Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Colombiana de Ciencias Pecuarias
Print version ISSN 0120-0690On-line version ISSN 2256-2958
Rev Colom Cienc Pecua vol.23 no.3 Medellín July/Sept. 2010
Casos clínicos
Protective compromise of great omentum in an asymptomatic uterine rupture in a bitch: a case report¤
Compromiso protector del omento mayor en una ruptura uterina asintomática en una perra: reporte de un caso
Empenho protetor do omento maior em uma rotura uterina asymptomatica numa cadela: um porte de caso
María S González-Domínguez1*, MV, Esp.; Carlos A Hernández1 , MV, MS; Juan G Maldonado-Estrada2, MVZ, MS, PhD.
1Grupo de Investigación INCA-CES Facultad de Medicina Veterinaria y Zootecnia, Universidad CES; Medellín, Colombia. 2Grupo de Investigación CENTAURO, Escuela de Medicina Veterinaria, Universidad de Antioquia. Medellín, Colombia.
(Recibido: 19 agosto, 2009; aceptado: 15 julio, 2010 )
Summary
The great omentum plays an important role in protecting the peritoneal cavity from bacteria and contaminating material and providing the peritoneum with leukocytes from the omental milky spots (OMS). However, there are no reports on the existence of OMS in dogs. In this report an unusual case of asymptomatic uterine rupture (UR) is described in a 16 month old pointer bitch that was admitted at the CES University Veterinary Clinic in Medellin (Colombia) for elective neutering. In the abdominal surgical plane, the great omentum was found sequestering abundant macerated fetal debris and uterine content released near the ruptured uterine wall. A severe congestive and brown-like appearance of peritoneum suggesting a protective inflammatory process was observed. All uterine contents, uterus and compromised great omentum were completely removed. The dog recovered satisfactorily with no clinical complications after a long term postsurgical period. Additionally we discuss the existence of OMS in the canine omentum.
Key words: asymptomatic uterine rupture, canine epyplon, elective neutering.
Resumen
El omento mayor juega un papel importante en la protección de la cavidad peritoneal contra infecciones bacterianas y material contaminante proporcionando leucocitos al peritoneo producidos en los puntos lechosos del omento (OMS). Sin embargo, en la literatura científica no hay reportes sobre la existencia de los OMS en caninos. En este reporte es descrito un caso poco usual de ruptura uterina (UR) asintomática en una perra de la raza pointer de 16 meses de edad, que fue atendida en la consulta del Centro de Medicina Veterinaria y Zootecnia de la Universidad CES en Medellín (Colombia) para ser sometida a ovariohisterectomía electiva. Una vez fue alcanzado el plano quirúrgico abdominal el omento mayor fue encontrado recubriendo una cantidad abundante de restos fetales macerados y otro contenido uterino que había sido liberado a la cavidad peritoneal cerca al sitio de ruptura de la pared uterina. El omento presentaba un aspecto de congestión severa de color parduzco, que sugería una reacción inflamatoria intensa. Todo el útero y el contenido revertido a la cavidad fueron removidos quirúrgicamente, como también el omento mayor. La perra se recuperó de manera satisfactoria sin complicaciones clínicas después de un largo periodo posterior a la cirugía. En la discusión es planteada la existencia de los OMS en el omento canino.
Palabras clave: epiplón canino, omento mayor, ovariohisterectomía electiva, ruptura uterina asintomática.
Resumo
O Omento desempenha um papel importante na protecção da cavidade peritoneal contra infecções bacterianas y material contaminante fornecendo no peritónio dos leucócitos produzidos dos pontos leitoso do omento (OMS). No entanto, na literatura científica não relata sobre a existência do OMS em caninos. Este reporte de caso descreve um caso incomum de rotura uterina (UR) assintomática em uma cadela da raça Pointer de 16 meses de idade, que foi notificado ao Centro de Medicina Veterinária y Zootecnia da Universidad CES en Medellín (Colômbia) para ser submetida à ovariohisterectomia electiva. Assim que o avião cirurgia abdominal foi atingido, o omento maior foi encontrado cobrindo uma generosa quantidade de remanescentes da maceração fetal e outros conteúdos uterinos tinham sido liberados na cavidade peritoneal, perto do local da ruptura da parede uterina. O Omento demonstraou um grave congestionamento de cor marrão, sugerindo uma intensa reacção inflamatória. A totalidade do útero e conteúdo revertida para a cavidade foi retirada cirurgicamente, bem como o omento maior. A cadela recuperou-se satisfatoriamente, sem complicações clínicas após um longo período após a cirurgia. Na discussão é levantada na existência do OMS no omento canino.
