Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Colombiana de Ciencias Pecuarias
Print version ISSN 0120-0690On-line version ISSN 2256-2958
Rev Colom Cienc Pecua vol.23 no.4 Medellín Oct./Dec. 2010
Osteoconductive and osseointegration properties of a commercial hydroxyapatite compared to a synthetic product
Comparación de las propiedades de osteoconducción y osteointegración de una hidroxiapatita reabsorbible comercial con una hidroxiapatita reabsorbible sintetizada
Comparação das propriedades da osteocondução e osteointegração de uma hidroxiapatita reabsorvivel comercial sintetizada
Carlos D Jaramillo1*, DVM, Clin SP; Jairo A Rivera2, DVM-ASc, DSc DVM; Alejandro Echavarría3, Met Eng, PhD; Johan O'byrne4, Farm Chem student; Diego Congote5, VM student; Luis F Restrepo¹, Stat.
1Professor, School of Veterinary Medicine, Faculty of Agricultural Sciences, University of Antioquia, AA 1226, Medellín, Colombia. 2independent researcher in the area of health. Medellin, Colombia. 3Professor, Faculty of Engineering, University of Antioquia, AA 1226, Medellín, Colombia. 4Pharmaceutical Chemistry student, Faculty of Pharmaceutical Chemistry, University of Antioquia, AA 1226, Medellín, Colombia. 5Veterinary Medicine student, School of Veterinary Medicine, Faculty of Agricultural Sciences, University of Antioquia, AA 1226, Medellín, Colombia.
(Received: 25 september, 2008; accepted: 12 march, 2009)
Summary
The effects of two types of hydroxylapatite on bone synthesis and properties were evaluated. An osteoconductive resorbable hydroxyapatite (OseoU), synthesized at two different temperatures of calcination (Type A and Type B) was compared with a commercial mixture of hydroxyapatite (Osteogen®), commonly used in several surgical procedures involving bone loss. The synthesis was performed in the laboratories of the University of Antioquia by precipitating a mixture of calcium nitrate and ammonium phosphate. The products obtained and the commercial hydroxyapatite were characterized by scanning electron microscopy (SEM), X- ray diffraction (XRD), Fourier transform infrared spectroscopy (FTIR), and energy dispersive spectroscopy (EDS). Osteoconductive and osseointegration characteristics were measured according to the products ability to induce local cell differentiation into bone forming cells. These characteristics were evaluated in hydroxyapatite implants performed in 70 New Zealand breed rabbits distributed into seven groups of 10 animals each, tested at 7, 14, 21, 28, 42, 60 and 90 days after the surgical procedure.
Key words: bone regeneration, hydroxyapatite, osteoconduction, osseointegration, synthetic bone substitutes.
Resumen
En el presente artículo se evalúan las propiedades de osteoconducción y osteointegración de una hidroxiapatita reabsorbible (OseoU), procesada a dos temperaturas diferentes de calcinación (Tipo A y Tipo B), con el propósito de compararlas con un preparado comercial de hidroxiapatita (Osteogen®), utilizado para múltiples procedimientos quirúrgicos en los cuales se involucra la pérdida de tejido óseo. La síntesis se realizó en los laboratorios de la Universidad de Antioquia por el método de precipitación acuosa de la mezcla de nitrato de calcio y de fosfato de amonio. Los productos obtenidos y la hidroxiapatita comercial fueron caracterizados por microscopia electrónica de barrido (SEM), difracción de rayos X (DRX), espectroscopia de infrarrojo transformada de Fourier (FTIR) y espectrometría por energía dispersiva (EDS). Las características de osteoconducción y osteointegración fueron medidas de acuerdo a la capacidad de los productos para inducir la diferenciación de células locales a células formadoras de hueso. Dichas características, se evaluaron en implantes de hidroxiapatita realizados en 70 conejos de la raza Nueva Zelanda distribuidos en siete grupos de 10 animales cada uno, evaluados a los 7, 14, 21, 28, 42, 60 y 90 días de efectuado el procedimiento quirúrgico. Los resultados obtenidos demostraron que el OseoU y el Osteogen®, presentaron características similares en cuanto a la estructura cristalina, la composición química y la adsorción, con apreciables diferencias morfológicas con respecto a la forma de las partículas. Al realizar el análisis de varianza no se encontraron diferencias estadísticas significativas para las variables histopatológicas evaluadas en las dos hidroxiapatitas (p>0.05), indicando que las hidroxiapatitas sintetizadas en la Universidad de Antioquia (OseoU) tuvieron el mismo resultado que la hidroxiapatita comercial (Osteogen®) en la osteoconducción y la osteointegración del tejido óseo.
Palabras clave: hidroxiapatita, osteoconducción, osteointegración, regeneración ósea, sustitutos sintéticos de hueso.
