Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Revista Colombiana de Ciencias Pecuarias
versão impressa ISSN 0120-0690versão On-line ISSN 2256-2958
Rev Colom Cienc Pecua v.23 n.4 Medellín out./dez. 2010
Composition and antibacterial activity of essential oils obtained from plants of the Lamiaceae family against pathogenic and beneficial bacteria¤
Composición y actividad antibacteriana de aceites esenciales obtenidos de plantas de la familiaLamiaceae contra bacterias patógenas y benéficas
Composição e atividade antibacterina de azeites essenciais obtidos de plantas da família Lamiaceae contra bactérias patogênicas e benéficas
Lina P Roldán1, MV; Gonzalo J Díaz, MV, PhD1* ; Jennifer M Duringer2, PC, PhD
1Laboratorio de Toxicología, Facultad de Medicina Veterinaria y de Zootecnia, Universidad Nacional de Colombia, Bogotá, D.C. Colombia. 2Department of Environmental and Molecular Toxicology, Oregon State University, Corvallis, Oregon, United States.
(Received: 27 june, 2010; accepted: 31 august, 2010)
Summary
The qualitative composition and antibacterial activity of six essential oils obtained from plants cultivated in the Colombian Andes (Mentha spicata, Mentha piperita, Ocimum basilicum, Salvia officinalis, Rosmarinus officinalis and Thymus vulgaris) and a commercial essential oil of Origanum vulgare subsp. hirtum were investigated. The essential oil composition was determined by gas chromatography-mass spectrometry (GC-MS), while the antibacterial activity of the essential oils against Escherichia coli, Salmonella enteritidis, Salmonella typhimurium, Lactobacillus acidophilus and Bifidobacterium breve was measured as the minimum bacte icidal concentration (MBC) using the agar dilution method. The chemical analysis revealed the presence of 16-28 compounds in each oil, corresponding mainly to phenols, oxygenated and hydrocarbon monoterpenes. O. vulgare and T. vulgaris oils were active at low MBCs (MBC ≤ 5 mg/ml) against all bacteria evaluated, including beneficial microorganisms. In contrast, O. basilicum oil was more active against pathogenic bacteria (MBCs ≤ 10mg/ml) than beneficial bacteria (MBCs of 80 mg/ml). The present study shows that the antimicrobial potential of essential oils depends not only on the chemical composition of the oil but also on the targeted microorganism. This has important practical implications for essential oils intended to be used as feed additives with antibacterial properties for animal nutrition or pharmaceutical products with natural compounds.
Key words: antibacterial activity, essential oils, Lamiaceae family.
Resumen
Se investigó la composición cualitativa y la actividad antibacteriana de seis aceites esenciales obtenidos de plantas cultivadas en los Andes Colombianos (Mentha spicata, Mentha piperita, Ocimum basilicum, Salvia officinalis, Rosmarinus officinalis y Thymus vulgaris) y un aceite esencial comercial de Origanum vulgare subsp. hirtum. La composición de los aceites esenciales fue determinada por cromatografía de gasesespectrofotometría de masas (CG-EM), mientras que la actividad antibacteriana de los aceites esenciales contra Escherichia coli, Salmonella enteritidis, Salmonella typhimurim, Lactobacillus acidophilus y Bifidobacterium breve, fue medida como la concentración mínima bactericida (CMB) usando el método de dilución en agar. Los análisis químicos revelaron la presencia de16 - 28 compuestos en cada aceite, correspondiendo principalmente a monoterpenos fenolicos, oxigenados e hidrocarbonos. Los aceites de O. vulgare y T. vulgaris fueron activos contra todas las bacterias evaluadas, incluyendo microorganismos benéficos a CMBs bajas (CMB ≤ 5 mg/ml). En contraste, el aceite de O. basilicum fue más activo contra bacterias patógenas (CMBs ≤ 10 mg/ml) en comparación de bacterias benéficas (CMBs de 80 mg/ml). El presente estudio demostró que el potencial antimicrobiano de los aceites esenciales no depende solo de la composición química del aceite sino también del microorganismo por sí mismo. Estos resultados tienen implicaciones prácticas para los aceites esenciales usados como aditivos alimenticios con propiedades antibacterianas para la nutrición animal o productos farmacéuticos con compuestos naturales.
Palabras clave: aceites esenciales, actividad antimicrobiana, familia Lamiaceae.
