylation and renal excretion (8, 19, 26). Ketamine acts as an antagonist of glutamate receptors (NMDA), and blocking neuronal transport of serotonin, dopamine and noradrenalin, so it causes depression of thalamus-neocorticals projections, showing central vagolytic effects but without depressing ventilatory response to hypoxia (9). It also causes dissociative effects achieving animal indifference to external environment, very useful in achieving immobilization during the induction of the anesthesia. Ketamine mixed with xylacine provides a sedative and analgesic effect (14, 19, 26).
Xylacine is one of the most used analgesic sedatives in veterinary medicine. It stimulates alpha 2 adrenergic receptors of presynaptic terminations in the central nervous system, and it has a relaxant effect on muscle by inhibiting conduction of intercalar motor neurons in the spinal cord. Xylacine may produce throwing up, increase in uterine contraction and auriculoventricular blockade with bradycardia (3, 5, 12).
Propofol (2,6-diisopropilfenol) is a medication for intravenous use, short action beginning and lasting, with absence of accumulative effects; recovery is quick and calm so that makes it ideal to induce and conduct general anesthesia. It has been, used in lagomorphs, canines and feline, but there are no reports of their use in complex and long lasting interventions as in swine (6, 15, 23). It is stable at room temperature but should not be stored at temperatures higher than 25 ºC, neither sould be frozen; causes central nervous system depression by increasing GABA’s activity (4, 25, 26). This highly lipophylic drug crosses promptly the cellular membrane and takes about 2-4 minutes for establishing blood-brain balance, causing a rapid induction of anesthesia. It is metabolized in liver to form glucuronid and sulfate conjugates. The main elimination route is urine (half life of elimination 30 min) and in lesser quantities in stools (16, 18, 20).
Propofol lacks of vagolytic activity and instead may generate central vagotonic effects; it alters slightly the normal breathing patterns modifying central chemoreceptive response which is sensitive to CO2 levels, sensitive peripheral chemoreceptors to O2 levels and lung receptors. Hypercapnia and acidosis have been documented (6, 15, 22). We could consider el propofol as a safe anesthesic for continuous perfussion, but laking analgesic effect the combination with opioids is strongly recomended for anesthesic-quirurgic. It could be used with preanesthesic medication, neuromuscular blockers or inhaled anesthesics (10, 13, 17)
Our aim of this study was to use a combination of xylacine and ketamine, as premedication and evaluate propofol as a general anesthetic in ten donors and ten recipient pigs in which trachea transplant would be done, that should be spontaneously breath during the tracheal resection and transplant.
Materials and methods
Bioethical considerations
All the methodology and handling care for pigs used to in this research as for macro- and micro environmental, procedures and approaches of end point selection, were under the approval of the Committee of Ethics for the Experimentation with Animals of the University of Antioquia and comply with the Colombian laws on experimentation (Act Nº 14, September 9 2004).
Type of study
A descriptive study was carried out in the operating room at the School of Veterinary Medicine, University of Antioquia (Medellín, Colombia) by the Airway Team of the "San Vicente de Paúl" University Hospital and the research team CENTAURO at the same University. In Colombia, SPF swines does not exist, therefore animals coming from an industrial hatchery from the Servicio Nacional de Aprendizaje (SENA) that has a standard plan with vaccines and permanent antiparasitic medicaments where used for the study.
Pigs were bred under environmental temperatures of 18 to 20ºC, percentage of humidity of 60%, controlled airflows and a 12 hours relation of light /darkness; the corral have an area of 25 m2 for five animals and hard cement floor. Pigs were fed with 16% crude protein, 6% fibra and 3200 Mcal/kg (Solla, Medellín, Colombia) food and water were given ad libitum.
Surgical procedure for trachea transplantation
Before the surgery, female pigs were in stormed of 6 hours for solids and 1 hour for liquids; once finished the transplant, each swine passed to an intensive care unity with similar characteristics of macro and micro atmosphere, but in individual corrals of 4 m2.
Because female’s urethra is easier to canalize, in order to facilitate the evacuation of bladder, in this study we used 10 donor and 10 recipient female Yorkshire’s swine’s having a body weight of about 30 kg. We performed the trachea extraction from a donor and the transplant to a recipient during each the same surgical procedure.
