Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Colombiana de Ciencias Pecuarias
Print version ISSN 0120-0690On-line version ISSN 2256-2958
Rev Colom Cienc Pecua vol.23 no.4 Medellín Oct./Dec. 2010
Equine Viral Arteritis: epidemiological and intervention perspectives¤
Arteritis Viral Equina: perspectivas epidemiológicas y de intervención
Arterite Viral Eqüina: Perspectivas Epidemiológicas e de Intervenção
Julián Ruiz-Sáenz1*, MV, MSc
1Grupo de Investigación CENTAURO, Facultad de Ciencias Agrarias, Universidad de Antioquia, A.A. 1226, Medellín, Colombia.
(Received: 10 march, 2009; accepted: 14 september, 2010)
Summary
Equine viral arteritis is an infectious viral disease of horses that causes serious economic losses due to the presentation of abortions, respiratory disease and loss of performance. After the initial infection males can become persistently infected carriers scattering infection through semen, a situation that brings indirect economic losses by restrictions on international trade of horses and semen from breeding and from countries at risk of infection with the virus. Outbreaks of infection have been reported in several American countries with which Colombia has active links of import and export of horses and semen. This article focuses on creating awareness of the key points of infection and on examples of how infection has been distributed in countries that have developed horse breeding, such as Argentina, a country aware of the origin and distribution of the infection. This study will help us understand how to assess, prevent and/ or control the entry of such a devastating infection for the equine industry.
Key words: equine arteritis virus, prevention and control, viral infection.
Resumen
La arteritis viral equina es una enfermedad viral infectocontagiosa de los equinos que puede llegar a causar graves pérdidas económicas debidas a la presentación de abortos, enfermedad respiratoria y pérdida del desempeño; luego de la primoinfección los machos pueden resultar infectados persistentemente convirtiéndose en portadores los cuales diseminan la infección a través del semen; hecho que acarrea pérdidas económicas indirectas por las restricciones en el comercio internacional tanto de ejemplares como de semen procedente de criaderos y países con riesgo de infección con el virus. Se han reportado brotes de infección en diversos países de América con los cuales Colombia tiene activos vínculos de importación y exportación de equinos y semen. El presente artículo trata de dar a conocer los puntos clave de la infección y ejemplos de cómo se ha distribuido la infección en países con sistemas de explotación de equinos tales como Argentina, en el cual se conoce el origen de la infección y su distribución; cuyo estudio, nos ayudará a entender cómo debemos evaluar, prevenir y/o controlar la entrada de una infección devastadora para la industria equina nacional.
Palabras clave: infección viral, prevención y mitigación, virus de la arteritis equina.
Resumo
A arterites viral eqüina é uma doença infecto-contagiosa dos eqüinos que pode chegar a causar graves perdas económicas devidas à apresentação de abortos, enfermidade respiratória e perda do desempenho; logo da primoinfecção, os machos podem resultar infectados persistentemente, convertendo-se em portadores e disseminam a infecção a través do sémen; fato que acarreia perdas económicas indirectas pelas restrições no comercio internacional de reprodutores e sémen procedente de criatórios e países com risco de infecção com o vírus. Tem-se reportado surtos de infecção em diversos países da América, com os quais Colômbia tem activos vínculos de importação e exportação de equinos e sémen. O presente artigo trata de dar a conhecer os pontos chave da infecção e exemplos de como tem-se distribuído a infecção em países com sistemas de exploração de eqüinos como Argentina, na qual se conhece a origem da infecção e sua distribuição; para o qual o estudo, nos ajudará a entender como devemos avaliar, prevenir e/ou controlar a entrada da infecção devastadora para a industria eqüina nacional.
Palavras chave: vírus, infecção viral, prevenção e mitigação.
¤ To cite this paper: Ruiz Sáenz J. Equine Viral Arteritis: epidemiological and intervention perspectives. Rev Colomb Cienc Pecu 2010;23:501-512.
* Correspondence author: Julián Ruíz Sáenz. Unidad de Diagnóstico, Facultad de Ciencias Agrarias. Universidad de Antioquia. Carrera 75 No.65-87.Ciudadela de Robledo. Medellín, Colombia.Tel:(574)219 91 60.E-mail: julianruizsaenz@gmail.com.
Introduction
Equine viral arteritis (EVA) is a contagious disease of horses caused by an enveloped RNA virus, known as Equine arteritis virus (EAV). EAV is the prototype virus of the genus Arterivirus, family Arteriviridae, order Nidovirales (Cavanagh, 1997; ICTVdB, 2006). Based on their genetic structure and replication strategy, three additional viruses have been classified in the same genus and family. They are of great importance, one of them causing Porcine Reproductive and Respiratory Syndrome (Plagemannand Moennig, 1992).
Based on extensive comparative studies at the genomic and antigenic level of EAV, only one serotype has been detected, usually called the Bucyrus strain (McCollum, 1969). However, temporal and geographic diversity among EAV isolates has been demonstrated (Murphy et al., 1992). There also exists some variability between the strains based on their pathogenicity, some strains being capable of causing a wide range of clinical symptoms, collectively referred to as EVA in susceptible horses, whereas others produce only a slight fever (Balasuriya et al.,1995)
Although through the years it has been recognized that EAV causes contagious abortion in pregnant mares (Doll et al., 1957), and that the virus causes abortion outbreaks (Balasuriya et al., 1998), some evidence suggests that not all strains of the virus have abortigenic properties, some strains causing only clinically mild or asymptomatic diseases such that they are considered strong candidates to be used as vaccine strains (Moore et al., 2002, Moore et al., 2003). However, from a practical standpoint, it is difficult to distinguish between EAV strains that can cause abortion and those which do not; additionally, there are no standard techniques to classify the strains based on their abortive properties. Therefore, from a clinical point of view, all strains of the virus should be considered potentially abortigenic unless proved otherwise (Timoney,2003).