Palavras chave: omento canino, omento maior, ovariohisterectomia electiva, rotura uterina asintomatica.
¤ Para citar este artículo. González-Domínguez MS, Hernández CA, Maldonado-Estrada JG. Protective compromise of great omentum in an asymptomatic uterine rupture in a bitch: a case report; 2010. Rev Colomb Cienc Pecu, 2010; 23:369-376.
* Corresponding author: Grupo de Investigación INCA-CES, Facultad de Medicina Veterinaria y Zootecnia, Universidad CES. Phone (+574) 3360260. Medellín, Colombia. E-mail: mgonzalez@ces.edu.co
Introduction
Uterine rupture (UR) is a rarely diagnosed clinical entity in bitches and queens which is frequently related to trauma, hit by a car (Foster, 2009), abnormal uterine horn development (Schulman and Bolton, 1997), and pyometra (Esquivel, 2008; Foster, 2009; Lucas et al., 2003). Sporadic cases of UR could occur as a consequence of a postpartum complication when the dam had received oxytocin and/or prostaglandin for whelping induction, and for treatment of metritis and/or dystocia. UR occurs also as an unusual complication of trauma during late pregnancy or even during normal whelping (Linde-Forsberg, 2007). Clinical symptoms of UR include severe abdominal pain, distended abdomen and rapid detrimental of the dog's general condition. UR is frequently fatal and most of the cases remain undiagnosed (Hayes, 2004). A definitive diagnosis of UR is established only by exploratory laparotomy. Neutering accompanied with intravenous fluid therapy and antibiotics is the most accepted treatment for bitches affected by UR (Linde-Forsberg, 2007). There are no evidences of fetal death or fetal maceration as a cause of UR, neither its association with gravid uterine torsion, or abdominal trauma, have been documented so far (Linde-Forsberg, 2007). Abnormal uterine invasion by trophoblast cells during placenta development has also been related to UR in bitches (Foster, 2009). Peritonitis should be a critical complication of UR peritonitis if the peritoneal cavity is fulfi ll with contaminated material or reverted uterine contents as a consequence of the rupture.
Great omentum plays an important immune function consisting of protecting the peritoneal cavity from blood-born invading bacteria and from other contaminating material (Borisov, 1964). Great omentum provides the peritoneal cavity with rapid migrating leukocytes, mainly macrophages, T cells and B cells (Beelen et al., 1980). Competent immune cells are organized in great omentum into small lymphoid structures known as omental milky spots (OMS) (Shimotsuma et al., 1989; Shimotsuma et al., 1993). OMS were first reported in adult human great omentum (Borisov, 1964) and their presence was later confirmed in an eight-month-old infant's omentum (Shimotsuma et al., 1993).
Although great omentum was initially referred as a specialized omentum-associated lymphoid tissue (Shimotsuma et al., 1993), it consists of aggregated immune cells lacking the typical interdigitating cells and dendritic cells characteristics of the secondary lymphoid organs (Krist et al., 1995), although atypical dendritic cells functioning as antigen presenting cells (APC) has been reported (Carlow et al., 2005). The presence of OMS have been confirmed in great omentum of rats (Skurzak and Dux, 1971) and mice (Szaniawska, 1974; Szaniawska, 1975) in which these structures are responsible for the omental differentiation of macrophage colonies in response to peritoneumborne injuries (Ratajczak et al., 1987).
The presence of OMS in sheep and goats was evidenced by Brandt and Schnorr (1983). In the guinea-pig OMS were evidenced in experiments related to cytokine-induced lipolysis (Mattacks and Pond, 1999) suggesting that OMS can also exists as specialized immune structures in the guineapig peritoneum. Unfortunately there are no reports in the literature about the existence of OMS in domestic and companion animals, particularly in the dog. Here we report a UR in a 16 month old pointer bitch attended for an elective neutering, in which the asymptomatic course of the rupture could have been related to a protective compromise of great omentum.
