Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Colombiana de Ciencias Pecuarias
Print version ISSN 0120-0690On-line version ISSN 2256-2958
Rev Colom Cienc Pecua vol.24 no.1 Medellín Jan./Mar. 2011
Artículos originales
Production of an extracellular protease by an Antarctic bacterial isolate (Bacillus sp. JSP1)as a potential feed additive¤
Proteasa extracelular de un aislado bacteriano Antartico (Bacillus sp. JSP1) con uso potencial como aditivo alimenticio para animales
Produção de proteasa extracelular por bactérias antárcticas isoladas, Bacillus sp. JSP1 como um aditivo potencial em concentrado
Inkyung Park1, Animal Scientist, PhD ; Jaiesoon Cho2*, Feed Biotechnologist, PhD.
1Animal Resources Research Center, College of Animal Bioscience and Technology, Konkuk University, 1 Hwayang-dong, Gwangjin-gu, Seoul 143-701, Korea
2Department of Animal Sciences and Environment, College of Animal Bioscience and Technology, Konkuk University, 1 Hwayang-dong, Gwangjin-gu, Seoul 143-701, Korea.
(Recibido: 29 julio, 2010; aceptado: 18 enero, 2011)
Summary
Extracellular proteolytic activity was found in JSP1, an Antarctic bacterial isolate. The strain was related to Bacillus sp, based on 16S rRNA gene sequence analysis. The JSP1 protease was partially purified by ammonium sulfate precipitation. Optimal enzyme activity occurred at 40 C and pH 7.4. Enzyme activity was significantly enhanced in the presence of Mg2+ and Ca2+ and was completely inactivated in presence of Cu2+, Zn2+, Hg2+, EDTA and SDS. The enzyme hydrolyzed casein the most effectively among the protein substrates tested. The enzyme also exhibited relatively high activity on keratin and gluten, and was active against peptidyl conjugates such asL-Leu-p-Nitroanilide and N-Succinyl-L-Phe-p-Nitroanilide. This study suggests that JSP1 protease could be utilized as a potential environmentally-friendly feed additive in animal production.
Key words: antarctic, Bacillus sp., feed additive, keratin, livestock production, protease.
Resumen
Se reporta haber encontrado actividad proteolítica extracelular en una bacteria antártica denominada JSP1. Con base en el análisis de secuencia genética 16S ARNr, la cepa fué relacionada con Bacillus sp. La proteasa JSP1 fué parcialmente purificada por precipitación con sulfato de amonio. La actividad óptima de la enzima se produjo a 40 ºC y pH 7,4. La actividad enzimática fue significativamente mayor en presencia de Mg2+ y Ca2+ y se inactivó completamente en presencia de Cu2+, Zn2+ , Hg2+ , EDTA y SDS. Entre todos los sustratos ensayados, el más eficientemente hidrolizado por la enzima fue la caseína. La enzima tuvo actividad relativamente alta sobre la queratina y el gluten, y participó activamente contra conjugados peptídicos como la L-Leu-p-nitroanilida y la N-succinil-L-fenilalanina-p-nitroanilida. La enzima podría ofrecer potencial para su uso como aditivo alimenticio ecológico en producción animal.
Palabras clave: antártida, aditivo alimenticio, Bacillus sp., producción animal, proteasa queratina.
Resumo
A actividade proteolítica extracelular foi encontrada a partir de uma bactéria antárctica denominada JSP1, mediante uma análises de sequencia do gene 16S rRNA, a cepa foi relacionada para Bacillus sp. A proteasa JSP1 foi parcialmente purificada por precipitação com sulfato de amônia. Uma óptima actividade enzimática ocorreu em 40 ºC e pH 7,4. A actividade da enzima foi significativamente maior na presença de Mg2 + e Ca2 +, e foi completamente inactivada em presença de Cu2 + Zn2 +, Hg2 +, EDTA e SDS. A enzima hidrolisada de caseína foi mais eficaz entre os substratos de proteína testados, apresentando maior actividade na queratina e no glúten e foi activo contra os peptidil conjugados como L-Leu-p Nitroanilida e N-succinil-L-Phe-p-Nitroanilida. A enzima pode ser utilizado como aditivo ambiental na alimentação animal.