EAV is not particularly resistant to exposure to the environment and is rapidly inactivated by a wide range of physico-chemical conditions, mainly temperature, surviving only 20-30 minutes at 56 ºC and 2-3 days at 37 °C. Additionally, it can survive up to 75 days at 4 ºC, and even years in fluids and tissues preserved at -70 ºC. However, the virus can rapidly be inactivated by low humidity, sunlight, and the use of lipid solvents, or by using common inactivating detergents (Burky, 1966, Shirai et al., 2000). Yet, since virus viability upon refrigeration or freezing is high, EAV can remain infective in frozen semen for long periods of time, even years (Timoney, 2000).
Background
More than a century ago, reports were published in the European veterinary literature concerning a horse disease whose clinical features were consistent with those described for EVA. However, the virus was not isolated until 1953, during an outbreak of abortions and respiratory illness in a "Standardbred" horse breeding ranch near Bucyrus, Ohio (Doll,1957)
The most important outbreak of EVA in America occurred in 1984, when the disease struck many racing thoroughbred farms in Kentucky. This outbreak led to two very important findings regarding the EVA: the efficiency of venereal transmission of the virus by an infected stallion, and the high tendency of transmission as a carrier that a stallion has after a natural infection (Timoney, 1984). The fear that a highly pathogenic strain had emerged, joined with the belief that most horse populations are susceptible to the virus, led to an increased evaluation of the importance of the disease worldwide, imposing severe restrictions on the movement of horses with positive titers of antibodies against the virus (Timoney and McCollum, 1993a). These measures were subsequently gradually decreased, with today a greater emphasis on controlling the international trade of carrier stallions and infected semen, which have frequently been implicated in the spread of the virus within and between countries (Timoney, 2000b)
According to the World Animal Health Organization-OIE, a carrier stallion has serological positive for antibodies to the virus using the virus neutralisation test or an appropriately validated enzyme-linked immunosorbent assay (ELISA) and also positive virus isolation from the semen (Timoney, 2004).
Transmission
EVA is primarily a respiratory disease, transmitted through inhalation of viral particles between horses during the acute stage of infection mainly during transport of the animals for trade, exhibition or racing. The virus can be excreted by nasal secretions until 16 days post-infection, and this is a source of infection for horses that are in closecontact(McCollum, 1971).
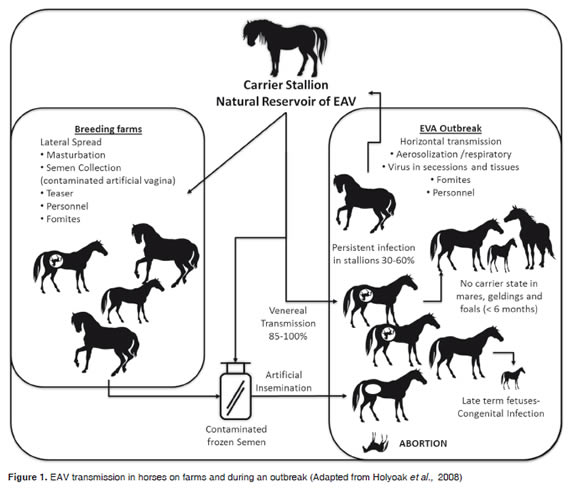
However, unlike other respiratory diseases, EAV can also be transmitted as a venereal disease during insemination, either by natural mating or artificial insemination, because the virus may be excreted in the semen, which is a major source of infection (Figure 1). When a mare, a gelding or a sexually immature colt catches the disease, the animal excretes the virus through respiratory airways and develops a strong immunity to re-infection (Fukunaga et al., 1981). In contrast, adult males are very likely to become carriers of the virus for long periods of time and can transmit the virus to mares during mating (Neu et al., 1988).Although the mare easily eliminates the virus, a pregnant EAV-infected mare cantransmit the virus tothe fetus.
Vertical transmission can occur by the congenital route, and depending on how advanced the pregnancy is, the fetus can become infected, die and be aborted between 9 and 30 days after infection. At times a live but congenitally infected and sick foal is born (Vaala et al., 1992); in such cases, the placenta, the placental fluids and the fetus are important sources of infectious virus (Figure 1). Additionally, indirect transmission can occur in an iatrogenic manner by using utensils contaminated with nasal secretions/excretions or abortion tissues orsecretions(Timoney and McCollum, 1993b)
Clinical findings
There are considerable variations in the clinical symptoms and in severity of infection, and although many horses infected with EAV do not show symptoms, it has been demonstrated that experimental infection can be fatal (Glaser et al., 1996, MacLachlan et al., 1996). As in most infectious diseases, certain host factors determine the severity of the disease, such as age, immune status, sex which determines the possibility of being a potential carrier, etc. Other factors depend on the agent such as the route of exposure, viral strain and infective dose.