Patient's examination
Anamnesis
A 16 month old Pointer female dog 26 kg of body weight was attended for elective neutering at general consultation service of “Centro de Medicina Veterinaria y Zootecnia” at CES University at Medellín (Colombia). The bitch had a complete vaccination schedule and had no history of any recurrent or allergic disease or clinical compromise.
The owner informed during the last estrous the bitch had been bred resulting in a fertile mating whose pregnancy was later on confirmed by ultrasound. Three months after breeding the dog had not shown whelping behavior. However, the dog's career reported to have seen a slight bloody vulvar secretion several days before consultation (no specific data was provided). Under the suspicion of abortion and cannibalism of the aborted fetuses the bitch was administered with antibiotics (no specific product or treatment schedule was provided) and then the owner decided for neutering the dog.
Clinical findings
At consultation for elective neutering the dog was subjected to a complete pre-surgical examination. At clinical examination the dog showed no clinical signs or hematological findings suggestive of a systemic inflammatory process or sepsis. Then the dog was programmed for surgery according to the routine of the hospital for elective neutering of a bitch.
Diagnostic aids
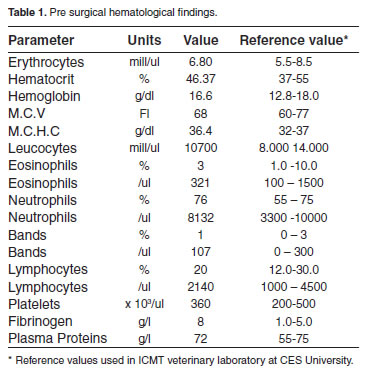
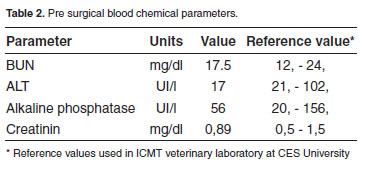
Blood samples were taken from jugular vein in sterile vacuum recipient with or without anticoagulants for complete and differential blood counts, and for pre surgical blood chemistry exams. All hematological and biochemical parameters indicated the dog had a healthy clinical condition (Tables 1 and 2).
Treatment schedule
Surgical procedure
Neutering was performed according to the standard schedule of the Clinics for non-pregnant bitch as previously reported (Ruiz et al., 2008). Briefly: Premedication was established with azepromazine (0.05 mg/kg, IM) and tramadol (1 mg/kg, IM). Anesthesia induction was done with ketamine (3 mg/kg I.V.) and propofol (3 mg/kg I.V.). Deep anesthesia maintenance was reached by continuous isofluorane inhalation using a Datex-Ohmeda S/5 Aespire anesthesia machine (Louisville, Kentucky, USA). Surgical planes were completed by a standard ventro-medial laparotomy (Fossum et al., 2007). When the surgical procedure was completed the muscular layer was sutured with a single continuous pattern (Using absorbable 3-0 suture). Non-absorbable 3-0 suture was used for skin suture in a simple continuous pattern. All suture material was from Novartis-Ethicon (Johnson & Johnson, EUA).
Clinical monitoreum during anesthesia
During anesthesia the patient was monitored by electrocardiogram (ECG), capnography (mm/Hg), oxymetry (% of saturation), non invasive arterial pressure (mm/Hg), body temperature (°C), cardiac frequency (Beats/min), and respiratory frequency (Respirations/min) using a Datex-Ohmeda S/5 Aespire anesthesia machine.
Postoperative care
For post-operatory controlling of pain the patient was given ketoprofeno (1 mg/kg p.o. c/24 h/2 d). No post-operatory complications were observed in the patient and cephalexin (20 mg/kg given twice orally during 5 days) was prescribed for preventing post-operatory infections. The dog recovered satisfactorily during the standard 10 days post operatory period.