Palavras chave:antarctic,Bacillus sp., feed additive, keratin, livestock production, protease.
¤ Para citar este artículo: Park I, Cho J. Production of an extracellular protease by Antarctic isolate, Bacillus sp. JSP1 as a potencial feed additive. Rev. Colomb Cienc Pecu 2011; 24:3-10
* Corresponding author: Jaiesoon Cho. Department of Animal Sciences and Environment, College of Animal Bioscience and Technologu, Konkuk University, 1 Hwayang-dond, Gwangjin.gu, Seoul 143-701, Korea Tel) +82-2-450-3375 Fax) +82-2-455-1044. E-mail: chojs70@konkuk.ac.kr
Introduction
Proteases have attracted a great deal of attention due to their broad range of applications in the detergent, food, pharmaceutical, chemical, leather, paper, and pulp, and silk industries (Rai and Mukherjee, 2009). Microbial proteases comprise approximately 40% of the worldwide production of enzymes (Jaouadi et al., 2008). In the field of animal nutrition, exogenous proteases have been used as feed additives. For instance, proteases help degrade soybean proteins such as glycinin and β-conglycinin, along with some protein anti-nutritional factors (lectins and trypsin-inhibitors) in inadequately processed soybean meal (Thorpe and Beal, 2001). Moreover, the combined use of proteases together with carbohydrate-degrading enzymes such as xylanase and amylase was found to enhance the nutritional value of a corn and soybean meal-based diet for poultry (Hong et al., 2002; Marsman et al., 1997).
In particular, Bacillus species, a type of exogenous spore-forming bacteria, are prolific producers of extracellular proteases (Rao et al., 1998). Currently, Bacillus licheniformis, Bacillussubtilis, and Bacillus pumilus are well-known species employed industrially for alkaline protease production (Gupta et al., 2002). Although they do not commensally colonize the gastrointestinal tract, Bacillus species have been shown to be effective in maintaining a favorable balance of microflora in the gastrointestinal tract and in improving the production performance of farm animals (Alexopoulos et al., 2004; Kritas and Morrison, 2005).
Some useful and unusual enzymes have been reported from so-called extremophiles inhabiting Antarctica (Demirjian et al., 2001). Considering that the number of microbes cultured to date remains only a tiny fraction of all microbial species on earth, the number of novel enzymes is expected to increase continuously (Park et al., 2007). In this report, data are presented concerning general properties of extracellular proteolytic activity derived from an Antarctic bacterial isolate, Bacillus sp. JSP1.
Materials and methods
Bacterial strain and culture conditions
A bacterial isolate, JSP1, derived from Antarctic soil samples was supplied from Korea Polar Research Institute, operating the King Sejong Station (South Korea) in Antarctica. Screening for protease activity was performed on selective agar plates [1.0% skim milk (Sigma), 0.45% (NH4)2SO4, 0.05% yeast extract (Difco), 0.07% KH2PO4, 0.01% MgSO47H2O, 0.01% NaCl, 0.01% CaCl22H2O, 0.001% MnSO44H2O, 0.001% FeSO47H2O, and 1.5% bacto agar (Difco), pH 7.4] at 28 ºC by observing a clear zone of hydrolyzed casein around the colonies, as previously described (Hutadilok- Towatana et al., 1999).
Growth and protease production were investigated in 100 mL of protease production medium [0.5% skim milk, 0.5% yeast extract, 0.07% KH2PO4, 0.01% NaCl, 0.01% CaCl22H2O, 0.01% MgSO47H2O, 0.001% MnSO44H2O and 0.001% FeSO47H2O (pH 7.4)] in a 500 mL Erlenmeyer flask, aerobically incubated with vigorous shaking (220 rpm), by monitoring the absorbance (O.D.600nm) for the cell growth and protease activity of the culture supernatant at 28 ºC at various time points.