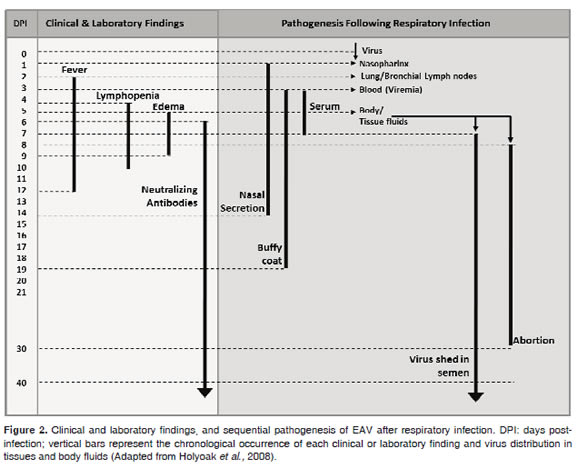
After an incubation period varying from 2-14 days (6-8 days after venereal exposure) the most prominent symptom is fever of about 41 °C that may last between 2 and 12 days, usually concomitant with depression and anorexia, conjunctivitis and rhinitis with nasal and ocular discharge, leucopenia, peripheral or supraorbital edema, and inflammation and edema of the scrotum, prepuce, mammary glands and limbs (Figure 2). Urticaria that may be localized in the face and neck or generalized in most of the body is also common (Timoney andMcCollum, 1993b)
Another symptom of infection in pregnant mares is abortion, that is not preceded by any characteristic or premonitory sign and may occur either at the end of the acute phase of illness or when the recovery phase begins (Coignoul and Cheville, 1984; Vaala et al., 1992). Abortions have been observed between 3 and 10 months of gestation in both natural and experimental infections and abortion rates in the different outbreaks reported have varied from low levels of 10% or up to dramatic levels of 50-60% (Timoney and McCollum, 1993b).
During acute infection, stallions may show temporal subfertility/infertility, associated with decreased libido, low sperm motility/concentration and low percentage of morphologically normal sperm; these changes are mainly due to the high scrotal temperature and not to a pathological effect induced by the virus. These changes persist for about 16 weeks, at which time the horse returns to its normal seminal parameters (Neu et al., 1992). Additionally, carrier stallions can excrete virus in the semen and exhibit a normal semen quality (Timoney et al., 1987, Timoney and McCollum, 1993b). As for other members of the Equidae family, outbreaks have not been reported among donkeys or mules, and although antibodies have been identified, there is little information on the clinical signs observed in these species (Timoney and McCollum, 1993b).
Pathogenesis
Two days after aerosol infection, EAV spreads rapidly to the bronchial and pulmonary lymph nodes, reaching the bloodstream and spreading throughout the body. It is therefore possible to isolate the virus in a wide variety of tissues and body fluids, making isolation of the virus possible 2 days post-infection in nasopharyngeal swab samples, 19 days in white cells, and up to 9 days in serum or plasma (Figure 2). Virus clearance coincides with the development of specific neutralizing antibodies against EAV (Timoney and McCollum, 1993b).
During primary viremia, EAV infects and replicates in endothelial cells causing strong multi-vessel damage to the endothelium, the subsequent internal elastic lamina and affecting the middle muscular layer of the vessels. Vasculitis is characterized by fibrinoid necrosis of small arteries with extravasation of red blood cells and proteinaceous material. Additionally, the virus infects macrophages, which in conjunction with endothelial cell damage leads to increased release of proinflammatory cytokines that are heavily involved in the pathogenesis of "arteritis" (Moore et al., 2003).
Experimentally, it has become clear that abortion is primarily the result of fetal infection, other than myometritis or damage to the placenta, leading to expulsion of the fetus; in addition, there is evidence that the tissues of the aborted fetus tissues have higher viral titers compared to the mother, stressing the high level of virus replication in the fetus (MacLachlan et al., 1996).
Virus reservoir
The natural reservoir of EAV infection is the carrier stallion, which ensures the permanence of the infection in equine populations. The carrier state has been identified only in the stallion, not in mares, geldings or sexually immature foals (Timoney et al., 1987). Virus can persist in the stallion for weeks, months or years, even for life in some individuals. About 60% of the stallions that acquire the virus by the respiratory route may become persistently infected; that is why the frequency of carriers in any stallion population can vary from less than 10% to over 70% (Timoney and McCollum, 1993b). In the persistently infected stallion, EAV is located in the accessory sexual glands and establishment and persistence of the carrier state is testosteronedependent (Little et al., 1992). Carrier stallions constantly eliminate the virus in the semen and consequently the risk of transmission of infection is limited to the time of mating. Transmission rates range from 85 to 100%,whether mating is naturalor artificial insemination isperformed (Figure 1).
A lesser percentage of long-term carrier stallions can clear the virus spontaneously from the reproductive tracts and do not show risk for transmission of infection (Timoney and McCollum, 2000). However it has been shown that carrier stallions are the natural source of genetic and phenotypic diversity of the virus, creating new risks of emergence of viral variants with new pathogenic potential (Balasuriya et al.,1999, Hedges et al., 1999, Balasuriya et al., 2004).
Diagnosis
Given the clinical similarity of EVA with other equine infectious and non-infectious diseases, any presumptive diagnosis should always be confirmed by laboratory tests (Holyoak et al., 2008). The presence of EVA must be suspected if respiratory symptoms are accompanied by abortions. Virus isolates can be obtained from nasopharyngeal swabs or washes, conjunctival swabs, samples of ejaculates, essentially the sperm-rich fraction, placentas, fetal fluids and tissues of aborted fetuses, such as lung, spleen and lymph nodes, and through blood samples with EDTAas anticoagulant.
The most widely used method for diagnosis is the evaluation of neutralizing antibodies, although the presence of such antibodies does not indicate active infection, but rather that the animal has been exposed to EAV. An active infection is diagnosed when high levels of antibodies in a single sample are found or when an increase in antibody titers occurs in two paired blood samples, obtained 1428 days apart (Holyoak et al., 2008). Although several types of immunoassays (ELISAs) have been developed, given the high specificity of the virus neutralization test to detect individuals with low serum antibody titers, this test has been established by the World Animal Health Organization-OIE as the gold standard for international transport of horses (Timoney, 2004)
Treatment
Since there is no specific treatment for EVA, once the infection is confirmed clinical management should include rest, fluids and in some cases, broadspectrum antibiotics to reduce the risk of secondary bacterial infections. Adult horses fully recover from the disease, leaving only the recovered stallions as carriers and sources ofinfection.