Resumo
Pesquisou-se a composição qualitativo e a atividade antibacteriana de seis azeites essenciais obtidos de plantas cultivadas nos Andes Colombianos (Mentha spicata, Mentha piperita, Ocimum basilicum, Salvia officinalis, Rosmarinus officinalis e Thymus vulgaris) e um azeite essencial comercial de Origanum vulgare subsp. hirtum. A composição dos azeites essenciais foi determinada por cromatografía de gases -espectrofotometría de massas (CM-EM), enquanto a atividade antibacteriana dos azeites essenciais contra Escherichia coli, Salmonella enteritidis, Salmonella typhimurim, Lactobacillus acidophilus e Bifidobacterium breve foi medida como a concentração mínima bactericida (CMB) usando o método de diluição em ágar. As análises químicas revelaram a presença de16 - 28 compostos em cada azeite, correspondendo principalmente à monoterpenos fenólicos, hidrocarbonetos e oxigenados. Os azeites de O. vulgare e T. vulgaris foram ativos contra todas as bactérias testadas, incluindo microorganismos benéficos a CMBs baixas (CMB ≤ 5 mg/ml). Em contraste, o azeite de O. basilicum foi mais ativo contra bactérias patogénicas do que bactérias benéficas (CMBs de 80 mg/ml). Este estudo demonstrou o potencial antimicrobiano dos azeites essenciais depende da composição química do azeite e o microorganismo próprio. Estes resultados têm implicações práticas para os azeites essenciais usados como aditivos alimentícios com propriedades antibacterianas para a nutrição animal ou produtos farmacêuticos com produtos naturais.
Palavras chave: atividade antibacteriana, azeites essenciais, familia Lamiaceae.
¤ To cite this paper: Roldán LP, Díaz GJ, Duringer JM. Composition and antibacterial activity of essential oils obtained from plants of the Lamiaceae family against pathogenic and beneficial bacteria. Rev Colomb Cienc Pecu 2010;23:451-461.
* Corresponding author: Gonzalo J Díaz. laboratorio de Toxicología, Facultad de Medicina Veterinaria y de Zootecnia, Universidad Nacional de Colombia, Bogotá, D.C., Colombia. E-mail: gjdiazg@unal.edu.co
Introduction
Manipulation of the gut function and antimicrobial habitat of domestic animals with feed additives has been recognized as an important tool for improving growth performance and feed efficiency (Collington et al., 1990). Since the prohibition of antibiotic growth promoters (AGPs) in the European Union (regulation EC/1831/2003 banned the use of in-feed antibiotics in the EU as from January 2006), a diverse group of phytogenic additives has been evaluated as potential substitutes of AGPs in order to maintain the same production standards. Among these compounds are the essential oils (EOs) obtained from several classes of plants (Hertrampf, 2001).
Essential oils are volatile secondary metabolites isolated from plant tissues either by hydro- or steam distillation. Among plant species containing large amounts of EOs are plants from the families Asteraceae, Apiaceae, Lamiaceae (Labiatae), Lauraceae, Liliaceae, Mirtaceae, Magnoliaceae, Rutaceae and Pinaceae (Jones, 2002). The main constituents of essential oils are mono-and sesquiterpenes and some of these compounds have shown antibacterial, antifungal and antioxidant activities (Lee and Ahn, 1998).
Studies conducted with poultry have shown that EOs are able to improve growth performance and prevent gastrointestinal diseases such as colibacilosis, necrotic enteritis and coccidiosis (William and Losa, 2001). Compounds of particular importance that have shown specific biological activities are the phenolic monoterpenes, carvacrol and thymol, which are particularly abundant in the EO from oregano and thyme (Basilico and Basilico, 1999). Other compounds with antibacterial properties found in EOs are eugenol, α-and β- pinene, R- and S-limonene, 1,8 cineole, borneol, estragol and p-cymene (Mourey and Canillac, 2002; Bagamboula et al., 2004).
In order to test EOs antimicrobial activity, human and food-borne pathogens are most frequently chosen. Commonly tested pathogenic bacteria include two Gram-positive bacteria (Bacillus subtilis and Staphylococcus aureus), as well as three Gram-negative bacteria (Escherichia coli, Salmonella spp. and Pseudomonas aeruginosa) (Kalemba and Kunicka, 2003). Benefic bacteria are rarely chosen, even though it is important to investigate the effects of EOs on the normal beneficial microflora.
The objectives of the present work were to characterize the EOs composition of six plants of the Lamiaceae family cultivated in the Colombian Andes (for gas chromatography-mass spectrometry) and to investigate the antimicrobial activity of these EOs against selected pathogenic and benefic microorganisms.
Materials and methods
Plant material
The following species from the Lamiaceae family were evaluated: Ocimum basilicum (basil), Salvia officinalis (sage), Rosmarinus officinalis (rosemary), Thymus vulgaris (thyme), Mentha spicata (spearmint) and Mentha piperita (mint). The plants were grown at the experimental station of the College of Agriculture, National University of Colombia in the Bogotá campus located at 2630 m above sea level, from September 2008 to January 2009. A commercially available essential oil (EO) from Origanum vulgare subsp. hirtum (Reganotm, Racol Nutrition Inc, Marshal, MN, USA) was also analyzed and its antibacterial activity compared to the EOs under study. EO from O. vulgare was chosen because this EO has been reported to have strong antimicrobial activity (Tsao et al., 2007).
