Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Colombiana de Ciencias Pecuarias
Print version ISSN 0120-0690On-line version ISSN 2256-2958
Rev Colom Cienc Pecua vol.25 no.1 Medellín Jan./Mar. 2012
Artículos originales
Embryonic development of Columba livia(Aves:Columbiformes) from an altricial-precocial perspective¤
Desarrollo embrionario de Columba livia(Aves: Columbiformes) desde una perspectiva altricial-precoz
Desenvolvimento embrionário de Columba livia(Aves: Columbiformes) a partir de uma perspectiva altricial-precoce
Gabriela B Olea1, Lic Cienc Biol; María T Sandoval1*, Lic Cienc Biol.
1Laboratorio de Herpetología. Departamento de Biología. Facultad de Ciencias Exactas y Naturales y Agrimensura – Universidad Nacional del Nordeste.Avenida Libertad,5470(3400) Corrientes, Argentina.
(Recibido:28enero,2011; aceptado: 29 septiembre, 2011)
Summary
Objective: this study characterized the morphogenesis of Columba livia and proposed a table with 43 embryonic stages. The ontogeny of this species was divided into three phases: early, middle and late. Methods and Results: the early phase (stages 1-26) includes the final period of segmentation, gastrulation, neurulation and somitogenesis, organization of the extraembryonic membranes, and the initial formation of the major organic systems. The middle phase (stages 27-36) was characterized mainly by the growth of limbs and the organization of the autopodium, the formation and growth of the peak, and the development of integumentary annexes such as feather germs and leg scales. The late phase (stages 37-43) shows the overall embryo growth, the final organization of the pterilosis pattern and growth of feather germs, as well as final consumption of the yolk, and hatching. Conclusions: these results allow making comparisons with the proposed developmental events of Gallus gallus domesticus, highlighting similarities and differences in the ontogenetic sequence of both species. Therefore we propose some hypotheses about possible heterochronic events related to altricial-precocial developmental models.
Key words: Columbidae, development patterns, embryonic stages, ontogeny.
Resumen
Objetivo: en el presente trabajo se caracteriza la morfogénesis de Columba livia y se propone una tabla con 43 estadios embrionarios. La ontogenia de dicha especie fue dividida en tres etapas: temprana, media y tardía. Métodos y Resultados: la etapa temprana (estadios 1-26) incluye el periodo final de la segmentación, la gastrulación, neurulación y somitogénesis, la organización de las membranas extraembrionarias y la formación de los esbozos de los principales sistemas de órganos. La etapa media (estadios 27-36) se caracteriza principalmente por el crecimiento de los miembros y la organización del autopodio, la formación y crecimiento del pico y el desarrollo de los anexos tegumentarios como plumones y escamas de las patas. En la etapa tardía (estadios 37-43) se evidencia el crecimiento general del embrión, la organización final del patrón de pterilosis y crecimiento de plumones, como así también el consumo final del vitelo y la eclosión. Conclusiones: en base a los resultados obtenidos se realizan algunas comparaciones con respecto a los eventos del desarrollo propuestos para Gallus gallus domesticus, destacando semejanzas y diferencias en la secuencia ontogénetica de ambas especies. A partir de esto se plantean algunas hipótesis acerca de posibles eventos heterocrónicos relacionados con los modelos de desarrollo altricial-precoz.
Palabras clave:: Columbidae, estados de desarrollo embrionario, ontogenia.
Resumo
Objetivo: no presente trabalho caracteriza-se a morfogênese de Columba livia, mediante uma tabela com 43 estágios embrionários. A ontogenia desta espécie foi dividida em três etapas: inicial, intermediária e final. Métodos y Resultados: a etapa inicial (estágios 1-26) incluiu o período final de segmentação, gastrulação, neurulação e somitogênese, organização das membranas extraembrionárias e formação dos esboços dos principais sistemas de órgãos. A etapa intermediária (estágios 27-36) foi caracterizada principalmente pelo crescimento dos membros e organização do autopódio, formação e crescimento do bico e desenvolvimento de anexos tegumentários como as penas e as escamas das patas. Na etapa tardia (estágios 37-43) foi caracterizada pelo crescimento geral do embrião, a organização final do padrão de pterilose e o padrão de crescimento das penas, assim como o consumo final do vitelo e eclosão. Conclusões: com base nos resultados obtidos, foram realizadas algumas comparações com respeito aos eventos do desenvolvimento proposto para Gallus gallus domesticus, destacando semelhanças e diferenças durante a sequência ontogenética de ambas espécies. A partir disso, algumas hipóteses são propostas acerca dos possíveis eventos heterocrônicos relacionados com os modelos de desenvolvimento altricial e precoce.
Palavras chave:: Columbidae, estádios de desenvolvimento embrionário, ontogenia, padrões de desenvolvimento.