Taxonomic identification of strain JSP1
Genomic DNA was extracted from strain JSP1 using a FastDNA kit (Qbiogene) according to the manufacturer`s protocol. The 16S rRNA gene was amplified from genomic DNA by PCR using the universal primers 27F (5`-AGAGTTTGATCCTGGCTCAG-3`) and 1492R (5`-GGTTACCTTGTTACGACTT-3`) (William et al., 1991). The amplified ed 1,427 bp sequences were determined by an automated ABI PRISM 3730 XL DNA analyzer (Applied Biosystems). The resulting sequences were compared with the GenBank database (NCBI) using BLAST (Altschul et al., 1990). Sequences showing a relevant degree of similarity were imported into the CLUSTAL W program (Thompson et al., 1994) and aligned. The evolutionary distances to other Bacillus strainswere computed using the Maximum Composite Likelihood method (Tamura et al., 2004), and the phylogenetic relationships were determined using the software MEGA, version 4.0 (Tamura et al., 2007).
Nucleotide sequence accession numbers
The nucleotide sequence of the 16S rRNA gene has been deposited in the GenBank database under Accession No. GU014529.
Partial purification of the enzyme
One liter of protease production medium [0.5% skim milk, 0.5% yeast extract, 0.07% KH2PO4, 0.01% NaCl, 0.01% CaCl22H2O, 0.01% MgSO47H2O, 0.001% MnSO44H2O and 0.001% FeSO47H2O (pH 7.4)] in two 2 L Erlenmeyer flasks was aseptically inoculated with a single colony of strain JSP1 and aerobically cultivated with vigorous shaking (220 rpm) for 96 h at 28 ºC. The culture medium containing secreted protease was centrifuged at 9000 g for 30 min at 4 ºC to remove the cells, and proteins in the supernatant were then precipitated with ammonium sulfate (75% saturation). The pellet was dissolved in 50 mM Tris-HCl (pH 8.0) and dialyzed overnight against 50 mM Tris-HCl (pH 7.4) at 4 ºC. The dialyzed solution was used as the protease source throughout this work to examine its catalytic properties.
Zymography
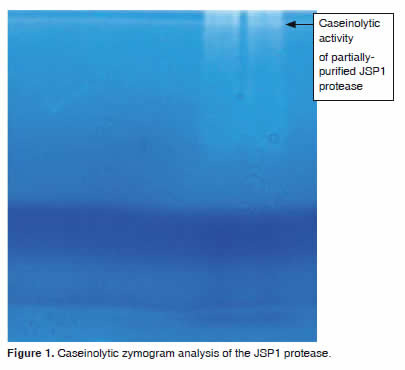
Native Polyacrylamide Gel Electrophoresis (PAGE) was carried out with a Modular Mini-Protein II Electrophoresis System (Bio- Rad, Hercules, CA, USA) according to the manufacturer`s instructions. Zymograms (0.1% casein in 10% polyacrylamide) were run for 6 h at 4 ºC and 60 V in buffer containing 25 mM Tris-HCl (pH 8.0) and 125 mM glycine. After electrophoresis, the gel was incubated overnight at room temperature in calcium proteolysis buffer (20 mM Tris-HCl, 20 mM CaCl2 ; pH 7.4) under gentle shaking. The gel was stained with SimplyBlueSafeStain (Invitrogen) for 30 min and destained overnight. The bands of caseinolytic activity appear white on a blue-stained background.
Preparation of keratin substrate
Keratin substrate was prepared from chicken feathers by the modification method of Nam et al. (2002). Briefly, ground chicken feathers (1 g) in 50 mL of dimethyl sulfoxide, were solubilized by heat treatment on a hot plate at 90 ºC for 2 h. Soluble keratin was then precipitated by addition of cold acetone (200 mL) at -70 ºC for 2 h followed by centrifugation at 10000 g for 20 min. The precipitate was washed twice with distilled water and then dissolved in 10 mM Tris-HCl buffer (pH 9.0).