Intrasurgical findings
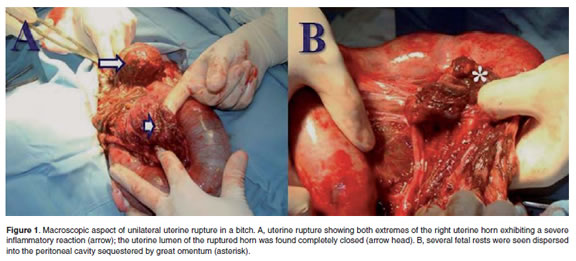
When the abdominal surgical plane was reached a complete rupture of the right uterine horn was observed (Figure 1A). Uterine content consisting of fetal debris was observed expelled into the peritoneum, including macerated fetuses (Figure 1B), and placental rests and debris. Interestingly, the great omentum was found actively protecting the peritoneal cavity by sequestering the fetal and placental rests.
The great omentum and peritoneum had a macroscopic evidence of a severe congestion but without evidence of an infectious inflammatory process. Great omentum was found covering fetal and placental debris and fetal fragments tightly adhered to the urinary bladder, small intestine, and macerated fetuses (Figure 1B). Finally, at both extremes of the ruptured uterine horn a severe inflammatory process covered by omentum was found suggesting a final healing process (Figure 1A).
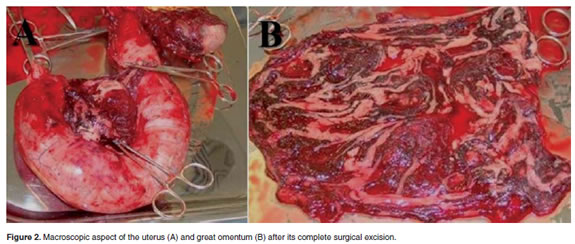
Once adherences were corrected the great omentum was excised and the surgical procedure was completed according to the standard procedure of the Clinics for OH (Ruiz et al., 2008). All tissues compromising adherences were surgically removed including the adherences of epyplon to the uterus and urinary bladder, small intestine, bowel and mesenterium, the left uterine horn containing non viable fetuses, and the remaining non ruptured uterus (Figure 2A). The peritoneum was observed severely irritated with no macroscopic evidence of infection, sepsis, necrosis, or purulent material (Figure 2B).
Discussion
In this case report a true asymptomatic UR is described in a bitch attended for an elective neutering. In several cases in which authors reported clinical cases of asymptomatic UR, they actually found several clinical signs, contrary to this report in which no clinical or laboratory evidence of UR was observed at consultation. Interestingly, the great omentum was found sequestering all fetal material and debris expelled by the rupture uterine horn into the peritoneal cavity, which evidence the protective role great omentum played in this case. The chronic nature of this finding is evidenced by the almost complete healing process found at the ends of the ruptured uterine horn. Curiously, the omentum exhibited a strong protective infl ammatory response not evidenced at clinical examination or by the results of clinical pathology (Tables 1 and 2). These findings are in agreement with a report in which a ruptured uterus showing a healing process compromising the omentum was found at necropsy in a pregnant queen that had received euthanasia (King and Amoroso 1983).
Unfortunately, no reports were found in the literature about true asymptomatic uterine rupture in bitches. In a recent report a post breeding symptomatic UR complicated with peritonitis was found in a bitch which presented abdominal pain, a bloody vaginal discharge, and lethargy at consultation (Morey, 2006). Uterine rupture has been reported in dogs in the following cases: 1) during surgical removing of a macerated fetus in a bitch (Hayes, 2004), 2) in a case in which fetal viscera of a mummified fetus was found adhered to omentum and other abdominal viscera during an elective surgical procedure practiced in a giant Schnauzer bitch (Hajurka et al., 2005), and 3) in a case of prolonged gestation in which seven fetuses expelled of the ruptured uterus were found adhered to bowel and bladder in a bitch (Banks, 1963). In all those cases clinical signs were evident at clinical exam and were suggestive of UR. On the contrary, according to the information provided by the owner, the patient of the present report had not history of a previous traumatic event, and no clinical signs of a previous injury were found at clinical examination, as supported by laboratory results (Tables 1 and 2). In cats, there is only one report of UR associated to trauma in a pregnant queen hit by a car (Lucas et al., 2003). These finding allowed us to conclude that great omentum probably accounted for the asymptomatic clinical condition found in the dog of this report.