The female pigs were prescribed with 10 mg/kg ketamina, 2 mg/kg xylacine, both by an intramuscular injection deeply between the semitendinous and semimembranous muscles. After the recumbency period, the marginal ear vein was canalized with an 18G or 20G angiocatheter and an intravenous (IV) anesthetic induction was performed with 5 mg/kg propofol, keeping spontaneous ventilation. Retrograde intubation or under direct laryngoscopic vision was performed with 6 to 7.5 mm-orotracheal tubes, depending on the size of the sow.
The tracheal was extracted from the donor sow after dissection of all adjacent anatomical structures. After the bench surgery ended, the tracheal was transplanted to the recipient sow, according to the protocol designed by the Airway Team.
Anesthesia was maintained with an average of 2 mg/kg/h propofol IV infusion, and as analgesic 2 ug/kg/h of fentanyl was used. Ventilation was sustained at an average volume of 8 to 10 ml/kg and 15 perfusion minute rates. At the end of surgery, the endotracheal tube was retired after the animal reached a normal breathing pattern, protective reflexs of the aerial way appeared, hemodynamic stability and no edema appeared in the airways. Postoperative analgesia consisted on a daily intramuscular injection of 0.5 mg/kg eltenaco (COX 2 inhibitor) during five days.
Follow-up
All swine were evaluated for temperature, breathing rate, heartbeat rate, mucus condition, time of capillary filling, corporal condition, progress, mood, behavior, and water and food consumption, before the surgery.
After ketamina - xylacine (K-X) administration, the recumbency time (TR), i.e. the time that the female pigs took in falling after propofol was given, was evaluated. The starting surgical time (TQ), which is the time lasted from the anesthetic’s initial administration until the time when the tracheal was extracted or transplant is finished and propofol was administered. The latency period (LP), which is the time each pig took loosing palpebral reflection once propofol was given, was also measured.
During the intravenous anesthesia a continuous non invasive monitoring of the mean arterial pressure (MAP), heartbeat rate (HR), O2 saturation (SO2) and temperature (T), was performed with a multiparameter monitor, model RGB Omicron FT A - 31, and the exhaled capnometry (PCO2) with a monitor model RGB Omicron FT A 22. After having finishing the surgery, time of esternal recumbecency (TER), i.e. the time that the animal took to be in esternal decubitus and the time to stand up (TS) or the time that took in standing up on its four extremities once the procedure was ended, were also measured.
Follow up after the intervention was carried out with daily evaluations of: body temperature, breathing frequency, heart rate, mucus conditions, time of capillary filling, corporal condition, mood, behavior and water and food consumption.
Statistical analysis
According to the objectives of this study statistical comparisons between donor or recipients groups were not done because euthanasia was practiced in donor pigs immediately after trachea extractions, then only descriptive statistics (average ± standard error) with maximums and minimums were performed for both groups when possible.
Results
The average body weight (BW) was 30 ± 2.92 kg for the entire group. In donor female pigs the BW was 30.20 ± 2.97 kg, while for the recipients it was 29.80 ± 3.01 kg.
The average TR for donors and recipients was 7.60 ± 2.32 min, and 8.90 ± 3.28 min and 8.25 ± 2.85 min respectively. The average latency period (LP) was 6.10 ± 2.08 min for donors, while for recipients was 6.00 ± 1.41 min. For both groups, the average LP was 6.05 ± 1.73 min. The average ST for donors and recipients was 80 ± 0.02 and 247 ± 0.02 min, respectively (See Table 1).
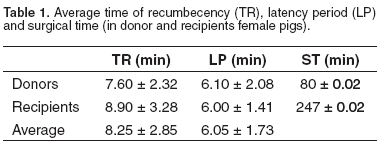
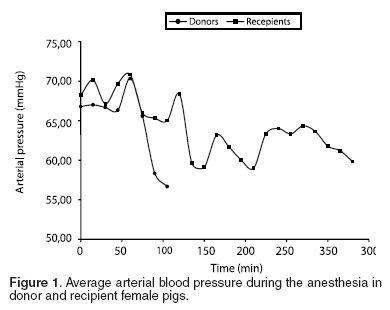
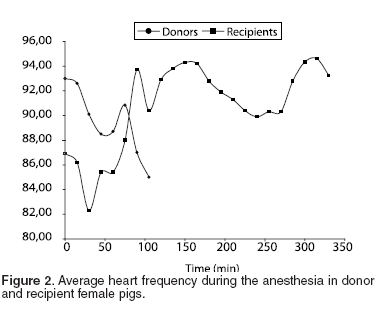
Variations in cardiovascular (PAM and HR) and ventilatory (SO2 and PCO2 ) parameters were measured during the anesthetic period. The meaning variations were correlated to the management of the proof of anesthetic procedures. The MAP for all the group was 65.47 ± 5.94 mmHg (See Table 2 and Figure 1), with an average of 66.76 ± 5.56 mmHg for the donor pigs and of the 64.19 ± 6.31 mmHg for the recipients. The HR average for donor female pigs was 90.30 ± 7.66 bpm, and 90.37 ± 9.00 bpm for recipients. The FC average for both groups was 90.34 ± 8.14 bpm (See Table 2 and Figure 2).