Prevention and control
Although in Colombia, EVA is an exotic disease, some prevention and control measures have been widely reported, that can make infection a manageable disease, and most importantly, can reduce the direct economic losses produced by disease outbreaks. There is currently an avirulent live vaccine (Arvac®, Fort Dodge Animal Health Laboratories, Iowa, USA) that is safe, effective and economical, and has been used in other Latin-American countries. The combination of vaccination with the use of isolation measures of unvaccinated animals can prevent the transmission of EAV. Since EAV-negative and properly immunized stallions cannot be carriers, every negative foal under 9 months of age should be vaccinated. It is worth mentioning that the use of this vaccine in pregnant mares has not been approved due to the possibility of causing abortion (USDA-APHIS, 2004). In addition, vaccination has been successfully used as a tool to control the spread of the disease during an outbreak, controlling the spread of infection and the severity of the symptoms (Timoney, 1988). It is necessary to mention that due to EVA is an exotic disease, vaccinations are not recommended in our country yet.
In breeders and sites dedicated to reproduction, it is necessary to perform bleeding and serological assays of all horses prior to mating; viral isolation should also be attempted on imported semen before use. It is very important to maintain strict hygiene and disinfection of instruments and equipment to minimize the risk of transmission of the virus. EAVnegative mares should mate only with semen from EAV-seronegative stallions, but not with semen from carriers that could be infected.
If the results of blood tests are positive for a stallion, but there is no official documentation of a negative state prior to vaccination, the stallion should be analyzed to determine its possible carrier state. Virus isolation should be attempted using semen of two separate ejaculations, or mating two EAV-negative mares with the same stallion. Twenty-eight days after mating, serological surveys should be performed on the mares to determine if neutralizing antibodies against EAV have developed (Holyoak et al., 2008).
Carrier stallions should mate only with EAVpositive or properly vaccinated mares. When a carrier stallion is paired with a positive mare or with a vaccinated mare, both mares should be isolated for 24 hours after mating to prevent mechanical transmission of the virus through traces of semen. If it is the first time that the mare has been paired with a carrier stallion, the mare should be isolated from other horses for 21 days due to the possibility of virus dispersion trough traces of semen. All vaccinated horses should receive yearly boosters to protect them against infection and in the case of stallions, to prevent the development of the carrier state. In a generation or two, these practices could eliminate the population of carrier stallions (USDAAPHIS, 2004).
Epidemiological status in America
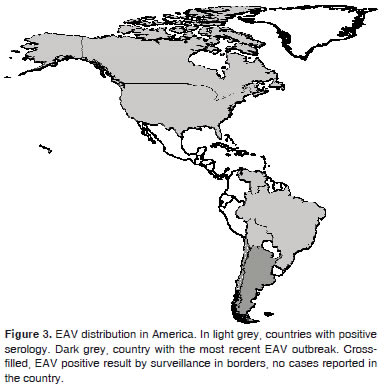
In America, the presence of EAV has been reported in different countries of South, Central and North America, mainly in Argentina, the United States and Canada where the virus has caused substantial economic losses and is today one of the main pathogens of veterinary importance (Figure 3).
Figure 3. EAV distribution in America. In light grey, countries with positive serology. Dark grey, country with the most recent EAV outbreak. Crossfilled, EAV positive result by surveillance in borders, no cases reported in the country.
The prevalence of EAV varies significantly among horse breeds. In the U.S., this infection is particularly common among Standardbreds. In a survey done in 1998, 24% of unvaccinated Standardbreds, 4.5% of Thoroughbreds, 0.6% of Quarter horses, and 3.6% of Warmblood horses had antibodies to this virus. Breed-related differences in seroprevalence might be due to genetic differences, but they are more likely to be caused by different management practices (USDA-APHIS, 2004). In experimentally infected horses, the breed has no apparent effect on susceptibility to infection or the establishment of carriers (Neu et al., 1992). For Latin American countries there arenotanofficialreportofprevalence due to there is only few studies and EAV serologic surveyofhorse'spopulationsdoesnotexist.
In Brazil, infection was first reported in 1993 in Ibiúna, state of São Paulo (Fernandes et al., 1997), and now prevalence rates have been reported, ranging from 0.85% in the state of Minas Gerais (Bello et al., 2007), to 18.2% in São Paulo (Lara et al., 2002) and to 23% in horses in the state of Rio Grande do Sul (Diel et al., 2006 ), indicating that the level of infection can be high if no adequate control measures areadopted.
However, studies conducted in the province of Urara in Brazil and in serum samples of horses belonging to different breeds and uses in Peru did not reveal seropositive animals (Rodríguez and Rivera, 1998, Heinemann et al., 2002). On the other hand, the virus was never isolated in Chile, but the border surveillance program detected the presence of two positive animals (Figure 3), one of which was killed and the other returned to its country of origin (Berrios, 2005).
In 2005, using neutralization assays, out of 1,008 equine serum samples taken from 5 different states of Venezuela, 2.48% seropositivity was reported, with a significantly greater proportion in imported horses compared with horses born in the country; this was the first laboratory evidence for the presence of EVA in the northern region of South America (Perozo, 2005), and highlights the importance of horse exchange on the border betweenVenezuelaand Colombia.