Resumo
No presente estudo foram avaliadas as propriedades de ostecondução e osteointegração de uma hidroxiapatita reabsorvivel (OseoU), processada a duas temperaturas de calcinaçao (Tipo A e B), com o propósito de serem comparadas com um produto comercial (Osteogen®), utilizado para múltiples procedimentos cirúrgicos nos quais se envolve a perda do tecido osso. Asíntese foi realizada nos laboratorios da Universidad de Antioquia pelo método de precipitação aquosa da mistura de nitrato de cálcio e fosfato de amônio. Os produtos obtidos e a hidroxiapatita comercial foram caracterizados por microscopia eletrônica de barrido (SEM), difração raios X (DRX), espectroscopia de infravermelho transformada de Fourier (FTIR) e espectrometria por energia dispersiva (EDS). As características de osteocondução e osteointegração foram mensuradas de acordo à capacidade dos produtos para induzir a diferenciação das células locais formadoras do osso. Estas características foram avaliadas em implantações de hidroxiapatita realizadas em 70 coelhos da raça Nova Zelândia distribuídos em sete grupos de 10 animais cada um, avaliados aos 7, 14, 21, 28, 42, 60 e 90 dias de efetuado o procedimento cirúrgico. Os resultados obtidos demonstraram que o OseoU e o Osteogen® apresentaram características similares em quanto à estrutura cristalina, a comparação química e à absorção, com apreciáveis diferenças morfológicas com respeito à forma das partículas. Ao realizar as análises de variâncias não foram encontradas diferencias estatísticas significativas para as variáveis histopatológicas avaliadas nas duas hidroxiapatitas (p>0.05), indicando que as hidroxiapatitas sintetizadas na Universidad de Antioquia (OseoU) tiveram o mesmo resultado que a hidroxiapatita comercial (Osteogen®) em osteocondução e osteointegração do tecido ósseo.
Palavras chave: hidroxiapatita, regeneração óssea, substitutos sintéticos para osso.
¤ To cite this paper: Jaramillo CD, Rivera JA, Echevarria A, O byrne J, Congote D, Restrepo LF. Osteoconductive and osseointegration properties of a commercial hydroxyapatite compared to a synthetic product Rev Colomb Cienc Pec. 2010, 23: 471-483
* Corresponding Author: Carlos David Jaramillo. Faculty of Agricultural Sciences, Carrera 75 No 65 -87, Ciudadela Robledo, Universidad de Antioquia, Medellín, Colombia. Email: cadaja78@hotmail.com.
Introduction
Degenerative diseases, aging, tumors, trauma, resorption in edentulous patients, congenital bone deformities, among other, are the cause of skeletal system conditions involving tissue loss, and requiring the use of biological grafts. These situations are common to various medical areas such as orthopedics and traumatology, dentistry, veterinary and human medicine, plastic and maxillofacial surgery (Dasso et al., 1998).
Biological grafts are materials temporarily or permanently placed in the body to assist or assume the role of a body part. They are made of relatively inert substances that stimulate a biological response. The body's response to these grafts depends on factors such as graft material (porosity, stiffness, shape, and graft type) and its microenvironment (Bay, 1985; Block and Kent, 1984; Wagner, 1990).
An autologous bone graft is the best material to help complete bone healing. However, its use is problematic due to donor site morbidity, insufficient amounts of the material, and uncontrolled resorption. For this reason, alternatives have been found to grafts taken from the individual himself. These alternative materials should have excellent osseointegration, minimal immunogenic response, and they should be biocompatible, nontoxic, and readily available (Callan and Rohrer, 1993; Cardona, 1997).
Due to the limited availability of autografts and the many complications experienced with the use of allografts (grafts from other individuals), other alternatives have being tested for bone reconstruction. Among these: demineralized bone, the application of bone-forming proteins, and the use of synthetic bone substitutes.
Demineralized bone results from taking allograft cortical bone, removing the surface lipids, and dehydrating the bone with ethanol and ethyl ether. The bone is then processed with hydrochloric acid to remove soluble proteins, which could cause rejection problems. This material is costly and is obtained from bone banks, which may lead to undesirable reactions on the implanted patient (Block et al., 1987; Echavarria et al., 1999).
Ceramic materials for bone replacement consist mainly of phosphate, calcium carbonate or sulfate, and have been widely used for bone repair. Their disadvantage is that some of these substances degrade. Thus, the space occupied by the ceramic mass cannot be filled by viable bone, and the ceramic particles introduce stress concentrators preventing vascularization (Callan et al., 1993; López et al., 2002; Rivera, 2004).
Ceramic materials may be an impediment to infiltration of soft tissues in the graft. Moreover, these ceramics take up space around the implant, competing with infiltrated soft tissue. This may prevent bone infiltration by the graft, so that these two circumstances may delay the consolidation of the graft. Additionally, these ceramics can also provide material to increase the volume of autogenous bone graft. Some ceramic substitutes require bone marrow combined with the implant before use (Callan et al., 1993; López et al., 2003; Rivera, 2004).