Essential oil extraction
Aerial parts (5 kg) of fresh plants were subjected to steam distillation in a semi-industrial stainless steel apparatus with recirculation of the condensed water for 2 hours in order to obtain the essential oils. The extracts were stored in amber vials and kept refrigerated at 4 oC prior to further analysis.
Gas chromatography-mass spectrometry (GCMS)
Samples were diluted 1:40 with ethyl acetate and a standard alkane mixture (C10-C40, Fluka Analytical, Sigma-Aldrich, Buchs, Switzerland) was added in order to determine the Kovat's retention indices (RI). GC-MS analysis was performed using a Perkin Elmer AutoSystem XL GC apparatus attached to a PE-5MS fused silica capillary 5% diphenyl/95% dimethylpolysiloxane column (30 m x 0.25 mm, 0.25 µm film thickness, Perkin Elmer). The column temperature was initially 40 ºC, held for 2 min, then ramped from 40-250 ºC at 3 ºC/min. Helium (1.0 ml/min) was used as the carrier gas. Line and injector temperature were set at 225 ºC and 250 ºC, respectively.
Samples (2 µl) were injected using a PSSI injector in the split mode (1:40). MS conditions were run in EI+ through a Perkin Elmer TurboMass Upgrade mass spectrometer as follows: ionization energy -70 eV; scan rate 1.6 scans/sec; interscan delay 0.01 sec; source temperature 200 ºC; mass range 20 to 400 m/z; solvent delay 3.00 min. The RI of the compounds were calculated based on the retention time of the C10n-alkanes. Quantitative -C40 data were calculated by obtaining the peak area from total ion chromatogram using the TurboMass 5.1 software program (Perkin Elmer Inc., Waltham, MA, USA), while qualitative data were obtained by comparing spectra to those in the Wiley NIST/EPA/ NIH Mass Spectral Library 2005.
Test organisms and preparation of the inocula
Bacteria were obtained from the culture collections of the National Laboratory of Veterinary Diagnostic of Colombian Agricultural Institute (CEISA-ICA) in Bogota, Colombia, which were American Type Culture Collection ATCC. The pathogenic bacterial strains used were the Gramnegative microorganisms Escherichia coli ATCC 25922, Escherichia coli O157, Salmonella enteritidis ATCC 13076, and Salmonella typhimurium ATCC 14028. Additionally, the Gram-positive beneficial bacteria Lactobacillus acidophilus ATCC 4356 and Bifidobacterium breve ATCC 15700 were also tested. Gram-negative strains were incubated in Tryptic Soy Broth (Merck, Darmstadt, Germany) at 37 oC for 24 h. Gram-positive strains were incubated in MRS Broth (MRSB, Oxoid, Basingstoke, Hampshire, UK) at 37 oC for 48 and 72 h for L. acidophilus and B. breve, respectively. The bacterial cells were harvested, centrifuged to a pellet, washed, re-suspended in Peptone Buffer Solution and diluted to a concentration of 1x106 CFU/ml.
Determination of minimum bactericidal concentration (MBC)
For the determination of the MBC, the agar dilution susceptibility assay was used, as recommended by the National Committee for Clinical Laboratory Standards (NCCLS, 1999). MBC was defined as the lowest concentration where 99.9% o more of the initial inoculum is killed after an incubation time (Burt, 2004). A stock solution of 16% (w/v) of each EO was prepared with Tween 80 (Sigma-Aldrich, St Louis, MO, USA) and sterile water. Before agar dilution method was performed, the micro-dilution broth assay was conducted (NCCLS, 1999). In micro-dilution broth assay, all tests for Gram-negative bacteria were performed in Mueller Hinton Broth (MHB; Becton Dickinson, Sparks, MD, USA) while L. acidophilus and B. breve were tested in MRSB. A series of two-fold dilutions of each oil were carried out in 96-well microtitre plates over the range of 0.078 to 80 mg/ ml. The inocula were then added to the plates, which were incubated under normal atmospheric conditions, at 37 oC for 24 h and 48 h for Gramnegative bacteria and L. acidophilus, respectively.
B. breve was incubated in anaerobic conditions (Anaerogen, Oxoid, Basingstoke, Hampshire, UK) at 37 oC for 72 h. Bacterial growth was indicated by the presence of a white pellet at the well bottom. The lowest concentration that completely inhibits the visible growth of microorganisms was defined as the minimum inhibitory concentration (Delaquis et al., 2002).