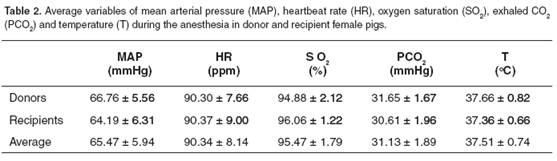
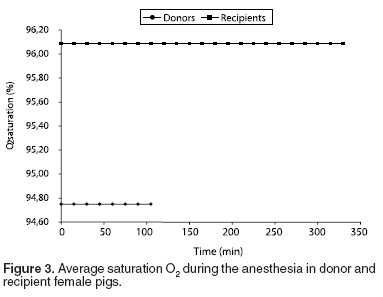
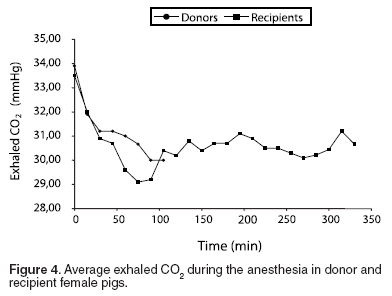
The average of O2 saturation (SO2) was 95.47 ± 1.79 %, and the exhaled PCO2 average was 31.13 ± 1.89 mmHg. For donor group female pigs SO2 was 94.88 ± 2.12%, while for recipients it was 96.06 ± 1.22% (See Table 2 and Figure 3). On the other hand, exhaled CO2 (PCO2) average for donor female pigs group was 31.65 ± 1.67 mmHg, and 30.61 ± 1.96 mmHg for recipient female pigs (See Table 2 and Figure 4).
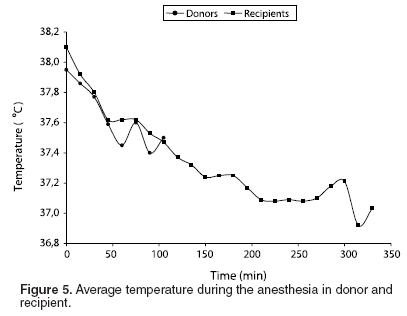
Average T for donor and recipient sow groups was 37.66 ± 0.82, and 37.36 ± 0.66 ºC, respectively; the average T for both groups was 37.51 ± 0.74oC (See Table 2 and Figure 5).
The average time of esternal recumbecency (TRE) for donor female pigs was 16.50 ± 4.09 min, whereas the average time to stand up (TS) for recipient female pigs was 30.70 + 3.27 min.
Discussion
Tracheal transplant in swine is a complex surgical procedure that requires some special conditions of handling of the aerial way and anesthetic drowsiness with hemodynamic stability during prolonged times, besides an excellent analgesia as much as intra- and post-operative (13). At different moments of the surgery procedure it is necessary that the animal breathing would be spontaneous; and under general anesthesia or with intravenous anesthetic agents we should provide these conditions, when normal air way and intubation are interrupted as it happens to extract tracheal and transplant it.
To our knowledge propofol fulfills the required conditions for the tracheal transplant. It should be considered that this is the first report on porcine to be surrendered to long period anesthetic procedures with propofol.
Average weight of female pigs used during this investigation was uniform for both groups (donors and recipients). This is a fundamental aspect when analyzing similar variables in animal experimental models.
After of K-X administration recumbency time, or time that the female pigs took in falling, it was shorter for the donor group possibly because it was administered around noon and the caloric diestrés accelerated absorption and distribution of the medications; K-X administration to recipients female pigs was in the afternoon for some animals, so they were rested and calm a bit. For both groups the TR was short because of the quick action, sedative and relaxant of the ketamina with xylacine combination (12).
Premedicated animals were easy to manipulate, and they were infused the propofol, the latency period (LP). In transplanted female pigs , anesthetic induction was quick, soft and free of excitement, which is attributed to propofol quick distribution in the organism, initially to the brain and highly perfused tissues and later on to other fewer irrigated (17, 21), it was found that propofol provides a quick induction of general anesthesia despite of pre-anesthetic medication.