Enzyme assay and general catalytic properties
Unless otherwise stated, the assay was performed at 40 ºC for 1 h in a reaction mixture containing 460 μL of 50 mM Tris-HCl (pH 7.4), 140 μL of 3% azocasein as a nonspecific substrate, and 100 μL of enzyme. The reaction was terminated by addition of 700 μL of 10% trichloroacetic acid. One unit of the azocaseinolytic activity was defined as the amount of enzyme required to produce an increase in absorbance at 366 nm of 0.01 per minute under the given assay conditions. Protease substrate specificity was examined with case in (Sigma, St. Louis, MO, USA), gelatin (Sigma, St. Louis, MO, USA), collagen (Sigma, St. Louis, MO, USA), bovine serum albumin (Sigma, St. Louis, MO, USA), gluten (Sigma, St. Louis, MO, USA), and chicken feather keratin by a modified method of Wang et al. (2005). Briefly, 200 μL of enzyme was added to a reaction mixture containing 3% of each substrate in 360 μL of 50 mM Tris-HCl (pH 7.4) and incubated at 40 ºC. The reaction was stopped by adding 700 μL of 10% trichloroacetic acid and centrifuged at 10000 g for 10 min.
The protein remaining in the supernatant was determined by Folin-phenol reagent (Folin and Ciocalteau, 1927). One unit of protease activity was defined as the amount of nzyme that liberated 1μg of tyrosine per minute under the defined assay conditions. Peptidolytic activity was also assayed at 40 ºC using 2 mM each of L-Leu-p-Nitroanilide, N-Succinyl-L-Ala-Ala-Ala-p-Nitroanilide, and N-Succinyl-L-Phe-p-Nitroanilide as substrates in 50 mM Tris-HCl (pH 7.4). The amount of p-Nitroaniline liberated was determined from the samples. absorbance at 405 nm.
Determination of pH and temperature optima on protease activity
To study the temperature optimum and enzyme activity, the enzyme reaction mixture was incubated at different temperatures from 0 to 80 ºC in 50 mM Tris-HCl (pH 7.4) buffer sing azocasein as a substrate. The pH optimum for protease activity with azocasein substrate was determined at 40 ºC in 50 mM glycine-HCl (pH 3), 50 mM sodium acetate (pH 4-5), 50 mM Bis-Tris-HCl (pH 6-7), and 50 mM Tris-HCl (pH 7.4-9.0) buffers.
Effect of reagents on enzyme activity
The effects of metal ions and inhibitors on protease activity were examined with azocasein as a substrate. Each additive (5 mM) was pre-incubated with the enzyme for 30 min at 40 ºC before the standard assay was performed, and the residual activity was measured.
Results
Identification of isolated strain JSP1, protease production and partial purification of the enzyme
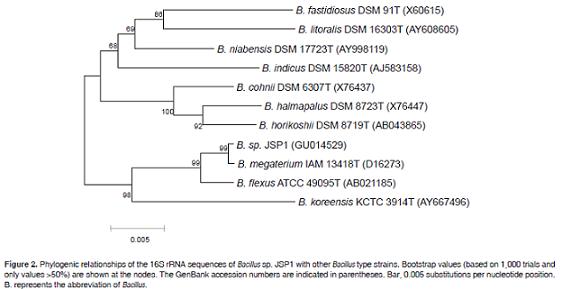
To identify the isolated strain JSP1 that shows protease activity (Figure 1), we cloned its 16S rRNA gene and compared the sequence with those available in the database. A phylogenic tree based on the 16S rRNA gene sequences from 10 bacterial Bacillus strains show ed that the JSP1 strain shared 99.7% sequence identity with the type strain, Bacillus megaterium IAM 13418 (Figure 2).
Therefore, it was named Bacillus sp. JSP1.