After incubation, 10 µl of each well were seeded on McConkey Agar (Oxoid) in the case of Gramnegative bacteria and MRS Agar (Oxoid) for Lact. acidophilus and Bif. breve, after which they were incubated again for 24, 48 and 72 h, respectively. Total absence of bacterial colonies on the agar plate was determined as the MBC. Both growth controls (containing inocula but no EOs) and negative control (containing EOs but not inocula) were included into each microtitre and agar plates. Streptomycin was used as antibacterial control. Every assay was carried out in triplicate.
Results
Essential oil yields
The EO yield of each plant was expressed as a percentage (v/w) in relation to fresh plant material weight. In general, all plants yielded less than 1% EO and the average yields values were as follows: Rosmarinus officinalis: 0.82%, Salvia officinalis: 0.64%, Thymus vulgaris: 0.48%, Ocimum basilicum: 0.1%, Mentha piperita: 0.1% and Mentha spicata: 0.08%.
GC-MS analysis
Table 1 summarizes the results of the seven EOs analyzed (six selected plants plus the commercial oregano oil). The components were organized by elution time from a PE-5MS column. The compounds identified account for 94-99% of the chemical components in the EOs. In all EOs analyzed, the majority of the compounds corresponded to monoterpenes, either phenols, oxygenated or hydrocarbon.
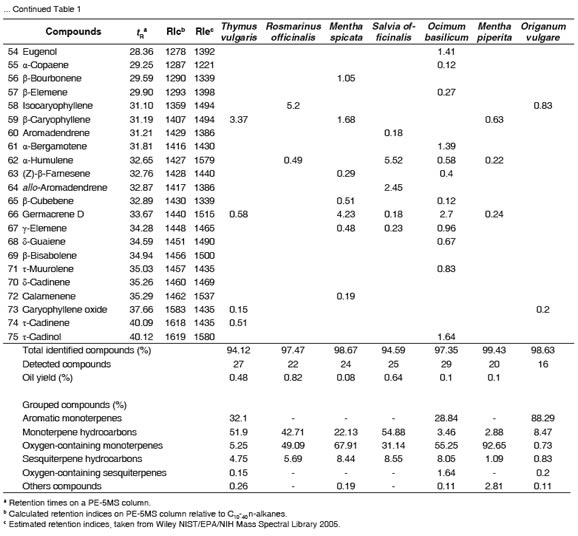
The monoterpenes hydrocarbons, α-and β-pinene and β-myrcene were present in most of the EOs analyzed. Sesquiterpenes were found in much lesser amounts (a range of 0.8-8.6%). In T. vulgaris and O. vulgare EOs, 28 and 16 compounds were detected and identified, respectively. The major components of these EOs were the monoterpene phenols thymol and carvacrol. Thymol (30.61%) and γ-terpinene (27.31%) were the major components of T. vulgaris EO and carvacrol (85.28%) and p-cymene (4.75%) the major ones of O. vulgare EO. In R. officinalis EO a total of 22 compounds were identified. Oxygen containing monoterpenes such as 1,8-cineole or eucalyptol (28.05%) and camphor (12.25%), were the major components. The analysis of M. spicata, S. officinalis and O. basilicum EOs showed the presence of 24, 25 and 28 different compounds, respectively. The M. spicata EO, was found to be highly rich in oxygenated monoterpenes (67.91%), mostly D-carvone (61.53%) and 1,8-cineole (3.37%). Another important component of this EO was limonene (12.57%) a monoterpene hydrocarbon. In S. officinalis EO, α-thujone (29.53%) and 1,8-cineole (21.79%) were the major compounds present, while β-linalool (46.67%) and estragole (27.43%) were the major compounds of O. basilicum EO. Finally, oxygenated monoterpenes such as pulegone (44.54%) and iso-menthone (26.15%) were the main compounds found in M. piperita EO.
Minimum Bactericidal Concentration (MBC)
Table 2 summarizes the antimicrobial activity of the EOs evaluated. All bacterial strains showed sensitivity to the EOs tested. Some EOs had greater antibacterial activity than others or showed a differential activity depending on the type of microorganism (pathogenic or beneficial). S. officinalis EO was active against all bacteria concentrations tested with MBCs of ≥ 40 mg/ ml; R. officinalis EO was also active against all bacteria but had greater activity against E coli than S. officinalis EO. M. spicata and M. piperita EOs were the only ones that had no activity against the beneficial bacterium L. acidophilus at the tested (up to 80 mg/ml). O. basilicum EO was more active against Gram-negative pathogenic bacteria (MBC ≤ 10mg/ml) than Gram-positive beneficial bacteria (MBCs of 80 mg/ml). O. vulgare and T. vulgaris EOs were the most efficient bacterial growth inhibitors, with MBCs of ≤ 5 mg/ml for all strains tested. None of the EOs tested had greater antibacterial activity than streptomycin, but the O. vulgare EO inhibited S. enteritidis growth at the same concentration of streptomycin (0.078 mg/ml). In general, Gram-negative bacteria were more sensitive to the EOs evaluated than to Gram positive.