Polymer materials are also used for bone replacement. These polymers are made of natural or synthetic collagen. As with any graft material, it occupies a space in the defect that can be filled with bone. This material usually breaks down in response to a mild chronic inflammatory reaction or giantcell attack. This inflammation can be nonspecific, thereby preventing new bone formation and eroding the adjacent viable t bone (Callan et al., 1993; De Campos, 1999; Ricci et al., 1992).
Collagen materials used as implants commonly have a medium grade of immunological rejection that can prevent the formation of new bone. These collagen materials are of limited use (Callan et al., 1993; De Campos, 1999; Ricci et al., 1992).
Hydroxyapatite is an advance in the synthesis of bioceramic materials as an alternative to autologous grafting. This material is the main inorganic component of tissues such as bone, teeth, nail and some pathological calcifications such as dental and urinary stones in all vertebrates (Echavarría et al., 1999; Ricci et al., 1992; Tobón et al., 2002). It is a calcium phosphate compound, stable at room temperature, with a calcium/phosphorus ratio of 1.67. Crystallinity depends on the calcination temperature used (Linkow and Wagner, 1993).
Hydroxyapatite is an osteoconductive structure. This allows the material to be invaded by connective tissue from the surrounding bone, which will ossify later, keeping inside the characteristics of its origin. The material is an ideal platform on which new bone can grow, providing excellent osseointegration characteristics (López et al., 2003; Pinholt, 1995; Quintana, 1998).
According to Wagner (1990), different applications of this material continue to generate great interest in medicine.
The objective of this study was to evaluate osteoconductive and osseointegration properties of a resorbable hydroxyapatite, synthesized at the University of Antioquia, and to compare it with a commercial hydroxyapatite product (Osteogen®).
Materials and methods
Bioethics Committee
This study was approved by the Ethics Committee for Animal Experiments of the University of Antioquia, as recorded in Act No 39.
Synthesis of hydroxyapatites at the University of Antioquia
Resorbable hydroxyapatites were synthesized at the Biomaterials Laboratory, (Faculty of Engineering), with a hydro-thermal process described by Rivera (2004) y Rivera (2005), using pure Ca (NO3)2.4H2O (Merck, NJ) and NH4H2PO4 (Merck, NJ).
To ensure the quality of the final product, preliminary tests were conducted. Six batches of material (80 g each) were obtained for each hydroxyapatite type (A and B), subsequently analyzing the product by characterization tests set out later.
Calcium nitrate and ammonium phosphate solutions were prepared with the mentioned reagents. A reaction between both solutions yielded a supernatant which was washed several times with distilled water, obtaining a final pellet used to determine the percentage of solids in the sample, based on the wet and dry weights.
Once obtained, hydroxyapatite was dried and synthesized at two different temperatures (A and B), generating OseoU Types A and B.
Material characterization
Both OseoU resorbable hydroxyapatites and Osteogen® were analyzed by the following techniques:
1. Morphology and distribution of particle size, as well as energy dispersive spectroscopy (EDS) were analyzed by scanning electron microscopy (SEM) using a JEOL JSM-590LV equipment (Japan).
2. A Rigaku Miniflex equipment (Japan) was used for the X-ray diffraction analysis (XRD), with the following parameters: scanning: 2, speed: 2 deg / min, copper lamp radiation, wavelength 1.5418 ° A and 100 counts per second.
3. Fourier transform infrared spectroscopy (FTIR), appropriate method to determine the functional groups present in the hydroxyapatite, was performed with a Perkin Elmer Spectrum One DTGS detector (PerkinElmer, NJ).
4. Finally, mycological and bacteriological cultures were conducted in both synthesized hydroxyapatites before being implanted in the animals. The tests were total count of mesophilic bacteria, and YGC agar for fungi.
Animals
A total of 70 adult male New Zealand white rabbits were used. The average weight was 1.9 kg. Animals underwent clinical examination at the time of purchase, finding they were healthy. The rabbits were randomized in a uniform distribution in seven groups of 10 animals each. Five animals per group were implanted in the right proximal tibias OseoU Type A, and the left proximal tibias with Osteogen®.
The remaining five animals were implanted in the right proximal tibias OseoU Type B and the left proximal tibias with Osteogen®.
Surgical Procedure
Individuals were prepared for surgery according to protocols established in the operating rooms of the School of Veterinary Medicine, Faculty of Agricultural Sciences, University of Antioquia. After shaving and washing the area with chlorhexidine gluconate (0.2%), the animals were sedated and anesthetized with Diazepam (0.2 mg/kg) and ketamine (8.5 mg/kg).