§ To cite this article: Olea GB, Sandoval MT. Embryonic development of Columba livia (Aves: Columbiformes) from an altricial-precocial perspective. Rev Colomb Cienc Pecu 2012; 25:3-13.
* Corresponding author: María T Sandoval. Avenida Libertad, 5470 (3400) Corrientes, Argentina. E-mail: mtsandoval@exa.unne.edu.ar
Introduction
The embryology of vertebrates has been extensively studied in model organisms such as Danio rerio (Kimmel et al., 1995), Xenopus laevis (Keller, 1991), Bufo arenarum (Del Conte and Sirlin, 1951; Echeverria and De López, 1981), and Rattus norvegicus (Tam and Beddington, 1987) for which there is extensive information regarding the ontogenetic sequence and the morphology of different stages. In the case of birds, most of the knowledge on embryonic morphology has been derived from studies of Gallus gallus domesticus. Based on embryonic morphological characteristics, Hamburger and Hamilton (1952) established a table with 46 stages of development, which is used as a benchmark for many studies that have birds as the experimental model (Bellairs, 1958; Chevallier, 1977; Brush et al., 1972; Couly et al., 1992; Caprioli et al., 1998).
Other studies on bird development have addressed precocial bird species such as Colinus virginianus (Hendrickx and Hanzlik, 1965), Anas boschas domestica (Koecke 1958), Meleagris gallopavo (Mun and Kosin, 1960), Coturnix coturnix japonica (Ainsworth et al., 2009), and altricial species such as Lonchura striata (Yamasaki and Tonosaki, 1988) and Tyto alba (Kˆppl et al., 2005), although only partial information on their embryonic ontogeny is currently available. These reports indicate existing developmental variations in certain structures between species, which are related to the status of the chicks at hatching or, according to Yamasaki and Tonosaki (1988), are influenced by altricial or precocial developmental models. There are marked differences between early and altricial species specifically during the period when ontogenetic events occur. Starck & Ricklefs (1998) state that precocial species have higher rates of embryonic development so that at the time of hatching their organs are in a more advanced state of functional maturity as compared with altricial species. These differences demonstrate that the correspondence or assignment of a birdís embryonic stage based on a single reference table as established by Hamburger and Hamilton (1952) would not be valid for all taxa.
Columba livia is a cosmopolitan and flying species with urban and suburban habits and a type- 2 semialtricial development model (Starck and Ricklef, 1998). Given the ease of egg collection, laboratory incubation, and biological peculiarities, this species is a good model for embryological studies and has been used in many studies on the development of organ systems (Chevallier, 1977; Teillet, 1978; Caprioli et al., 1998, Pardanaud and Dieterlen-LiËvre, 1999). Currently, this species is used in genetic and developmental biology studies (Kuroda et al., 1990; Schultheiss et al., 1995; Petitte et al., 2004; Petitte, 2006), which require the identification of embryonic stages. However, there is no information on the ontogeny of Columba livia so the assignment of embryonic stage allocations is based on the Gallus gallus domesticus table. In this paper we address the study of the developmental sequence of Columba livia with the aim of producing a table of embryonic stages based on external morphological characters that would facilitate its identification. Additionally, the differences and/or similarities in the embryonic sequence of this species with the sequence proposed for Gallus gallus domesticus were analyzed in order to provide data that will allow the identification of patterns related to altricial and precocial models.
Materials and methods
Materials
Fertile eggs from Columba livia were collected on the Facultad de Ciencias Exactas y Naturales y Agrimensura campus of the Universidad Nacional del Nordeste (Corrientes, Argentina) during the nesting period. At this location the animals build their nests in holes and depressions on the roof and lay 2 eggs on alternating days. For the present study 10 nests were randomly selected to be checked daily or weekly for fertilized eggs from September to March 2008-2009 and 2009-2010. The eggs were collected early in the morning to ensure they were recently laid. Eggs were artificially incubated in a culture stove at 35-37 °C with approximately 40-45% humidity to obtain embryos in different stages of development. Fixation of the embryos in 10% formalin was performed according to the following protocol: every 1-4 hours during the first 5 days, then every 6-10 hours, until the tenth day and then every 12-24 hours, to complete the development. To slaughter the specimens, the standard method established in the Guidelines for Animal Euthanasia proposed by the IACUC (Institutional Animal Care and Use Committee) was followed. A stereoscopic microscope was used to separate yolks from embryos, which were cleaned and subsequently stained with methylene blue. The analyzed material became part of the scientific collection of the animal embryology course of the Department of Biology, Facultad de Ciencias Exactas y Naturales y Agrimensura of the Universidad Nacional del Nordeste.
Embryo analysis
A total of 190 embryos in various stages of development were analyzed: 100 in early phase (stages 1-26), 55 in middle phase (stages 27-36) and 35 in late phase (stages 37-43). Each stage was characterized based on the external morphological traits considered by Hamburger and Hamilton (1952) in addition to other traits not considered by these authors (see text). Stage 2 was divided into sub-stages a, b, and c, considering they represent the same process (formation of the primitive streak) in different periods.