Discussion
In general, the chemical composition of the EOs obtained from the plants of the Lamiaceae family cultivated in the Colombian Andes and selected for the present study was comparable with previous reports from other countries. However, some important differences were found. Previously reported chromatographic profiles of EOs obtained by hydrodistillation, steam distillation or ethanol extraction of aerial parts of T. vulgaris, O. vulgare, M. spicata, S officinalis and O. basilicum are similar to the ones obtained in the present study (Adam et al., 1998; Aligiannis et al., 2001; Lee et al., 2005; Sokovic et al., 2009; Dob et al., 2007; Chauhan et al., 2009). However, the main components of R. officinalis and M. piperita EO were different to previous reports. In R. officinalis EO, α-pinene had been reported as the major component (30-35%) followed by 1,8-cineole (14-20%) and camphor (7-12%) (Djeddi et al., 2007; Özcan and Chalchat, 2008; Jamshidi et al., 2009); menthol and menthone (>25%) had been reported as the major components of M. piperita EO (Iscan et al., 2002; Yadegarinia et al., 2006).
In contrast, in the present study, 1,8-cineole (28.05%) and pulegone (44.45%) were the major components of R. officinalis and M. piperita EO, respectively. Genetic and biochemical differences among specific cultivars of the same botanical species could explain these differences (Putievsky, et al., 1988; Tholl, 2006; Degenhardt et al., 2009). Other factors that may influence the chemical composition of a particular EO are climatic, seasonal and geographic conditions (Baydar et al., 2004). Additionally, both the oil yield and the relative composition of the constituents of an EO may vary greatly according to the developmental phase of the plant (Miguel et al., 2004). The Lamiaceae family is one of the most important ones in regards to the production of EOs with antimicrobial and antioxidant properties (Tsao et al., 2007). The content of active substances in the EO determines its in vitro and in vivo efficacy. However, the susceptibility of a microorganism to an EO depends not only on the properties of the EO but also on the microorganism itself. It is generally accepted that EOs are more active against pathogenic Gram-positive than against pathogenic Gram-negative bacteria (Lemos et al., 1990, Smith-Palmer et al., 1998, Mitsch et al., 2004, Burt and Reinders, 2003); however, in some studies, Gramnegative bacteria have been more sensitive (Kim et al., 1995, Hayes et al., 1997).
In the present study, all EOs tested were active against the Gram-negative pathogenic bacteria tested. In regards to beneficial bacteria, Horosova et al. (2006), found that oregano EO exhibited a strong bactericidal effect against chicken lactobacilli. The present study supports this finding since O. vulgare EO (and also T. vulgaris EO) had the lowest MBC (<5 mg/ml) against all strains tested, including the beneficial bacteria. This strong antibacterial action has been attributed to the phenolic monoterpenes carvacrol and thymol, which have similar, synergistic, and non-selective antimicrobial activity (Michiels, 2009). Additionally, there is also a possible synergistic effect with other minor components such as the monoterpene hydrocarbons γ-terpinene and p-cymene (Burt, 2004), which are biosynthetic precursors of thymol and carvacrol (Burt, 2004; Ultee et al., 2002). For example, p-cymene is a very weak antibacterial compound but it swells bacterial cell membranes to a greater extent than carvacrol does. By this mechanism p-cymene probably enables carvacrol to be more easily transported into the bacterial cell so that a synergistic effect is achieved when both compounds are simultaneously present (Ultee et al., 2002; Rota et al., 2008).
The results of the present study confirm previous studies where oregano and thyme EOs had been highly active against important pathogenic bacteria such as Escherichia coli, Salmonella typhimurium and Clostridium perfringens (Hammer et al., 1999; Kamel, 2000; Marino et al., 2000; Dorman and Deans, 2000, Burt and Reninders, 2003). On the other hand, the present study also shows that oregano and thyme EOs are highly active against the beneficial bacteria Lactobacillus acidophilus and Bifidobacterium breve, which is an undesirable effect. These findings however, are in contrast of those of Si et al., (2006) who reported that eugenol, cinnamon, thymol and carvacrol were less active against lactobacilli and bifidobacteria in relation to pathogen bacteria (Escherichia coli and Salmonella typhimurium). A possible explanation for this discrepancy is the differences in the methodology employed by Si et al., (2006) and the use of a purified compound rather than the whole essential oil, which contains a diverse mixture of compounds (16 for oregano and 28 for thyme in this study).