The case of Argentine has been largely discussed and documented in the scientific and technical literature, as it is the South American country with the largest number of reported cases. Until 1998, there were no reported cases of the disease in Argentina and the virus had never been isolated in samples from nasal swabs or abortions in horses suffering from respiratory or reproductive disorders. In parallel with the approval of the import of horses from EVA-infected countries, Argentina initiated serological monitoring of imported horses and semen as part of the EVA Epidemiological Surveillance Program; this revealed one positive sample of semen imported in April 1998, and led to the destruction of the entire lot of semen, and to official intervention of the two farms involved in the import and use of the semen. In paired samples carried out in all of the horses existing in the two farms, no infection was found,andthisdeterminedtheriseofhealthactivities and restrictive measures in the movement of horses (movementrestriction)(DelaSota et al., 2008).
In October 1998, of three stallions imported to Argentina, one was seropositive, so that the farm was restricted and required sanitation. In this site and during the implementation of these health activities a high serological prevalence was detected and the two other stallions were confirmed by biological assays as virus reservoirs and were consequently castrated to prevent the spread of the infection (Dela Sota et al., 2008).
There have been different serological tests, to try to assess the presence of infection in different areas and horse populations fromArgentina, most of them yielding negative results, such as in breeders that had imported horses to Argentina since 1995, and also in horses present in farms adjacent (in a radius of 10 km) to the first farm in which the infection was first detected (De la Sota et al., 2008).
Three year later, in October 2001, the requirement to diagnose EVA in native, imported horses or seminal material was established. Out of the 1774 samples included in this study, 99.83% were free of infection; 14 stallions were positive for EVA. Of these, 10 were thoroughbreds imported from the U.S. and one was a Welsh Pony imported from France; all of them had been vaccinated, the three remaining stallions were Show Jumping and Dressage horses, and were removed from the reproductive activity and castrated to prevent the reproductive spread of the infection(González et al., 2003).
InJune 2002, two farms epidemiologically related with the initial outbreak of EVA were evaluated by virus neutralization. Bleeding was performed on all the horses in order to detect possible cases of seroconversion. Eight of the 439 horses evaluated tested positive, corresponding to eight mares that came from the farm in which the initial outbreak had occurred(González et al., 2003).
As a result of positive serological diagnoses, interventions were conducted in six establishments. Serological surveys were conducted in all the horses, two serial diagnostic tests with an interval of 14 days, to verify the absence of viral activity. Of a total of 1080 equines sampled in nine farms, 306 (30.6%) were positive, indicating that once the infection enters a herd it can easily be distributed within it. However, none of the symptoms reported the presence of reproductive or respiratory clinical signs consistent with those caused by EAV infection. A consolidated analysis of the results obtained between 1998 and 2002 showed that of 7722 samples tested, 319 were positive (4.13%). In addition, when the origin of the horses was exanimated, the presence of infection was restricted to three of 23 tested provinces, Buenos Aires, Cordoba and Entre Rios(Dela Sota et al., 2008).
From the epidemiological investigations carried out, it was concluded that the possible entry of the virus that occurred through a stallion imported in 1996, could have spread the infection to other susceptible horses on the farm. Additionally it was concluded that EAV has a restricted geographic distribution inArgentina, being present in just a few provinces, equine categoriesand production systems due mainly to high risk factors such as horse importations (De laSota et al., 2008).
Phylogenetic analyses of strains isolated in 2002 from semen samples of animals belonging to herds with a history of import of animals from Europe and from a testicle stored at -20 ºC since 2000, showed that the Argentinian strains of EAV have strong phylogenetic relationships with European strains and that there are at least two different virus "populations" circulating in Argentina (Echeverria et al., 2007, Metz et al., 2008b). Other recent and larger studies identified two major groups of sequences confirming that South American strains have multiple origins, one of them being European strains (Metz et al., 2008a, Metz et al., 2010).
Additionally, studies of viral neutralization in vitro have shown that the strains isolated in Argentina have different neutralization patterns compared with a reference strain, and that the reference strain used in diagnostic tests and vaccines, is neutralized to a lower lever by heterologous antisera (Echeverría et al., 2010). This result is very important if one considers that American commercial vaccines use strains which may not generate adequate immunity in our countries.
EVA multi-state outbreak in U.S.
As in Latin America, in U.S. EVA has cause significant economic loses; in the last years a strong multi-state outbreak reaches 18 states. This outbreak, began with an outbreak of abortions in early June 2006 in a Quarter Horse ranch in the state of New Mexico where one of four stallions developed fever, depression, preputial and forehead limb edemas, with subsequent loss of fertility and virus isolation from semen. Subsequently, a second stallion developed pyrexia, but maintained adequate fertility. Prior to the diagnosis of EAV, semen had been sent to different states in the US, that all reported positive for EAVafter shipment and use. In the same breeder farm, the two remaining stallions were confirmed as positive, but only one showed light pyrexia and both excreted virus in the semen. In total and associated with the first case, there were 30 reported abortions, representing about half the population of pregnant mares exposed to the first infected stallion(Timoney et al., 2006).
Additionally, due to shipping of semen or movement of mares, the outbreak spread to 18 states: 69.5% corresponded to those infected through direct exposure to insemination with infected semen, 29% corresponded to mares and foals that were exposed indirectly by visiting infected herds during the outbreak and 1.5% in both insemination and indirect exposure. Moreover, in some states such as Utah, the outbreak resulted in respiratory illness, abortions, neonatal pneumonia and death of several animals. It should be noted that initial studies of the outbreak concluded that the infection reached approximately 90% seroprevalence in the infected farms, indicating the efficiency of spread of infection in a susceptible population (Timoney et al., 2006).