A proximal medial tibial longitudinal incision (1.5 cm) was made in both hind legs to expose the bone in order to drill a hole (diameter 5 mm) using a dental handpiece. OseoU or Osteogen® was deposited directly into the holes (0.05 g of each product). Then the periosteum was sutured (4/0 polyglactin 910), and Cephalexin was administered (8 mg/kg, by IM) every 12 hours for 3 days. Daily cleaning was performed in the incision using 0.2% chlorhexidine.
Histopathology
Euthanasia of all animals was conducted in the facilities of the SENA La Salada Agricultural Center, using a sodium pentobarbital and phenytoin mixture (80 and 10 mg, respectively, by IV).
Animals were euthanized under a schedule as follows:
Group 1: at 7 days after implantation.
Group 2: at 14 days of implantation.
Group 3: at 21 days of implantation.
Group 4: at 28 days of implantation.
Group 5: at 42 days of implantation.
Group 6: at 60 days of implantation.
Group 7: at 90 days of implantation.
Tissue samples were fixed in neutralized formalin and stabilized at 10%, decalcified in 5% nitric acid, and embedded in paraffin for histopathology. Then, cuts (4 μm thick) were conducted and then stained with hematoxylin-eosin for evaluation under the light microscope at 400 X.
Selection of the evaluation areas was performed on the medial tibial histological section at 40X magnification to visualize the implant (or the repair or remodeling processes when the implant was not evident). Five areas around the implant or the mentioned areas were evaluated at 400X for the presence of cellular elements and connective matrix related to inflammation, repair or remodeling. Presence of these elements was categorized as follows:
1. Mild: when the described elements were focally distributed in less than 30% of the area.
2. Moderate: elements were multifocal distributed in up to 60% of the area.
3. Severe: elements distributed diffusely over 60% of the area.
Histopathological evaluation was performed at the Laboratory of Animal Pathology, Faculty of Agricultural Sciences, University of Antioquia, by a certified veterinary pathologist.
Microscopic description considered the following variables: macrophages, fibrin, lymphocytes, edema, congestion, fibroblasts, fibrosis, vascularization, osteoprogenitor cells, osteoid, trabeculae, osteocytes, osteoclasts, cartilage, lagoons, osteons and presence of implant.
Statistical analysis
MANOVA (multivariate analysis of variance) was used for the experimental design to compare whether there was statistical osteointegration and osteoconductive differences between OseoU Types A and B, with regard to Osteogen® (validating the assumptions of normality, randomness and independence of experimental errors, homogeneity of variances, independence of means and variances).
Unidimensional ANOVA complemented the analysis, along with a Tukey comparison test (5% significance level) to detect statistical difference between treatments for each variable using version 9.0. of the SAS program.
Results
Characterization of hydroxyapatites
Tests demonstrated that OseoU (A and B) had similar characteristics in terms of crystal structure and functional groups, with respect to Osteogen®.
However, they had morphological differences as to the shape of the particles.
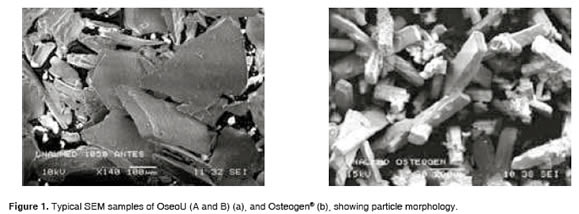
In terms of distribution, OseoU (A and B) showed sub-angular features, while Osteogen® had angular granules in rosette-shaped clusters with a clearly defined hexagonal crystalline distribution. In OseoU (A and B) there was no surface porosity, and a compact structure was evident (Figure 1).
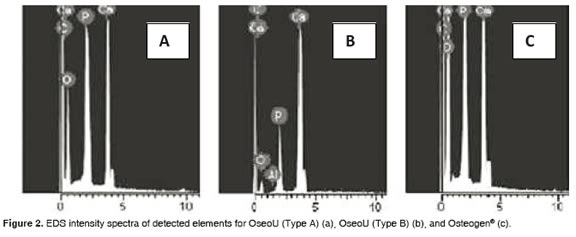
Figure 2 shows the EDS characterization used in OseoU (A and B) and Osteogen®. Presence of Ca, P and O confirm that samples are Hydroxyapatite. The presence of carbon suggests the existence of carbonates. OseoU (A) and Osteogen® have a similar Ca/P ratio, but lower than OseoU (B).
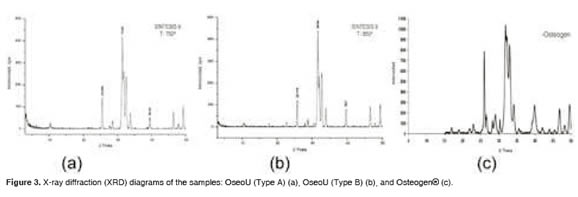
Figure 3 shows the XRD results of OseoU (A and B) and Osteogen®. According to diffractograms, the three cases failed to achieve high crystallinity (the three peaks characteristic of hydroxyapatite, lying between 31.5 and 33.5 °2θ, are not clearly defined). OseoU (B) shows an incipient peak for Osteogen®, associated with an undetermined type of calcium phosphate. According to these results, we conclude that the crystals of the hydroxyapatites evaluated are similar.