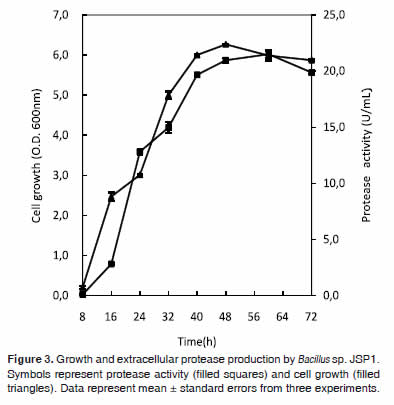
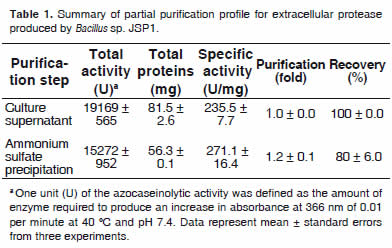
Time courses of cell growth and extracellular protease activity were shown in figure 3. Protease activity was nearly proportional to cell growth during cultivation. The enzyme was steeply produced after 16 h of incubation, showing a maximum activity (21.5 ± 0.1 U/mL) at 60 h of incubation. The partial purification profile of the extracellular protease produced by Bacillus sp. JSP1 was summarized in table 1.
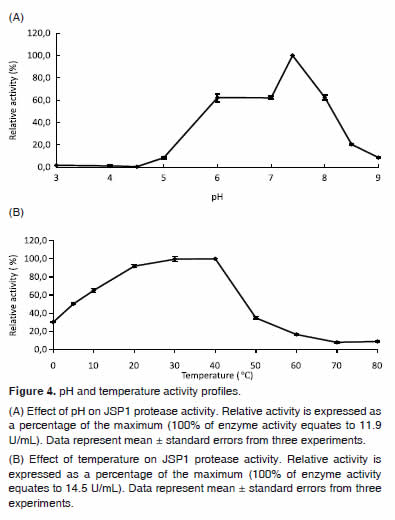
Effect of pH and temperature on enzyme activity
Optimal protease activity occurred at pH 7.4, while over 60% of the peak activity was achieved between pH 6.0 and 8.0. However, activity was nearly completely inactivated at acidic pH (pH 3.0-5.0) and above pH 8.5 (Figure 4A). As shown in figure 4B, JSP1 protease showed optimal activity at 20 — 40°C and retained more than 35% of the activity at 5 — 50°C.
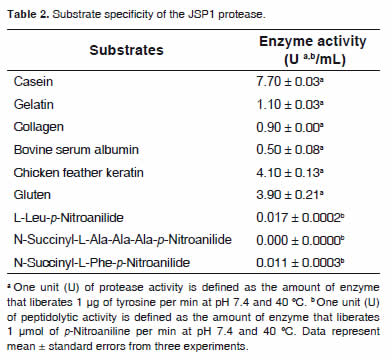
Substrate specificity
JSP1 protease hydrolyzed casein the most effectively among the protein substrates tested (Table 2). The enzyme also showed relatively high activity on keratin, which is the most abundant structural protein in skin, hair, wool, and feathers (Tatineni et al., 2008) and gluten, which is a useful protein source in cattle feed (Firkins et al., 1985; Ohajuruka and Palmquist, 1989). Low levels of hydrolysis were observed with gelatin, collagen, and bovine serum albumin (Table 2). Among the peptidyl-p-Nitroanilide substrates tested, the enzyme was active against L-Leu-p-Nitroanilide and N-Succinyl-L-Phe-p-Nitroanilide which is cleaved by subtilisin-like and chymotrypsin-like enzymes (Hutadilok-Towatana et al., 1999), and exhibited no activity on N-Succinyl-L-Ala-Ala-Ala- p-Nitroanilide.
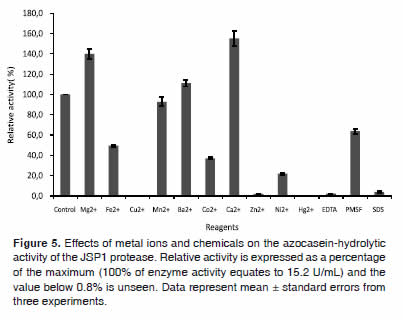
Effect of various reagents on enzyme activity
The protease activity in the presence of various metal ions or chemicals was shown in figure 5. Amongst the metal ions, Mg2+ and Ca2+ were highly effective at stimulating JSP1 proteperase, increasing activity by 40% and 55%, respectively. In contrast, enzyme activity was significantly reduced (50-78% of the control activity) in the presence of Fe2+, Co2+, and Ni2+. Although Cu2+, Zn2+, and Hg2+ almost completely inactivated the enzyme, no important effect on the activity was observed with Mn2+ and Ba2+. The enzyme was also completely inactivated by EDTA and Sodium Dodecyl Sulfate (SDS), but moderately inhibited by Phenyl Methyl Sulfonyl Fluoride (PMSF).