Of particular interest in our report is the finding of great omentum sequestering all the fetal material that had been expelled of the ruptured uterine horn. As reported in human and mice, great omentum actively participates in the peritoneal response to infectious contamination or injuries (Shimotsuma et al., 1993; Platell et al., 2000). Great omentum exerts such protective functions by sequestering of the contaminating material and by absorption of contaminating bacteria. Because of its abundant blood supply great omentum exhibits a non negligible angiogenic potential and absorption capability (Platell et al., 2000). Curiously, surgical removing of the omentum has not considerable clinical consequences (Kirby, 2003). Accordingly, an intensive reaction of the great omentum was found in our patient, the affected omentum was surgically removed (Figure 2B), and dog did not present any clinical complication after a long term follow up.
Although several evidences exist on the experimental and therapeutic use of great omentum in veterinary surgery (Valat and Moisonnier, 2001) no reports were found on the existence of canine OMS. We suggest that the severe inflammatory reaction and the asymptomatic course of UR in our patient could provide evidence on the function of great omentum as an important lymphoid organ in cases of UR. Experimental and descriptive evidence are required to confirm the structure and function of OMS in dogs.
Because no reports about the existence of OMS in canine omentum was found in the literature, we shall refer to several studies in human and in laboratory animals in order to provide some insights into the importance of these structures in protecting the peritoneal cavity with a rapid responsive lymphoid structure. In species in which OMS have been studied the classical structure of a secondary lymphoid tissue has been described. In a study by Wilkosz et al., (2005) an ultra structural comparison between human and mice greater omentum was performed by phase contrast microscopy, scanning electron microscopy (SEM), and transmission electron microscopy (TEM). Authors found in both species that omentum share similar structure and is composed by two distinct types of tissue: One, well vascularised adipose-rich tissue covered by a continuous mesothelial cell layer excepting at the sites of OMS, and a translucent and membranous poorly vascularised tissue containing abundant fenestrations. The authors concluded that further studies are needed to investigate the compromise of the fenestrated tissue in adherence formation during abdominal pathologies (Wilkosz et al., 2005).
In mice, OMS express the β7 integrinMAdCAM-1 that facilitates the migration of specific B2 cells from blood stream into the peritoneal cavity (Berberich et al., 2008) in mice. T cells and B cells counterflow between the blood stream and the peritoneal cavity trough omentum a process mediated by the presence of dentritic cells sharing the CD8-/CD11bhigh/Major Histocompatibility Complex (MHC) class IIhigh/ CD11chigh phenotype, which is related to a rapid priming of T cells entering the OMS (Carlow et al., 2009). One of the components of omentum is the omental fat band (OFB), which has been recently reported as a very important immune compartment responsible for rapid T cell activation, the presence of abundant dentritic cells functioning as antigen presenting cells (APC), and in which both types of cells can interact to present cognate and exogenous antigens for early (extra-thymic) T cell differentiation and for activation of T cells against foreign antigen, respectively (Carlow et al., 2005). OMS can support B1 lymphocytes development (A special group of B cells responsible for T-independent immune responses). In addition the migration of CD4+ and CD8+ T cells and of effector T cells differentiated in other lymphoid organs cal also be sustained, supporting the concept that OMS are responsible for mounting the immune response against peritoneal invading antigens (Rangel-Moreno et al., 2009).
Histological changes in number and size of OMS were studied in experiments designed to study the response of the peritoneal cavity to the stimulus of peritoneal dialysis (PD), dialysis catheters and dialysis fluids in rabbits, and in omental biopsies from patients treated by PD, and peritoneal biopsies from patients with sclerosing peritonitis. Authors reported that in rabbits receiving intra peritoneal infusion of glucose (1.38% and 3.86%) the number and size of OMS significantly increased after 30 days treatment. In addition peritonitis induced a dramatic increase in OMS in human patients, whereas only slight changes were observed in OMS from patients with sclerosing peritonitis. Authors concluded that OMS functions as true secondary lymphoid organs, and that increased OMS was associated with augmented diameter of cells and number of vessels, suggesting an active process of angiogenesis in response to immunogenic stimuli (Di Paolo at el., 2005). These findings were further confirmed in a report by Litbargo et al. (2007) who found that Sprague Dawley rats receiving polydextran particle slurry intraperitoneally to activate the omentum, undergo several fold increases of the OMS, blood vessels, angiogenic factors like Vascular Endothelial Growth Factor (VEGF) and chimiokines like Stromal Derived-Factor 1 (SDF-1) and its receptor CXCR4, as well as cells harboring a regenerative phenotype (CXCR4, WT1) (Litbargo et al. 2007).