The gut microflora (bifidiobacteria and lactobacilli) are often considered to play an important role in metabolic activities that result in salvage of energy and absorbable nutrients, important trophic effects on the intestinal epithelium and on immune structure and function. Also, these bacteria protect the colonized host against invasion by alien microbes. The imbalance of native gut flora might also be an essential factor in certain pathological disorders, including multisystemic organ failure, colon cancer, and inflammatory bowel diseases (Lee and Ahn, 1998; Guarner and Malagelada, 2003). Due to these protective and positive roles, it is highly desirable that growth promoter substances do not have an inhibitory effect on these bacterial populations. Interestingly, even though O. basilicum EO inhibited beneficial bacteria, the MBCs required (80 mg/ml) were much higher than those required to inhibit pathogenic bacteria (5-10 mg/ml). O. basilicum might therefore be used to control pathogenic bacteria without affecting beneficial bacteria, provided that the right dose is used.
M. spicata and M. piperita EOs showed intermediate MBCs (5-40 mg/ml) in regards to their effect on pathogenic bacteria and did not inhibit L. acidophilus growth. However, B. breve was inhibited with MBCs of 10 and 40 mg/ml for M. spicata and M. piperita, respectively. R. officinalis and S. officinalis EOs were active against all bacteria evaluated, but their antibacterial activity was low (high MBCs) and non-selective (about the same against both pathogenic and beneficial bacteria). This activity is consistent with the chemical composition of these EO, characterized by the presence of monoterpene hydrocarbons (limonene, α-pinene and α-thujone) and oxygen containing monoterpenes (menthone, carvone, 1,8-cineole and camphor). These compounds have shown weaker antimicrobial activity compared with phenolic monoterpenes (Kim et al., 1995; Helander et al., 1998; Dorman and Deans, 2000). The antimicrobial action of EO components is determined by the lipophilicity of their hydrocarbon skeleton and the hydrophilicity of their major functional groups. The antimicrobial activity of EO components has been ranked as follows: phenols > aldehydes > ketones > alcohols > ethers > hydrocarbons (Kalemba and Kunicka, 2003).
In summary, the results of the present study indicate that the locally grown Lamiaceae plants selected for this study are capable of producing EOs with variable antibacterial activity. The "model" EO used (commercial Origanum vulgare EO), as well as the antibiotic selected as control, showed high antibacterial activity against both pathogenic and beneficial bacteria. The chemical composition of the EOs evaluated is consistent with previous studies from other countries, with a few exceptions. Some of the EOs tested are highly active against pathogenic bacteria but also against beneficial bacteria, an evident undesirable characteristic. O. basilicum EO, however, had an interesting antibacterial activity since it inhibited preferentially pathogenic bacteria. However, its yield was one of the lowest obtained (0.1%).
More studies are needed to investigate the effect of the EOs tested using in vivo models in order to determine if these oils (alone or in combination) can be used to prevent gastrointestinal diseases in animals as natural alternatives to antibiotics. The type of essential oil, yield, chemical composition, concentration needed to obtain a biological effect and bioavailability are all aspects that need to be taken into consideration for their potential use as feed additives in animal nutrition.
Acknowledgments
Thanks are due to Jairo Cuervo of the College of Agriculture, National University of Colombia for providing the experimental plants, and to Rocio Patiño of CEISA-ICA, for her support in the microbiological assays.