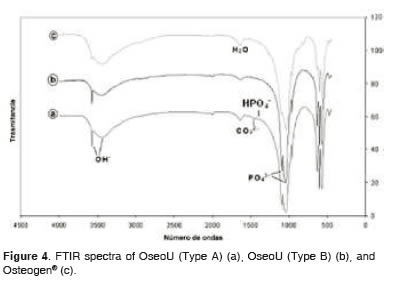
Figure 4 shows that all FTIR spectra are similar among samples, clearly identifying the contributions of functional groups PO4-3 and OH-, although less evidently, the presence of HPO4-2 groups and CO3- 2 in OseoU (A).
Mycological and bacteriological cultures
Cultures performed on all hydroxyapatites used for OseoU implants (A and B) and Osteogen®, showed no growth of any of these organisms.
Surgical Procedure
All animals recovered well from the bilateral surgery and reached date of programmed euthanasia without adverse events. There were no signs of inflammation or infection in the animals throughout the study.
Histology
There were no differences between the hydroxyapatites synthesized at the University of Antioquia or between each one (A or B) and the commercial product (p>0.05) for the histological variables examined (macrophages, fibrin, lymphocytes, edema, congestion, fibroblasts, fibrosis, vascularity, osteoid, osteoblasts, trabeculae, osteocytes, osteoclasts, cartilage, lagoons, osteons and presence of implant).
The Tukey found no difference among the three treatments. Below is the histological pattern of the hydroxyapatites assessed:
OseoU (Tipe A) cellular response for different evaluation periods
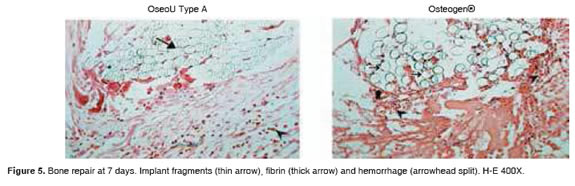
Macrophages. At 7 days there were high rates of absence of such cells. After 14 days, their presence was high in 25% of the cases, rising to 33.3% on day 21, and as time was progressing they began to disappear. At day 60, they were absent in 100% of the cases.
Fibrin. Was absent at 21 days in 100% of the cases.
Lymphocytes. Disappeared at 28 days.
Congestion. Some cases at 7, 14 and 21 days. Completely disappears at 60 days.
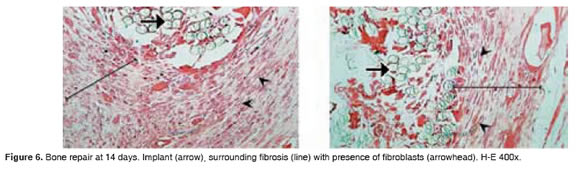
Fibroblasts. At 60 days there was a lack of fibroblasts, but until day 42 there were mild to moderate levels.
Fibrosis. At day 21, 83.3% did not show this type of material and it disappeared completely after 90 days.
Vascularization. It was present until day 42.
Progenitor cells. Its presence was high until 14 and 21 days in 50% of the cases. Thereafter it decreased.
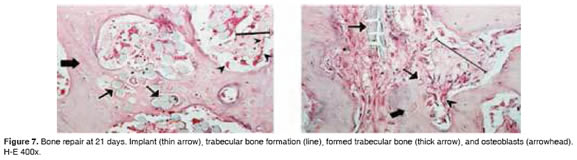
Osteoid. Moderate to high presence at day 21 in 50% of cases, tending to decrease over time.
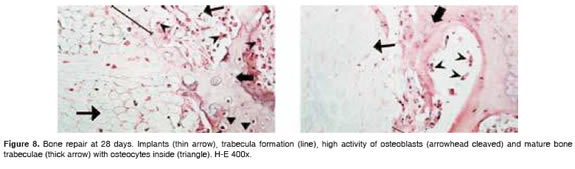
Trabeculae. Moderate from day 21 in 50% of cases. Presence increased to 100% of cases at day 60.
Osteocytes. After 21 days begins a moderate to high increase, being 90 to 100% at day 60.
Osteoclasts. High presence at 21 days in 50% of cases. Tended to decrease with time, finally disappearing at 60 days.
Lagoons. There was a moderate to high presence at day 60. Osteon. Presence of 100% at day 90.
Cartilage. moderate presence at day 28 in 100% of the cases, maintaining this value until day 90.
Implant. Presence in 100% of cases. It was low until day 42 becoming moderate at days 60 and 90.