Discussion
In the present study, the extracellular protease secreted by an Antarctic strain, Bacillus sp. JSP1, was partially characterized, and most of its properties were found to be distinct from those of other proteases from Bacillus strains. The JSP1 protease is nearly a neutral protease, with an optimal pH of 7.4, while most known Bacillus species produce commercial proteases that are highly active at pH 7.0 and 11.0, with an optimum around pH 8.0-10.0 (Davail et al., 1994; Hutadilok-Towatana et al., 1999; Jaouadi et al., 2008).
The optimum temperature of 40 ºC for the protease is similar to the temperature (35 ºC) for the serine protease of the psychrophilic bacterium, Colwellia sp. NJ341 (Wang et al., 2005). Moreover, the JSP1 protease maintains 30% of its highest activity at 0 ºC, which is one of the typical characteristics found in cold-active enzymes (Wang et al., 2005; Zhang and Zeng, 2008).
The JSP1 protease showed the highest activity on casein, much like the alkaline serine proteases from Bacillus pumilus CBS (Jaouadi et al., 2008), Bacillus stearothermophilus F1 (Rahman et al., 1994) and Bacillus sp. KSM-K16 (Kobayashi et al., 1995). Unexpectedly, the enzyme could hydrolyze keratin which, like other insoluble proteins, is an unacceptable substrate for common proteases such as trypsin and pepsin (Letourneau et al., 1998; Papadopoulos et al., 1986). In the animal feed industry, feather waste can be a potential alternative to more costly dietary ingredients for animal feedstuffs (Shih, 1993). Worldwide, commercial poultry processing produces millions of tons of feathers per year, which are currently converted to feather meal through steam pressure and chemical treatment (Shih, 1993). Although chemical treatment renders keratin waste more digestible, it is high-priced and destroys certain amino acids (Papadopoulos et al., 1986).
The nutritional enhancement of feather meal by the enzymatic treatment might significantly improve amino acid availability of feather keratin (Odetallah et al., 2003). Until now, known keratinolytic enzymes have been mainly produced by mesophilic fungi (Santos et al., 1996), actinomycetes (Böckle et al., 1995), some thermophilic Bacillus sp. (Kim et al., 2001), and some thermophilic anaerobes (Nam et al., 2002). To our knowledge, little has been known about keratinolytic activity detected in Antarctic Bacillus strains.
Calcium ions are generally known to be involved in maintaining the activity of Bacillus serine proteases (Hutadilok-Towatana et al., 1999; Jaouadi et al., 2008). However, the JSP1 protease seems to belong to the metalloprotease rather than to the serine protease family, because the activity of this enzyme was bly inhibited by a chelating agent, EDTA, but only partially inhibited by PMSF, a well-known inhibitor of serine proteases (Hutadilok-Towatana et al., 1999). This reasoning may be also supported by the observed Zn2+-dependent inhibition, because excess zinc inhibits some metalloproteases (Auld, 1995). It is interesting that the enzyme exhibited keratinolytic activity, despite the fact that metalloproteases are not frequently associated with keratinolytic activity (Tatineni et al., 2008). The enzyme was sensitive to anionic SDS addition, indicating that hydrogen bonds ay play a pivotal role in maintaining enzyme activity (Wang et al., 2005).