References
1. Adam K, Sivropoulou A, Kokkini S, Lanaras T, Arsenakis, M. Antifungal activities of Origanum vulgare subsp. hirtum, Mentha spicata, Lavandula angustifolia and Salvia fruticosa essential oils against human pathogenic fungi. J Agric Food Chem 1998; 46:1739-1745. [ Links ]
2. Aligiannis N, Kalpoutzakis E, Mitaku S, Chinou I. Composition and antimicrobial activity of the essential oils of two Origanum species. J Agric Food Chem 2001; 49:4168-4170. [ Links ]
3. Bagamboula CF, Uyttendaele M, Debevere J. Inhibitory effect of thyme and basil essential oils, carvacrol, thymol, estragol, linalool and p-cymene towards Shigella sonnei and S. flexneri. Food Microbiol 2004; 21:33-42. [ Links ]
4. Basilico MZ, Basilico JC. Inhibitory effects of some spice essential oils on Aspergillus ochraceus NRRL 3174 growth and ochratoxin A production. Lett Appl Microbiol 1999; 29:238-241. [ Links ]
5. Baydar H, Sagdic O, Ozkan G, Karadogan T. Antibacterial activity and composition of essential oils from Origanum, Thymbra and Satureja species with commercial importance in Turkey. Food Control 2004; 15:169-172. [ Links ]
6. Burt SA, Reinders, RD. Antibacterial activity of selected plant essential oils against Escherichia coli O157:H7. Lett Appl Microbiol 2003; 36: 162-167. [ Links ]
7. Burt S. Essential oils: their antibacterial properties and potential applications in foods - a review. Int J Food Microbiol 2004; 94:223-253. [ Links ]
8. Collington GK, Park DS, Armstrong DG. The influence of inclusion of either an antibiotic and a probiotic in the diet on the development of digestive activity in the pig. Brit J Nutr 1990; 64:59-70. [ Links ]
9. Chauhan RS, Kaul MK, Shahi AK, Kumar A, Ram G, Tawa A. Chemical composition of essential oils in Mentha spicata L. accession [IIIM(J)26] from North-West Himalayan region, India. Ind Crop Prod 2009; 29:654-656. [ Links ]
10. Degenhardt J, Köllner TG, Gershenzon J. Monoterpene and sesquiterpene synthases and the origin of terpene skeletal diversity in plants. Phytochemistry 2009; 70:1621-1637. [ Links ]
11. Delaquis PJ, Stanich K, Girard B, Mazza G. Antimicrobial activity of individual and mixed fractions of dill, cilantro, coriander and eucalyptus essential oils. Int J Food Microbiol 2002; 74:101-109. [ Links ]
12. Djeddi S, Bouchenahn N, Settar I, Skaltsa H. Composition and antimicrobial activity of the essential oil of Rosmarinus officinalis from Algeria. Chem Nat Compds 2007; 43:487-490. [ Links ]
13. Dob T, Berramdane T, Dahmane D, Benabdelkader T, Chelghoum C. Chemical composition of the essential oil of Salvia officinalis from Algeria. Chem Nat Compds 2007; 43:491-494. [ Links ]
14. Dorman HJD, Deans SG. Antimicrobial agents from plants: antibacterial activity of plant volatile oils. J Appl Microbiol 2000; 88: 308-316. [ Links ]
15. Guarner F, Malagelada JR. Gut flora in health and disease -review. Lancet 2003: 361:512-519. [ Links ]
16. Hammer K., Carson C, Riley T. Antimicrobial activity of essential oils and other plant extracts. J Appl Microbiol 1999; 86: 985-990. [ Links ]
17. Hayes AJ, Leach DN, Markham JL, Markovic B. In vitro cytotoxicity of Australian tea tree oil using human cell lines. J Essent Oil Res 1997; 9: 575-582. [ Links ]
18. Helander IM, Alakomi HL, Latva-Kala K, Mattila-Sandholm T, Pol I, Smid EJ, Gorris LGM and Wright A. Characterization of the action of selected essential oil components on Gramnegative bacteria. J Agric Food Chem 1998; 46:3590-3595. [ Links ]
19. Hertrampf JW. Alternative antibacterial performance promoters. Poult Int 2001; 40:50-55. [ Links ]
20. Horosova, K., Bujnakova D, Kmet V. Effect of oregano essential oil on chicken lactobacilli and E. coli. Folia Microbiol 2006; 51:278-280. [ Links ]
21. Iscan G, Kirimer N, Kürkcüoglu K, Baser H,, Demirci F. Antimicrobial screening of Mentha piperita essential oils. J Agric Food Chem 2002; 50:3943-3946. [ Links ]
22. Jamshidi R, Afzali Z, Afzali D. Chemical composition of hydrodistillation essential oil of rosemary in different origins in Iran and comparison with other Countries. American-Eurasian J of Agric Environ Sc 2009; 5: 78-81. [ Links ]
23. Jones G. Phytogenic Additives, edition no. 1; Biomin Gesunde Tierernährung International GmbH; Herzogenburg, Austria, 2002; Vol no. 1, pp 9-21. [ Links ]
24. Kalemba D, Kunicka A. Antibacterial and antifungal properties of essential oils. Curr Med Chem 2003; 10:813-829. [ Links ]
25. Kamel CA. Novel look at a classic approach of plant extracts. Feed Mix Special 2000; 249:19-25. [ Links ]