OseoU (Tipe B) cellular response for different evaluation periods:
Macrophages. High rates of absence at 7 and 14 days. Mild to moderate increases until day 42, being absent from day 60.
Fibrin. Absent at 14 days in 100% of the cases.
Lymphocytes. Disappear within 14 days of treatment.
Congestion. Disappears completely at day 60 and 90 of treatment.
Fibroblasts. Up to day 42 showed mild to moderate patterns. There is an absence of fibroblasts at 60 days.
Fibrosis. It is high at 21 and 42 days (50% cases), and disappears at 60 and 90 days.
Vascularization. Observed until day 60 of treatment.
Progenitor cells. Present in 50% of the cases. High to moderate at 14, 21 and 28 days, then decrease to slight presence at day 90.
Osteoid. Moderate to high from 21 to 28 days in 40% of cases.
Trabeculae. Moderate from day 21 in 33.33% of cases, and increasing to 100% at 60 days.
Osteocytes. Increase from 21 days between moderate and high, being 100% at 60 and 90 days.
Osteoclasts. Presence between moderate and high in 50% of cases between 21 and 28 days, ending as mild at 60 and 90 days.
Lagoons. High presence with 100% at day 90.
Osteon. 100% at day 90.
Cartilage. 100% of the cases were present as moderate at day 28 days, and continuing so until day 90.
Implant. Moderate presence until day 60 and 90 of treatment.
Osteogen® cellular response for different evaluation periods:
Macrophages. 44% absent at 7 days, going to 33% mild at 21 days, decreasing progressively to be absent from day 60.
Fibrin. Absent from day 21 in 100% of the cases.
Lymphocyte. Absent from day 21.
Congestion. Mild to moderate the first 7 days, to disappear completely at day 60 and 90.
Fibroblasts. Mild to moderate until day 42, and absent on day 60 and 90.
Fibrosis. Between moderate and high until day 28. High at day 42, in 72% of cases.
Vascularization. It occurs until day 60 of treatment.
Progenitor cells. Moderate to high until day 28, where it starts to decrease.
Osteoid. Between moderate and high at 21 days in 30% of cases, decreasing thereafter.
Trabeculae. Increased in time, becoming moderate (40%) at 42 days, and 100% at 60 days.
Osteocytes. Increased over time to be high (100% cases) at 90 days.
Osteoclasts. Mild to moderate at 42 days (30% cases), and mild at 90 days.
Lagoons. Moderate to high at day 90.
Osteon. Present in 100% cases at day 90.
Cartilage. Mild at day 28 (100% cases), and no presence at day 90.
Implant. Mild until day 42 (100% of the cases), ending as moderate on day 90.
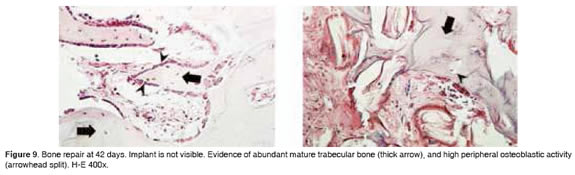
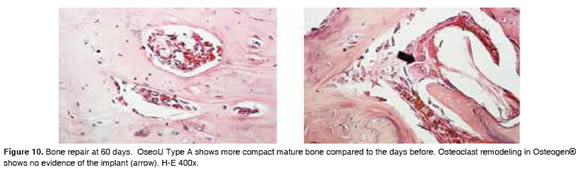
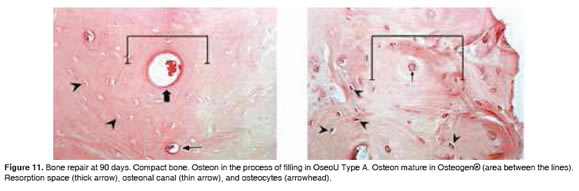
Figures 5 to 11 show the histological condition of bone tissue at different periods of assessment after implantation.
Discussion
Osteoconductive and osseointegration properties are important to evaluate an implant. This involves a thorough assessment of the materials that could be implanted, because if they do not meet the characteristics needed for a good repair, patient's condition may not improve and therefore rather than a benefit, it could cause a harmful situation.
Although there was a difference in shape for the hydroxyapatite particles in the University of Antioquia material (OseoU Types A and B) in comparison to Osteogen®, the final response of the implants was similar as a result of the similarity in the particle size (300 μm in average for all implants). This particle size is the most commonly used by surgeons (López et al., 2002). Despite differences in form, osteoconduction and osseointegration response were similar for all the materials evaluated in this study.