The JSP1 protease may offer potential for use as an environmentally-friendly feed dditive to improve the production performance of farm animals, due to its broad substrate specificity and relatively desirable activity levels at physiologically relevant pH and temperature. Additionally, the keratinolytic activity of the enzyme will help to conduct biotechnological processes of the keratinous biomaterials from poultry and leather industries. A more detailed characterization of the enzyme such as gene cloning, protein engineering, and fermentation technology is warranted to maximize the catalytic efficiency and productive yield of the enzyme.
References
1. Alexopoulos C, Georgoulakis IE, Tzivara A, Kyriakis CS, Govaris A, Kyriakis SC. Field evaluation of the effect of a probioticcontaining Bacillus licheniformis and Bacillus subtilis spores on the health status, performance, and carcass quality of grower and finisher pigs. J Vet Med A Physiol Pathol Clin Med 2004; 51:306-312. [ Links ]
2. Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol 1990; 215:403-410. [ Links ]
3. Auld DS. Removal and replacement of metal ions in metallopeptidases. Meth Enzymol 1995; 248:228-242. [ Links ]
4. Böckle B, Galunski B, Müller R. Characterization of a keratinolytic serine protease from Streptomyces pactum DSM40530. Appl Environ Microbiol 1995; 61:3705-3710. [ Links ]
5. Davail S, Feller G, Narinx E, Gerday C. Cold adaptation of proteins: purification, characterization, and sequence of the heat-labile subtilisin from the Antarctic psychrophile Bacillus TA41. J Biol Chem 1994; 269:17448-17453. [ Links ]
6. Demirjian DC, Moris-Varas F, Cassidy CS. Enzymes from extremophiles. Curr Opin Chem Biol 2001; 5:144-151. [ Links ]
7. Firkins JL, Berger LL, Fahey Jr. GC. Evaluation of wet and dry distillers. grains and wet and dry corn gluten feeds for ruminants. J Anim Sci 1985; 60:847-860. [ Links ]
8. Folin O, Ciocalteau V. On tyrosine and tryptophan determinations in proteins. J Biol Chem 1927; 73:627-650. [ Links ]
9. Gupta R, Beg QK, Lorenz P. Bacterial alkaline protease: molecular approaches and industrial applications. Appl Microbiol Biotechnol 2002; 59:15-32. [ Links ]
10. Hong D, Burrows H, Adeola O. Addition of enzymes to starter and grower diets for ducks. Poult Sci 2002; 81:1842-1849. [ Links ]
11. Hutadilok-Towatana N, Painupong A, Suntinanalert P. Purification and characterization of an extracellular protease from alkaliphilic and thermophilic Bacillus sp. PS 719. J Biosci Bioeng 1999; 87:581-587. [ Links ]
12. Jaouadi B, Ellouz-Chaabouni S, Rhimi M, Bejar S. Biochemical and molecular characterization of a detergent-stable alkaline protease from Bacillus pumilus CBS with high catalytic eficiency. Biochimie 2008; 90:1291-1305. [ Links ]
13. Kim JM, Lim WJ, Suh HJ. Feather-degrading Bacillus species from poultry waste. Process Biochem 2001; 37:287-291. [ Links ]
14. Kobayashi T, Hakamada Y, Adachi S, Hitomi J, Yoshimatsu T, Koike K, Kawai S, Ito S. Purification and properties of an alkaline protease from alkalophilic Bacillus sp. KSM-K16. Appl Microbiol Biotechnol 1995; 43:473-481. [ Links ]
15. Kritas SK, Morrison RB. Evaluation of probiotics as a substitute for antibiotics in a large pig nursery. Vet Rec 2005; 156:447-448. [ Links ]
16. Letourneau F, Soussotte V, Bressollier P, Branland P, Verneuil B. Keratinolytic activity of Streptomyces sp. S.K 1-02: A new isolated strain. Lett Appl Microbiol 1998; 26:77-80. [ Links ]