Despite of surgical time for recipient female pigs was about 3.1 times longer than for donors; the hemodynamic and breathing parameters were properly stable during all long the procedure. When analyzing PAM for both groups, we find that the average MAP during the first hour was 65.47 mmHg for donor and 64.19 mmHg for recipient female pigs. The HR stayed about of 90.37 bpm during the whole procedure. These two variables allow us to conclude an appropriate hemodynamic stability during a long surgical procedure; with some individual variations that depend on the surgical moment which were they are subjected, for example, tracheal resection or carotid clamping that renders variable stimulation.
Average oxygen saturation in the donor and recipient groups was 94.88, and 96.06%, respectively. It is important to state that the lower saturation value found in donors was due to a fall during the tracheal’s extraction, where euthanasia was administered. During the tracheal exchange and the transplantation of the tracheal, the saturation stayed around the normal average values because we allowed the female pigs spontaneously to breathe and they were given a transitory trans-tracheal ventilation jet with 100%, when SO2 decreased below 90% (15, 25).
The average of CO2 exhaled was 31.13 mmHg, during the whole procedure. Compared to the blood measured P CO2 (35 - 36 mmHg), we considered the exhaled CO2 remained on normal expected values. Knowing that the difference among the breathed and the one measured by sanguine gases (35-36 mmHg) it is of 5 mmHg below the breathed air, we consider that female pigs remained between normal parameters during the whole surgical time.
The average temperature was 37.51 ºC, showing a time decreasing tendency, explained by exposure of the air road to ambient, the administration of cold endovenous liquids, the operating room temperature (21 ºC) and depression of the central nervous system. This temperature decreasing did not have implications in the animal’s final recovery.
As much the TRE as the TRT were short, post-anesthesia recovery was quick, and calm. Important factors in recovery shortened period are the quick metabolic rate in liver and assumingly other places of extra-hepatic metabolism, and the effective elimination of propofol from blood stream (10, 21, 23).
Comparing our results with those obtained in canine by Smith 1993, it can be deduced that surgical anesthesia is related with the maintenance dose of propofol, achieving a good anesthetic effect for several hours. Use of anesthetic premedication influences the anesthesia time and it can also allow to reduce the dose of propofol used.
Finally, we demonstrated the use of K-X as a premedication and propofol as an intravenous anesthetic in continuous infusion for use in porcinis experimental models, useful because the rapid induction of anesthetic events, good muscular relaxation, but more important, propofol was hemodynamically safe. We propose it in research and procedures which required prolonged times of anesthesia.
Propofol can be considered as a safe anesthetic for use in continuous perfusion. Since it has not an analgesic effect it is strongly recommended to combine it with opioids during anesthetic-surgical procedures; it can be also used with neuromuscular preanesthetics, inhibitors or inhaled anesthetics.
Acknowledgments
Authors thanks to Dr. Gildardo Alzate, Mrs Pilar Vanegas and Mr Johny Stan from operating room of the Veterinary Medicine School, University of Antioquia and Mr. Heriberto Arias and Gildardo Bedoya from National Learning Service (SENA) Colombia.