In conclusion, OMS functions as a very important and immune-specialized tissue serving as a reservoir for resident and migrating peritoneal inflammatory cells, a storage site for lipid, and in regulating peritoneal fluid exchange (Wilkosz et al., 2005). The role of omentum and particularly of OMS in peritoneal cavity immune defenses, and the rapid adherence of omentum to areas of inflammation and peritoneal injuries, raised the question about the importance of developing research activities conducted to characterize OMS in dogs. In the present report we provided evidence suggesting the existence of omentum as a protective tissue for the defense against peritoneal injuries in dogs as described in human and rodents.
Acknowledgements
Author thanks to University CES for funding INCA-CES research activities and CODI at University of Antioquia for partially funding Centauro research group (JME).
References
1. Banks PN. Uterine rupture in the bitch. J Small Anim Pract 1963; 4:345-347. [ Links ]
2. Brandt A, Schnorr B. Blood supply of the greater omentum in sheep and goats. Z Mikrosk Anat Forsch 1983; 97:427-440. [ Links ]
3. Beelen RH, Oosterling SJ, van Egmond M, van den Born J, Zareie M. Omental milky spots in peritoneal pathophysiology (spots before your eyes). Perit Dial Int 2005; 25:30-32. [ Links ]
4. Berberich S, Dähne S, Schippers A, Peters T, Müller W, Kremmer E, Förster R, Pabst O. Differential molecular and anatomical basis for B cell migration into the peritoneal cavity and omental milky spots. J Immunol 2008; 180:2196-2203. [ Links ]
5. Borisov AV. Lymphatic capillaries and blood vessels of milky spots in the human greater omentum. Federation Proc Transplant Suppl 1964; 23:150-154. [ Links ]
6. Carlow DA, Gold MR, Ziltener HJ. Lymphocytes in the peritoneum home to the omentum and are activated by resident dendritic cells. J Immunol 2009; 183:1155-1165. [ Links ]
7. Di Paolo N, Sacchi G, Garosi G, Sansoni E, Bargagli L, Ponzo P, Tanganelli P, Gaggiotti E. Omental milky spots and peritoneal dialysis--review and personal experience. Perit Dial Int 2005; 25:48-57. [ Links ]
8. Esquivel CF. Alteraciones del aparato reproductor de la perra. 2008. URL: http://www.vet-uy.com/articulos/artic_can/050/0020/can0020.htm [Accessed on 6 October 2008]. [ Links ]
9. Fossum T, Willard MD, Hedlund CS, Johnson AL, Seim HB, Willard MD. Small Animal Surgery, 2nd Ed. Mosby, 2002. [ Links ]
10. Foster R. Surgical pathology of the canine female reproductive tract. 2009. URL: http://www.uoguelph.ca/~rfoster/repropath/surgicalpath/female/dog/female_dog.htm [Accessed on 13 March 2009]. [ Links ]
11. Hajurka J, Macak V, Hura V, Stavova L, Hajurka R. Spontaneous rupture of uterus in the bitch at parturition with evisceration of puppy intestine- a case report. Vet Med 2005; 50:85-88. [ Links ]
12. Hayes G. Asymptomatic uterine rupture in a bitch. Vet Rec 2004; 154:438-439. [ Links ]
13. King GJ, Amoroso EC. Unusual phenomena during pregnancy in the cat and cow. Can J Comp Med 1983; 47:379-381. [ Links ]
14. Kirby BM. Peritoneum and peritoneal cavity. In: Textbook of small animal surgery. Third ed. Ed D. Slatter. Saunders, Philadelphia. 2003. pp414-445. [ Links ]
15. Krist LF, Eestermans IL, Steenbergen JJ, Hoefsmit EC, Cuesta MA, Meyer S, Beelen RH. Cellular composition of milky spots in the human greater omentum: an immunochemical and ultrastructural study. Anat Rec 1995; 241:163-174. [ Links ]