The largest Ca/P ratio of OseoU (B), processed at a higher temperature than Type A, is related to the higher crystallinity as calcination temperature increases, which rises to the theoretical Ca/P = 10/6 = 1.67 at the maximum hydroxyapatite crystallinity. According to Lemhman and Rougraff (2002), the Ca/P ratio is determined by the solubility of hydroxyapatite in body fluids. Therefore, OseoU (A and B) are expected to have higher absorbability than a highly crystalline sample. This gives them the ability to be reabsorbed over time, given the lower Ca/P associated with lower crystallinity compared to the theoretical, which according to Artzi (2001), offers the advantage of serving as a reservoir of minerals. This also provides rigidity and serves as a bridge to the formation of new bone, and, as it is slowly reabsorbed, promotes angiogenesis, an essential factor for development and tissue repair which depends on the growth of blood vessels.
According to the histological analysis, the fibrosis percentage through evaluation periods for OseoU is only 30%, while the commercial product is 50%. According to Valenzuela et al., (2002), this feature is favorable and desired by many surgeons, because the fibrosis excess produce cystic barriers at the site of injury, slowing the bone regeneration process.
The histopathological changes described accompany the process of bone repair, which follows a similar pattern to the inflammatory process, with phenomena occurring in cascade to eliminate cellular debris, restore blood flow and form new bone tissue. These changes can be grouped into three stages (Rivera, 2004): 1) inflammatory, 2) repair, and 3) remodeling. The first two take approximately 21 days and the third takes approximately one year.
Studies report that after 6 to 12 months post implantation, Osteogen® particles are not reabsorbed (Tobón et al., 2002). In studies using dogs, other researchers reported approximately 80% of Osteogen® particles are resorbed at 12 weeks (Echavarría et al., 1999). In a human study, it was reported the presence of phagocytic (macrophageslike) cells and/or giant cells on the surface of Osteogen® particles 12 months later (López et al., 2003). This is probably due to qualitative differences in the repair process in humans, which is much slower (Tobón et al., 2002).
Regarding the resorption process of biomaterials, the theory states that all biomaterial can be reabsorbed after implantation by a process of physiological dissolution and/or a phagocyticdependent process (Dee and Puleo (2002); Tobón et al., 2002). It has not been determined which of the two processes is predominant. It is clear that Osteogen® triggers a strong cell-mediated response by mono-multtinucleated cells (Tobón et al., 2002). Comparing these results with those obtained in this study confirms the predominance of the cellular response to implanted biomaterials and emphasize that reabsorption at 90 days (12 weeks) was 60%. Nevertheless, Ricci et al., (1992) states that there is a low reabsorption of hydroxyapatite at 12 weeks.
The results of this research indicate that 50% repair was reached at 14 and 21 days, evident by new bone. A faster bone repair with OseoU was evident compared with other investigations which reported that 50% bone repair was achieved at 28 days.
At 90 days after implantation, we observed channel formation, thickening, lamellar remodeling, osteons and mature connective tissue, consistent with the histological changes reported by other investigators (Guerra y González, 1997).
Other research (Callan and Rohrer, 1993) reported evident bone formation of new bone by 20% at four weeks using hydroxyapatite and calcium phosphate granules. In this study, we found that bone formation rate was about 50% at four weeks of implantation. The above results ratify OseoU quality in terms of clinical response, not producing cellular reactions different to the imported commercial product, confirming OseoU compatibility and good integration with the bone tissue.
OseoU has the potential to be used in the alveolar implementation after tooth extraction to maintain and even improve ridge height, particularly in patients undergoing multiple extractions of periodontally affected teeth (Quintana, 1998; Tavera, 1993), so further research in this area is required.
Making OseoU means lower production costs, which will be reflected in the final price. This locally made product, of the same quality and lower cost than other available hydroxyapatites, may be used by professionals working with low income populations.
Considering that benefits obtained with OseoU Types A and B are similar in terms of osteoconductive and osteo-integration, we recommend the production and use of OseoU type A for its lower cost, associated with the lower calcination temperature used in the process, and lower Ca/P ratio.
Acknowledgements
We want to thank DVM Claudia Patricia Alzate Álvarez for her cooperation since the beginning of the project, DVM Héctor Augusto Jiménez Arboleda for reviewing and proofreading, to the staff of the Pathology and Microbiology Laboratories of the Faculty of Agricultural Sciences, University of Antioquia (Colombia) for processing and histological and microbiological analysis of samples, to the administrative staff, teachers and students of National Learning Service (SENA, sede La Salada, Caldas, Antioquia, Colombia) for the use of their facilities and animal handling during the project.