Recent phylogenetic analyses performed on samples of the same outbreak, confirmed that the national circulation of the virus originated from the dispersion of the original strain from New México and later that the virus mutated and changed its neutralizing phenotype, demonstrating the strong capacity of the virus to evolve and adapt through the persistence of the virus in carrier horses and through the passage from one animal to another during the outbreak (Zhang et al., 2010)
Is Colombia at risk?
Nowadays, Colombia has active import links of horses with countries in which EAV is present or has been reported, and has imported many horses from those countries. Examples of this are the massive import from Argentina of more than 700 Creole Argentine horses undertaken by the National Police in 1997, or the Paso Fino World Cups that have taken place in Medellín in 2003 and Pereira in 2009, using the figure of "temporary import" of animalsfor the international competitors.
The import of horses is a very important element of Colombia's livestock trade. Only during 2005 and 2006, 1079 horses were imported from Mexico, Panama,Perú,Portugal, Puerto Rico, the Dominican Republic, Guatemala, Honduras, Venezuela, Argentina, Aruba, Bolivia, Chile, Costa Rica, Ecuador, USA, France, Italy and the Netherlands with a value of USD 1.200.000. This is not to mention the importance of exports which during the same period was over USD 22.000.000 (Source: ColombianAgriculturalInstitute, ICA).
According to a 2008 equine census, published by ICA, Colombia currently has a population of 1.882.730 horses settled mainly in the departments of Cordoba, Antioquia, Casanare, Tolima and Cundinamarca, where it concentrates 42% of the national equine census. Given these figures, an infection could clearly be devastating to our susceptible populations and the risk is too high to be ignored.
Among the animal health requirements for permanent entrance of horses into Colombia, ICA requires that stallions be negative after two viral neutralization tests carried out during the 28 days prior to shipment and a 14 day interval between them; for seropositive stallions, the requirement is that they have mated two seronegative mares which remained negative after two neutralization tests, carried out first, the day of mating and second 28 days later. For females a negative result or stability of neutralizing titers in two serological, evaluated 28 days prior to shipment is required. Additionally, import is also allowed of geldings that were negative between the ages of 6 and 12 months and werethenvaccinated.
However "illegal" entry of equines across borders such as the Venezuelan border, for events that group populations such as fairs or for use as transport, can representa strong riskofdiseaseintroductioninto the country. This means that infection can be acquired via European strains imported to the Americas from South America, or via strains causing strong outbreaksinthenorthernhemisphere.
The background in the Latin American countries presented above highlights the importance of serological surveillance, which informs us that a new agent has come in contact with the susceptible population of horses, and allows us to establish preventivemeasures toavoid spread of the virus.
It is noteworthy that the ICA has recently temporarily suspended the entry into Colombia from horses from Argentina as a result of the national health warning issued by the National Health Service and Food Quality-SENASA following a positive diagnosis of EVAin the province of Buenos Aires (ICA, 2010).
Therefore we propose to strengthen technical scientific investigations and mainly diagnostic capacities to be prepared for early diagnosis and control, and avoid an infection that can be devastating for such a large and important industry as zootechnical exploitation of equines in our country.
Acknowledgment
The author thanks Dr. Anne-Lise Haenni for revision of English and critical reading of the manuscript.
References
1. Balasuriya UB, Evermann JF, Hedges JF, McKeirnan AJ, Mitten JQ, Beyer JC, McCollum WH, Timoney PJ, MacLachlan NJ. Serologic and molecular characterization of an abortigenic strain of equine arteritis virus isolated from infective frozen semen and an aborted equine fetus. J Am Vet MedAssoc 1998; 213:1586-1589. [ Links ]
2. Balasuriya UB, Hedges JF, Nadler SA, McCollum WH, Timoney PJ, MacLachlan NJ. Genetic stability of equine arteritis virus during horizontal and vertical transmission in an outbreak of equine viral arteritis. J Gen Virol 1999; 80:19491958. [ Links ]
3. Balasuriya UB, Hedges JF, Smalley VL, Navarrette A, McCollum WH, Timoney PJ, Snijder EJ, MacLachlan NJ. Genetic characterization of equine arteritis virus during persistent infectionof stallions.J Gen Virol 2004;85:379-390. [ Links ]
4. Balasuriya UB, Timoney PJ, McCollum WH, MacLachlan NJ. Phylogenetic analysis of open reading frame 5 of field isolates of equine arteritis virus and identification of conserved and nonconserved regions in the GL envelope glycoprotein. Virology 1995; 214:690-697. [ Links ]