26. Kim J, Marshall MR, Wei C. Antibacterial activity of some essential oil components against five foodborne pathogen. J Agric Food Chem 1995; 43:2839-2845. [ Links ]
27. Lee HS, Ahn YJ. Growing-inhibiting effects of Cinnamomum cassia bark derived materials on human intestinal bacteria. J Agric Food Chem 1998; 46:8-12. [ Links ]
28. Lee SJ, Umano K, Shibamoto T, Lee KG. Identification of volatile components in basil (Ocimum basilicum L.) and thyme leaves (Thymus vulgaris L.) and their antioxidant properties. Food Chem 2005; 91:131-137. [ Links ]
29. Lemos TL, Matos FJ, Alencar JW, Craveiro AA, Clark AM, McChesney JD. Antimicrobial activity of essential oils of Brazilian plants. Phytother Res 1990; 4:82-84. [ Links ]
30. Marino M, Bersani C,, Comi G. Impedance measurements to study the antimicrobial activity of essential oils from Lamiaceae and Compositae. Int J of Food Microbiol 2001; 67:187-195. [ Links ]
31. Michiels J, Missotten JAM, Fremaut D, Smet S, Dierick NA. In vitro characterization of the antimicrobial activity of selected essential oil components and binary combinations against the pig gut flora. Anim Feed Sc Tech 2009; 151: 111-127. [ Links ]
32. Miguel G, Simoes M, Figueiredo AC, Barroso JG, Pedro LG, Carvalho L. Composition and antioxidant activities of the essential oils of Thymus caespititius, Thymus camphorates and Thymus mastichina. Food Chem 2004; 86:183-188. [ Links ]
33. Mitsch P, Zitterl-Eglseer K, Köhler B, Gabler C, Losa R, Zimpernik I. The effect of two different blends of essential oil components on the proliferation of Clostridium perfringens in the intestines of broiler chickens. Poult Sci 2004; 83:669-675. [ Links ]
34. Mourey A, Canillac N. Anti-listeria monocytogenes activity of essential oils components of conifers. Food Control 2002; 13:289-292. [ Links ]
35. NIST 05, Mass spectral library (NIST/EPA, NHI). National Institute of Standards and Technology: Gaithersburg, MD; 2005. [ Links ]
36. NCCLS (National Committee for Clinical Laboratory Standards). Performance standards for antimicrobial susceptibility testing. 9th International Supplement. M100-S9, Wayne, PA; 1999. [ Links ]
37. Oviedo E, Hernández C, Williams P, Losa R. Responses of coccidia-vaccinated broilers to essential oil blends supplementation up to forty-nine days of age. J of Appl Poult Res 2005; 14:657-664. [ Links ]
38. Özcan MM, Chalchat JC. Chemical composition and antifungal activity of rosemary (Rosmarinus officinalis L.) oil from Turkey. Int J Food Sci Nutr 2008; 59: 7-8. http://www.ncbi.nlm.nih.gov/pubmed/18654909 [ Links ]
39. Putievsky E, Ravid U, Dudai N. Phenological and seasonal influences on essential oil of a cultivated clone of Origanum vulgare L. J Sci Food Agric 1998; 43:225-228. [ Links ]
40. Rota MC, Herrera A, Martínez RM, Sotomayor JA, Jordán MJ. Antimicrobial activity and chemical composition of Thymus vulgaris, Thymus zygis and Thymus hyemalis essential oils. Food Control 2008; 19:681-687. [ Links ]
41. Si W, Gong J, Tsao R, Zhou T, Yu H, Poppe C, Johnson R, Du Z. 2006. Antimicrobial activity of essential oils and structurally related synthetic food additives towards selected pathogenic and beneficial gut bacteria. J Appl Microbiol 2006; 100:296-305. [ Links ]
42. Smith-Palmer A, Stewart, Fyfe L. Antimicrobial properties of plant essential oils and essences against five important foodborne pathogens. Lett Appl Microbiol 1998; 26:118-122. [ Links ]
43. Sokovic MD, Vukojevic J, Marin PD, Brkic DD, Vajs V, Griensven LJ. 2009. Chemical composition of essential oils of Thymus and Mentha species and their antifungal activities. Molecules 2009; 14:238-249. [ Links ]
44. Tholl D. Terpene synthases, the regulation, diversity and biological roles of terpene metabolism. Curr Opin Plant Biol 2006; 9:297-304. [ Links ]
45. Tsao R., Zhou T. Natural antimicrobials from plant essential oils. New Biocides Development. American Chemical Society, New York. ACS Symposium series, 2007; Volume 967. Chapter 18, pp. 364-387. [Access date: 24 April 2009] URL: http://pubs. acs.org/doi/abs/10.1021/bk-2007-0967.ch018 [ Links ]
46. Ultee A, Bennik MHJ, Moezlaar R. The phenolic hydroxyl group of carvacrol is essential for action against the foodborne pathogen Bacillus cereus. Appl Environ Microbiol 2002; 68:1561-1568. [ Links ]
47. Williams P, Losa R. The use of essential oils and their compounds in poultry nutrition. World Poult 2001; 17:14-16. [ Links ]
48. Yadegarinia D, Gachkar L, Rezaei MB, Taghizadeh M, Astaneh S and Rasooli I. Biochemical activities of Iranian Mentha piperita L. and Myrtus communis L. essential oils. Phytochemistry 2006, 67:1249-1255. [ Links ]