References
1. Artzi Z, Nemcovsky CE, Tal H, Dayan D. Histopatological morphometric evaluation of 2 different hydroxyapatite-bone derivates in sinus augmentation procedures: A comparative study in Humans. J Periodontol 2001; 71:911-920. [ Links ]
2. Bay RA. The pathophysiology and anatomy of edentulous bone loss. En Fonseca R., Davis, W. (eds): Reconstructive Preprosthetic Oral and Maxillofacial surgery. Philadelphia, W. B. Saunders. 1985. [ Links ]
3. Block MS, Kent JN. Long term radiographic evaluation of hidroxilapatite augmentation of deficient mandibular alveolar ridges. J Oral Maxillofac Surg 1984; 42:793-796. [ Links ]
4. Block, MS, Kent, JN, Ardoin, RC, Davenport, W: Mandibular augmentation in dogs with hidroxilapatite combined with demineralizes bone. J Oral Maxilofac Surg 1987; 45:414-420. [ Links ]
5. Callan DP, Rohrer MD. Use of bovine derivared hydroxyapatite in the treatment of edentulous ridge defects. A human clinical and histopatological case report. J Periodontol 1993; 64:575-582. [ Links ]
6. Cardona JJ. Obtención y caracterización de hidroxiapatita sintética. Tesis de grado. Universidad de Antioquia. Facultad de Ingeniería. Departamento de ingeniería metalúrgica y materiales. Medellín 1997: 67. [ Links ]
7. Dasso G, Fernández MS, Arias JL. Reparación ósea mediante aloimplantes sometidos a diferentes métodos de conservación en conejos. Arch med vet 1998; 30:57- 64 [ Links ]
8. De campos V, Sinteses de hidroxiapatite e sua aplicacao como biomaterial. Tesis PhD.Universidad de São Paulo. 1999. [ Links ]
9. Dee K, Puleo D. An introduction to tissue biomaterial interactions. John Wiley & sons inc, Hoboken, New Jersey, 2002. p. 239. [ Links ]
10. Echavarría A, Riano C, Noreña A. Hidroxiapatita sintética de porosidad inducida. Comparación con el hueso calcinado. Rev Fac Ing 1999; 11:56-64 [ Links ]
11. Guerra LJ, González SR. Efectos de los sustituyentes en la estructura de las hidroxiapatitas biológicas. Rev CENIC, Ciencias Químicas, 1997; 28:158-168. [ Links ]
12. Lemhman D, Rougraff B. Recent advances in bone grafting 2002; [Marzo 23 de 2004] URL: http://www.medlib.iupui.edu/ bcr/recadv.htm. [ Links ]
13. Linkow LI, Wagner JR. Management of implant related problems and infections. J of Oral Implantol 1993; 29:321- 335 [ Links ]
14. López E, Echevarría A, Súarez R, Herrera N. Hidroxiapatita macroporosa obtenida en la U de A, síntesis, caracterización y comparación con el hueso esponjoso y calcinado de bovino. Rev Fac Ing 2003; 30:109-124. [ Links ]
15. López VC, Javer M, Arroya PS, Oyarzun D. Análisis ultraestructural de la formación ósea en relación con el osteogen. Av Periodoncia 2002; 14:29-36. [ Links ]
16. Pinholt M. Healing of experimentally created defects: A review. 1995. Br J Oral Maxillofac Surg 1995; 33:312-315. [ Links ]
17. Quintana D. Utilización de la hidroxiapatita en cirugía maxilofacial. Actualización bibliografica. Rev Cubana estomatol 1998; 35:16-20 [ Links ]
18. Ricci Jl, Blumenthal NC, Spivak JM, Alexander H. Evaluation of a low temperature calcium phosphate particulate implant material: physical -chemical properties and in vivo bone response. J Oral Maxilofacial surg 1992;50:969-978 [ Links ]
19. Rivera J. Obtención de proteínas morfogenéticas óseas (pmo) e hidroxiapatita sintética y evaluación de las características de dichos materiales y de sus mezclas, utilizados como material de injerto óseo en un modelo experimental lapino. Tesis doctorado. Universidad de León, España, 2005. 155p [ Links ]
20. Rivera J, Riaño CH, Echavarría A, Monsalve PA, Alzate G, Restrepo LF, Jaramillo CD. Injertos óseos -nueva alternativa. Fase III. Obtención, caracterización y evaluación de Hidroxiapatita sintética porosa - Proteínas MorfogenéticasÓseas en un modelo experimental lapino. Rev Colomb Cienc Pec. 2004; 17:21-28. [ Links ]
21. Tavera F. Patología general veterinaria. 2ed, México, ed interamericana; 1993. [ Links ]
22. Tobón SI, Arismendi JA, Marín ML, Valencia Ja. Comparison between a conventional technique and two bone regeneration techniques in periradiuclar surgery. Int Endod J 2002; 35:635-641. [ Links ]
23. Valenzuela C, et al. Análisis ultraestructural de la formación ósea en relación con el osteogen. Av Periodon Implantol. 2002; 14,1:29-36. [ Links ]
24. Wagner JR. A clinical and histological case study using resorbable hidroxylapatite for the repair of osseous defect prior to endosseous implant surgery. J Oral Implantol. 1989, 15:186-192. [ Links ]
25. Wagner JR. An osteoconductive resorbable hydroxylapatite graft material (OsteoGen®). J Florida State Dental Assn1990; 2:4c-5c. [ Links ]