17. Marsman GIP, Gruppen H, Van der Poel AFB, Kwakkel RP, Verstegen MWA, Voragen AGJ. The effect of thermal processing and enzyme treatments of soybean meal on growth performance, ileal nutrient digestibilities, and chime characteristics in broiler chicks. Poult Sci 1997; 76:864-872. [ Links ]
18. Nam GW, Lee DW, Lee HS, Lee NJ, Kim BC, Choe EA, Hwang JK, Suhartono MT, Pyun YR. Native-feather degradation by Fervidobacterium islandicum AW-1, a newly isolated keratinase-producing thermophilic anaerobe. Arch Microbiol 2002; 178:538-547. [ Links ]
19. Odetallah NH, Wang JJ, Garlich JD, Shih JCH. Keratinase in starter diets improves growth of broiler chicks. Poult Sci 2003; 82:664-670. [ Links ]
20. Ohajuruka OA, Palmquist DL. Response of high-producing dairy cows to high levels of dried corn gluten feed. Anim Feed Sci Tech 1989; 24:191-200. [ Links ]
21. Papadopoulos MC, El Boushy AR, Roodbeen AE, Ketelaars EH. Effect of processing time and moisture content on amino acid composition and nitrogen characteristics of feather meal. Anim Feed Sci Technol 1986; 14:279-290. [ Links ]
22. Park HJ, Jeon JH, Kang SG, Lee JH, Lee SA, Kim HK. Functional expression and refolding of new alkaline esterase, EM2L8 from deep-sea sediment metagenome. Protein Expres Purif 2007; 52:340-347. [ Links ]
23. Rahman RNZA, Razak CZ, Ampon K, Basri M, Zin WM, Yumus W, Salleh AB. Purification and characterization of a heat-stable alkaline protease from Bacillus stearothermophilus F1. Appl Microbiol Biotechnol 1994; 40:822-827. [ Links ]
24. Rai SK, Mukherjee AK. Ecological significance and some biotechnological application of an organic stable alkaline serine protease from Bacillus subtilis strain DM-04. Bioresource Technol 2009; 100:2642-2645. [ Links ]
25. Rao MB, Tanksale AM, Ghatge MS, Deshpande VV. Molecular and biotechnological aspects of microbial proteases. Microbiol Mol Biol Rev 1998; 62:597-635. [ Links ]
26. Santos RMD, Firmino AAP, Sá CM, Felix CR. Keratinolytic activity of Aspergillus fumigatus Fresenius. Curr Microbiol 1996; 33:364-370. [ Links ]
27. Shih JCH. Recent development in poultry waste digestion and feather utilization- a review. Poult Sci 1993; 72:1617-1620. [ Links ]
28. Tamura K, Dudley J, Nei M, Kumar S. MEGA4: Molecular Evolution Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol 2007; 24:1596-1599. [ Links ]
29. Tamura K, Nei M, Kumar S. Prospect for inferring very large phylogenies by using the neighbor-joining methods. Proc Natl Aca Sci USA 2004; 101:11030-11035. [ Links ]
30. Tatineni R, Doddapaneni KK, Potumarthi RC, Vellanki RN, Kandathil MT, Kolli N, Mangamoori LN. Purification and characterization of an alkaline keratinase from Streptomyces sp. Bioresour Technol 2008; 99:1596-1602. [ Links ]
31. Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 1994; 22:4673-4680. [ Links ]
32. Thorpe J, Beal JD. Vegetable protein meals and the effects of enzymes. In: Bedford MR, Partridge GG, editors. Enzymes in Farm Animal Nutrition. Wallingford, Oxon, UK: CABI Publ; 2001. p.125-143. [ Links ]
33. Wang QF, Miao JL, Hou YH, Ding Y, Wang GD, Li GY. Purfication and characterization of an extracellular cold-active serine protease from the psychrophilic bacterium Colwellia sp. NJ341. Biotechnol Lett 2005; 27:1195-1198. [ Links ]
34. William GW, Susan MB, Dale AP, David JL. 16S ribosomal DNA amplification for phylogenetic study. J Bacteriol 1991; 173:697-703. [ Links ]
35. Zhang JW, Zeng RY. Purification and characterization of a coldadapted α.amylase produced by Nocardiopsis sp. 7326 isolated from Prydz bay, Antarctic. Marine Biotechnol 2008; 10:75-82. [ Links ]