16. Linde-Forsberg C. Anomalías en la gestación, el parto y el período puerperal. In: Ettinger SJ, Feldman EC (Eds). Tratado de medicina interna veterinaria 6th ed. Elsevier, Madrid. 2007; pp1655-1667. [ Links ]
17. Litbarg NO, Gudehithlu KP, Sethupathi P, Arruda JA, Dunea G, Singh AK. Activated omentum becomes rich in factors that promote healing and tissue regeneration. Cell Tissue Res 2007; 328:487-497. [ Links ]
18. Lucas X, Agut A, Ramis G, Belda E, Soler M. Uterine rupture in a cat. Vet Rec 2003; 152:301-302. [ Links ]
19. Mattacks CA, Pond CM. Interactions of noradrenalin and tumour necrosis factor alpha, interleukin 4 and interleukin 6 in the control of lipolysis from adipocytes around lymph nodes. Cytokine 1999; 11:334-346. [ Links ]
20. Morey DL. Acute peritonitis secondary to traumatic breeding in the bitch. J Vet Emergency Crit Care 2006; 16:128-130. [ Links ]
21. Platell C, Cooper D, Papadimitriou JM, Hall JC. The omentum. World J Gastroenterol 2000; 6:169-176. [ Links ]
22. Rangel-Moreno J, Moyron-Quiroz JE, Carragher DM, Kusser K, Hartson L, Moquin A, Randall TD. Omental milky spots develop in the absence of lymphoid tissue-inducer cells and support B and T cell responses to peritoneal antigens. Immunity 2009; 30:731-743. [ Links ]
23. Ratajczak MZ, Jaskulski D, Pojda Z, Wiktor-Jedrzejczak W. Omental lymphoid organ as a source of macrophage colony stimulating activity in peritoneal cavity. Clin Exp Immunol 1987; 69:198-203. [ Links ]
24. Ruiz IC, Acevedo CM, Rodríguez M. Descripción y evaluación de una técnica de ovariohisterectomía laparoscópica en perras sanas. Rev Colomb Cienc Pecu 2008; 21:546-558. [ Links ]
25. Schulman ML, Bolton LA. Uterine horn aplasia with complications in two mixed-breed bitches. J South African Vet Assoc 1997; 68:150-153. [ Links ]
26. Shimotsuma M, Kawata M, Hagiwara A, Takahashi T. Milky spots in the human greater omentum. Macroscopic and histological identification. Acta Anat (Basel). 1989; 136:211, 216. [ Links ]
27. Shimotsuma M, Shields JW, Simpson-Morgan MW, Sakuyama A, Shirasu M, Hagiwara A, Takahashi T. Morpho-physiological function and role of omental milky spots as omentumassociated lymphoid tissue (OALT) in the peritoneal cavity. Lymphology 1993; 26:90-101. [ Links ]
28. Skurzak H, Dux K. Immunofluorescent studies of milky spots in rats inoculated with Ehrlich ascites tumor cells. Nowotwory 1971; 21:103-106. [ Links ]
29. Szaniawska B. Changes in the greater omentum of mice of different strains after intraperitoneal immunization with sheep erythrocytes. I. Production of IgM immunoglobulins in milky spots. Arch Immunol Ther Exp (Warsz). 1974; 22:585-593. [ Links ]
30. Szaniawska B. Changes in the greater omentum of mice of different strains following intraperitoneal strains following intraperitoneal immunization with sheep erythrocytes. III. Determination of the percentage of thymus-dependent cells in the omental milky spots in mice by the application of anti-o serum. Arch Immunol Ther Exp (Warsz). 1975; 23:19-24. [ Links ]
31. Valat B, Moisonnier P. The omentum "The surgeon's friend". Pract Med Chirurgic Anim Companion 2001; 36:91-103. [ Links ]
32. Wilkosz S, Ireland G, Khwaja N, Walker M, Butt R, de Giorgio-Miller A, Herrick SE. A comparative study of the structure of human and murine greater omentum. Anat Embryol (Berlin) 2005; 209:251-261. [ Links ]