5. Bello ACPP, Cunha AP, Braz GF, Lara MCSH, Reis JKP, Haddad JPH, Rocha MA, Leite RC. Frequency of equine viral arteritis in Minas Gerais State, Brazil. Arq Bras Med Vet Zootec 2007; 59: 1077-1079. [ Links ]
6. Berrios, P. Actualización sobre enfermedades virales de los equinos. MonElectr PatolVet 2005;2:34-59. [ Links ]
7. Burky F. Further properties of Equine Arteritis Virus. Arch Virol; 1966;19:123-129 [ Links ]
8. Cavanagh D. Nidovirales: a new order comprising Coronaviridae and Arteriviridae. Arch Virol 1997; 142: 629-633. [ Links ]
9. Coignoul FL, Cheville NF. Pathology of maternal genital tract, placenta, and fetus in equine viral arteritis. Vet Pathol 1984; 21:333-40. [ Links ]
10. De la Sota MD, González R, Chiricosta A. Situación de la arteritis viral equina en la Argentina. In: Curso Producción Equina I, Facultad de Agronomía y Veterinaria de la Universidad Nacional de Río Cuarto -Sitio Argentino de Producción Animal, 2008. Disponible en: http://www.produccionbovina.com Revisado en noviembre de 2008. [ Links ]
11. Diel DG, Almeida SR, Weiblen R, Frandoloso R, Anziliero D, Kreutz LC, Sauter Groff FH, Flores EF. Prevalence of antibodies to influenza virus, viral arteritis and herpesvirus in horses of the Rio Grande do Sul state, Brazil. Cienc. Rural, 2006;36:1467-1473. [ Links ]
12. Doll ER, Knappenberger RE, and Bryans JT. An outbreak of abortion caused by the equine arteritis virus. Cornell Vet 1957; 47:69-75. [ Links ]
13. Echeverría MG, Díaz S, Metz GE, Serena MS, Panei CJ, Nosetto E. Genetic typing of equine arteritis virus isolates from Argentina.Virus Genes 2007; 35:313-20. [ Links ]
14. Echeverría MG, Díaz S, Metz GE, Serena MS, Panei CJ, Nosetto E. Evaluation of neutralization patterns of the five unique Argentine equine arteritis virus field strains reported. Rev Argent Microbiol. 2010; 42:11-7. [ Links ]
15. Fernandes WR, Souza MCC, Timoney PJ. Ocorrência de surto de arterite viral dos eqüinos no Brasil. In: 52th Conferência Anual Da Sociedade Paulista De Medicina Veterinária, 1997, São Paulo. Anais..., São Paulo: Sociedade Paulista de Medicina Veterinária, 1997. p. 14. [ Links ]
16. Fukunaga Y, Imagawa H, Tabuchi E, Akiyama Y. Clinical and virological findings on experimental equine viral arteritis in horses. Bull Equine Res Instit 1981; 18: 110-118. [ Links ]
17. Glaser AL, de Vries AA, Rottier PJ, Horzinek MC, Colenbrander B. Equine arteritis virus: a review of clinical featuresand management aspects.Vet Q 1996;18:95-99. [ Links ]
18. Gonzalez LJ, De la sota M, Barrandeguy M, Trono K, Ayerbe M, Tarantelli M. Relevamiento serológico de arteritis viral equina en padrillos registrados en argentina. In Proceedings of the VIII Congreso of the World Equine Vet. Assoc., 2003, p. 194-195. [ Links ]
19. Hedges JF, Balasuriya UB, Timoney PJ, McCollum WH, Mac Lachlan NJ. Genetic divergence with emergence of novel phenotypic variants of equine arteritis virus during persistent infectionof stallions. JVirol 1999;73:3672-3681. [ Links ]
20. Heinemann MB, Cortez A, Souza MCC, Gotti T, Ferreira F, Homem VSF, Ferreira JS, Soares RM, Sakamoto SM, Cunha EMS, Richtzenhain LJ. Seroprevalence of equine infection anemia, equine viral arteritis and equine viralabortionin Uruará municipality, Pará State, Brazil. Braz J Vet ResAnim Sci 2002; 39:50-53. [ Links ]
21. Holyoak GR, Balasuriya UB, Broaddus CC, Timoney PJ. Equine viral arteritis: current status and prevention. Theriogenology 2008;70:403-14. [ Links ]
22. ICA-Instituto Colombiano Agropecuario. Resolución 001595, 11de Mayodel 2010. Bogotá.2010. [ Links ]
23. ICTVdB -03.004.0.01.002. Equine arteritis virus. In: ICTVdB - The Universal Virus Database, version 4. Büchen-Osmond, C. (Ed),Columbia University, NewYork, USA.2006. [ Links ]
24. Lara MCCSH, Fernandes WR, Timoney PJ, Birgel EH. Prevalence of equine arteritis virus specific antibodies in horses of São Paulo State, Brazil. Arq Bras Med Vet Zootec 2002; 54:223-227. [ Links ]
25. Little TV, Holyoak GR, McCollum WH, Timoney PJ. Output of equine arteritis virus from persistently infected stallions is testosterone dependent, p. 225-229. In: Plowright W, Rossdale PD, Wade JF. (ed.), Proceedings of the 6th International Conference on Equine Infectious Diseases, Cambridge, 1991. R &W Publications, Newmarket, England. 1992. [ Links ]
26. MacLachlan NJ, Balasuriya UB, Rossitto PV, Hullinger PA, Patton JF, Wilson WD. Fatal experimental equine arteritis virus infection of a pregnant mare: immunohistochemical staining of viral antigens.J Vet Diagn Invest 1996; 8:367-374. [ Links ]
27. McCollum WH. Development of a modified virus strain and vaccine for equine viral arteritis. JAVMA1969;155:318-322. [ Links ]
28. McCollum WH, Prickett ME, Bryans JT. Temporal distribution of equine arteritis virus in respiratory mucosa, tissues and body fluids of horses infected by inhalation. Res Vet Sci 1971; 12:459-464. [ Links ]
29. Metz GE, Serena MS, Díaz S, Echeverría MG. Caracterización de secuencias del virus de la arteritis equina obtenidas directamente de muestras de semen de equinos seropositivos. AnalectaVeterinaria 2008;28:21-26. [ Links ]
30. Metz GE, Serena MS, Ocampos GM, Panei CJ, Fernandez VL, Echeverría MG. Equine arteritis virus: a new isolate from the presumable first carrier stallion in Argentina and its genetic relationships among the four reported unique Argentinean strains.Arch Virol 2008b;153:2111-2115. [ Links ]