References
1. Al-Jahdari WS, Yamamoto K, Hiraoka H, Nakamura K, Goto F, et al. Prediction of total propofol clearance based on enzyme activities in microsomes from human kidney and liver. Eur J Clin Pharmacol 2006; 62:527-533. [ Links ]
2. Boschert K, Flecknell PA, Fosse RT, Framstad T, Ganter M, et al. Ketamine and its use in the pig. Recommendations of the consensus meeting on ketamine anaesthesia in pigs. Lab Anim 1996 Jul; 30:209-19.Adams HR. Veterinary pharmacology and therapeutics. 8th ed. Ames: Iowa State University press; 2001. p.209-219. [ Links ]
3. Botana LM, Landoni F, Jimenez T. Farmacologia y Terapéutica Veterinaria. Madrid: McGraw-Hill; 2002. 734 p. [ Links ]
4. Branson K, Gross, M. Propofol in veterinary medicine. JAVMA 1994; 204:1888-1890. [ Links ]
5. Cruz JI, Burzaco O, Campoy L, Ruiz J, Escartín A, et al. Anestesia total intravenosa para un modelo experimental de transplante ortotópico hepático en el cerdo. 5º Congreso Societat Catalana de Trasplantement. Barcelona, enero 1999:24-27. [ Links ]
6. Cullen L, Reynoldson J. Xylacina or metomidina premedication before propofol anestesia. Vet Rec 1993; 132:378-383. [ Links ]
7. Duke T. A new intravenous anesthetic agent: propofol. Can Vet J 1995; 36:181-183. [ Links ]
8. Guignard B, Coste C, Costes H, Sessler DI, Lebrault C, et al. Supplementing desflurane-remifentanil anesthesia with small-dose ketamine reduces perioperative opioid analgesic requirements. Anaesth Analg 2002; 95:103-8. [ Links ]
9. Hardam JG and Limbird L. Goodman and Gilman. The pharmacological basis of therapeutics. 11th ed. Nueva York: MacGraw-Hill, Interamericana; 2006. 2148 p. [ Links ]
10. Khursheed M, Hendrickson D. Pain management and anesthesia. Vet Clin North Am 2002; 1:204-207. [ Links ]
11. Ko JC, Williams BL, Smith VL, McGrath CJ, Jacobson JD. Comparison of telazo, telazol-ketamine, telazol-xylazine and telazol-ketamine-xylazine as chemical restraint and anesthetic combination in swine. Lab Anim Sci 1993; 43:476-480. [ Links ]
12. Ko JC, Williams BL, Rogers ER, Pablo LS, McCaine WC, et al. Increasing xylazine dose-enhanced anesthetic properties of telazol-xylazine combination in swine. Lab Anim Sci 1995 ; 45:290-294. [ Links ]
13. Kompatzki A. Nefrectomía laparoscópica donante vivo y autotrasplante en un modelo porcino. Rev Chi Urol 2003; 68:19-26. [ Links ]
14. Mathews KA. Perioperative use of nonsteroidal antiinflammatory analgesics. World Small Animal Veterinary Association. World Congress- Vancuver 2001. 215 p. [ Links ]
15. Matth N. Small animal practice clinical anesthesia. Vet Clin North Am 1999; 29:611-835. [ Links ]
16. Muñoz-Cuevas J, Cruz-Paz M, Olivero-Vásquez Y. Propofol ayer y hoy. Rev Mex Anest 2005; 28:148-158. [ Links ]
17. Murayama T, Sato Y, Wainai T, Enomoto A, Seo N, et al. Effect of continuous infusion of propofol on its concentration in blood with and without the liver in pigs. Tran Proc 2005; 37:4567-4570. [ Links ]
18. Padrid P. Small animal practice, respiratory medicine and surgery. Vet Clin North Am 2000; 30:1390 p. [ Links ]
19. Reichle F, Conzen P. Halogenated inhalation anesthetic. Bes Prac Res Clin Anes 2003; 7:29-46. [ Links ]
20. Schuttler J, Ihmsen H. Population pharmacokinetics of propofol: a multicenter study. Anesthesia 2000; 92:727-738. [ Links ]
21. Shafer S. Advances in propofol pharmacokinetics and pharmacodynamics. J Clin Anesth 1993; 5:14S-21S. [ Links ]
22. Short T, Young Y. Toxicity of intravenous anesthetic. Bes Prac Rese Clin Anes 2003; 7:77-89. [ Links ]
23. Skues M, Prys-Roberts C. The pharmacology of propofol. J Clin Anesth 1989; 1:387-400. [ Links ]
24. Smith J, Gaynor R, Bednarski R, Muir W. Advances effects of administration of propofol with various preanesthetic regiment. JAVMA 1993; 202:1111-1115. [ Links ]
25. Upton RN, Ludrook GI, Grant C. Cardiac output is a determinant of the initial concentration of propofol after short infusion administration. Anesthesia Analgesia 1999; 89:545-552. [ Links ]
26. Wright M. Pharmacologic effects of ketamine and its use in veterinary medicine. JAVMA 1982; 180:1462-1471. [ Links ]
¶ Para citar este artículo: Gaviria EJ, Restrepo JG, Marín JD, Arango G, Aramburo D, Franco F, Tintinago LF. Evaluation of propofol as an anesthetic in swine tracheal transplant surgery. Rev Col Cienc Pec 2007; 20:447-454.
* Autor para el envío de la correspondencia y la solicitud de separatas: Grupo de Investigación CENTAURO, Escuela de Medicina Veterinaria, Facultad de Ciencias Agrarias, Universidad de Antioquia. Medellín, Colombia. E-mail: jrestrepo03@yahoo.com