31. Metz GE, Ocampos GP, Serena MS, Gambaro SE, Nosetto E, Echeverría MG. Extended Phylogeny of the Equine Arteritis Virus Sequences Including South American Sequences. Intervirology 2010 6; 54:29-36. [ Links ]
32. Moore BD, Balasuriya UB, Hedges JF, MacLachlanNJ. Growth characteristics of a highly virulent, a moderately virulent, and an avirulent strain of equine arteritis virus in primary equine endothelial cells are predictive of their virulence to horses. Virology. 2002; 298:39-44. [ Links ]
33. Moore BD, Balasuriya UB, Watson JL, Bosio CM, MacKay RJ, MacLachlan NJ. Virulent and avirulent strains of equine arteritis virus induce different quantities ofTNF-alpha and other proinflammatory cytokines in alveolarand blood-derived equine macrophages.Virology 2003;314:662-70. [ Links ]
34. Murphy TW, McCollum WH, Timoney PJ, Klingeborn BW, Hyllseth B, Golnik W, Erasmus B. Genomic variability among globally distributed isolates of equine arteritis virus. Vet Microbiol 1992;32:101-115. [ Links ]
35. Neu SM, Timoney PJ, Lowry SR. Changes in semen quality following experimental equine arteritis virus infection in the stallion.Theriogenology 1992; 37: 407-31. [ Links ]
36. Neu SM, Timoney PJ, McCollum WH. Persistent infection of the reproductive tract in stallions experimentally infected with equine arteritis virus. In: Proceedings of the 5th Conf Equine Infec Dis1988; 149-154. [ Links ]
37. Perozo EA. Prevalencia de la arteritis viral equina en Venezuela a través de la prueba de seroneutralización en placa. Tesis de Maestría, Facultad de Ciencias Veterinarias, Postgrado de Reproducción Animal y Tecnología de la Inseminación Artificial. Universidad Central de Venezuela, Caracas, Venezuela 2005. [ Links ]
38. Plagemann PGW, Moennig V. Lactate dehydrogenase-elevating virus, equine arteritis virus, and simian hemorrhagic fever virus: A new group of positive-strand RNA viruses. Adv Virus Res 1992;41:99-192. [ Links ]
39. Rodríguez T, Rivera H. Seroprevalencia del virus arteritis equina en población de equinos en riesgo del peru. Rev Inv Pec IVITA(Peru) 1998;9:78-80. [ Links ]
40. Shirai J, Kanno T, Tsuchiya Y, Mitsubayashi S, Seki R. Effects of chlorine, iodine, and quaternary ammonium compound disinfectants on several exotic disease viruses. J Vet Med Sci 2000;62:85-92. [ Links ]
41. Timoney PJ, Creekmore L, Meade B, Fly D, Rogers E, King B. Multi-state occurrence of EVA. Report of the Committee on Infectious Diseases of Horses: Addressing equine viral arteritis in the United States. In: Proceedings of the one-hundred tenth annual meeting of the United States animal health association. Minneapolis, Minnesota: Pat Campbell & Associates and Spectrum Press; 2006. p.354-362. [ Links ]
42. Timoney PJ, McCollum WH, MurphyTW, Roberts AW, Willard JG, Carswell GD. The carrier state in equine arteritis virus infection in the stallion with specific emphasis on the venereal mode of virus transmission. J Reprod Fertility 1987;35:95-102. [ Links ]
43. Timoney PJ, McCollum WH. Equine Viral Arteritis - epidemiology and control. J Eq Vet Sci 1988; 8:54-9. [ Links ]
44. Timoney PJ, McCollum WH. Equine viral arteritis in perspective in relation to Int trade. J Equine Vet Sci 1993a;13:50-52. [ Links ]
45. Timoney PJ, McCollum WH. Equine viral arteritis. Vet Clin North Am Equine Pract 1993b; 9:295-309. [ Links ]
46. Timoney PJ, McCollum WH. Equine viral arteritis: Further characterization of the carrier state in stallions. J Reprod Fertility Suppl 2000; 56:3-11. [ Links ]
47. Timoney PJ. Clinical, virological and epidemiological features of the 1984 out break of equine viral arteritis in the Thorough bred population in Kentucky, USA. In: Proceedings of the Grayson Found Int Conf Thorough bred Breeders Org on EquineViral Arteritis1984; 24-33. [ Links ]
48. Timoney PJ. Equine viral arteritis. Am Assoc Equine Pract Report 2000a;7. [ Links ]
49. Timoney PJ. The increasing significance of international trade in equids and its influence on the spread of infectious diseases. Ann NYA cad Sci 2000b; 916: 55-60. [ Links ]
50. Timoney PJ. Equine viral arteritis Chapter 2.5.10. In OIE Manual of Diagnostic Tests and Vaccines for Terrestrial Animals. 5th Edn. Paris,World Organization forAnimal Health, OIE. 2004,1178pp. [ Links ]
51. Timoney PJ. Equine viral Arteritis. In: Equine Respiratory Diseases, P. Lekeux (Ed.) Publisher: International Veterinary Information Service (www.ivis.org), Ithaca, NewYork, USA. 2003. [ Links ]
52. USDA-APHIS. Equine viral arteritis uniform methods and rules effective April 19,2004. USDA-APHIS, 2004:1-19. [ Links ]
53. Vaala WE, Hamir AN, Dubovi EJ, Timoney PJ, Ruiz B. Fatal, congenitally acquired infection with equine arteritis virus in a neonatal Thorough bred.Equine Vet J 1992; 24:155-158. [ Links ]
54. Zhang J, Timoney PJ, Shuck KM, Seoul G, Go YY, Lu Z, Powell DG, Meade BJ, Balasuriya UB. Molecular epidemiology and genetic characterization of equine arteritis virus isolates associated with the 2006-2007 multi-state disease occurrence in the USA. J GenVirol 2010;91: 2286-2301. [ Links ]