The following were measured during stages 3-12: the length of the zona pellucida (LZP), the length of the primitive streak (LLP), and the total length (Lt) of the embryo pondered from the anterior end of the plate/neural tube to the posterior end of the primitive streak.
During stages 28-43 the length of the maxillary process (Lpmx) and length of mandibular process (Lpm) were measured. These measurements were made with an ocular micrometer incorporated in the stereomicroscope. For stages 37-42 a graduated cylinder was used to measure the embryo volume (Ve) and yolk volume (Vv). Using these data, total volume (Vt) was calculated as Vt = Ve + Vv. The morphology of lungs was described based upon the method proposed by Chuong et al. (2000). Photographs of the embryos were taken with a Canon A2000 IS digital camera.
Results
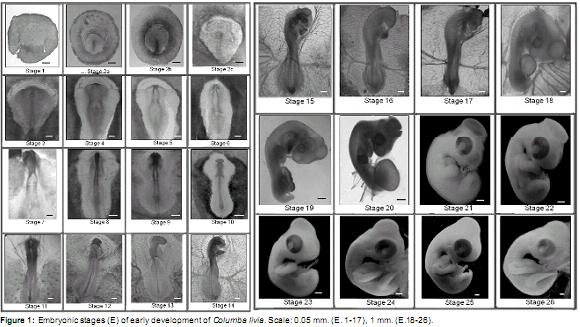
Early phase (Figure 1)
Stage 1 (± 3 h of incubation): zona pellucida and Zona opaca circularly shaped. Blastoderm in the central portion of the zona pellucida.
Stage 2a (± 8 h of incubation): zona pellucida inverted pear-shape. Primitive streak visible in the rear end of the zona pellucida. Llp LZP ± 25%.
Stage 2b (± 10 h incubation): zona pellucida inverted pear-shape. Llp LZP ± 50%.
Stage 2c (± 12 h incubation): zona pellucida inverted pear-shape. Llp LZP ± 70%. Primitive streak groove, and Hensenís node clearly distinguishable.
Stage 3 (± 14 h incubation): primitive streak similar to previous stage. Neural plate visible in front of the primitive streak.
Stage 4 (± 17 h of incubation): neural folds and neural groove visible in the anterior neural plate. Llp ± 75% of the Lt of the embryo. Blood islands are visible in the posterior region of the opaque area.
Stage 5 (± 18 h incubation): two pairs of somites. Deeper neural groove. Neural folds close together in the dorsal midline of the anterior region. Llp ± 65% of the Lt of the embryo. Blood islands as previously described.
Stage 6 (± 20 h incubation): three pairs of somites. Neural folds similar to the previous stage. Llp ± 50% of the Lt of the embryo. Patterns of vitelline vessels visible in the posterior region of the vitelline membrane.
Stage 7 (± 21 h incubation): four pairs of somites. Neural folds fused at the medial and posterior brain. Anterior region open. Llp ± 45% of the Lt of the embryo. Vitelline vessels extend to the middle region of the membrane sac.
Stage 8 (± 23 h incubation): five pairs of somites. Three delimited brain vesicles (forebrain, midbrain and hindbrain). Anterior neuropore is open. Neural folds of trunk unfused. Llp ± 37% of the Lt of the embryo. Vitelline vessels extending to the anterior region of the vitelline membrane.
Stage 9 (± 26 h incubation): six-seven pairs of somites. Forebrain expanded laterally to form optic vesicles. Anterior neuropore open. Neural folds of trunk and Llp as described above. Two endocardial tubes visible in the ventrally pharyngeal region of the embryo. Vitelline vessels extending to the anterior region of the vitelline membrane.
Stage 10 (± 27 h incubation): 9 pairs of somites. Forebrain with laterally expanded optic vesicles, well delineated midbrain and hindbrain. Anterior neuropore is closing. Neural folds of trunk fused at the level of the somites, unfused into the posterior region. Llp ± 20% of the Lt of the embryo. Endocardial tubes fused in the midline with vitelline veins projected laterally from the posterior region. Well-definedvitelline vesselsinthe vitelline membrane.
Stage 11 (± 28 h incubation): 11 pairs of somites. Telencephalon curved toward the anterior ventral region. Optic placodes distinguishable as thickenings on both sides of the optic vesicles. Neural folds of trunk unfused in the posterior region. Llp ± 20% of the Lt of the embryo. In ventral view, a tubular heart with a posterior widening corresponding to the venous sinus. Idem vitelline vessels.
Stage 12 (± 29 h incubation): 13 pairs of somites. More developed telencephalon and optic vesicles visible ventrally. More prominent midbrain, with six rombomeres in the hindbrain (r1-r6). Otic placodes in process of invagination visible dorsally at r5-r6. Llp ± 15% of the LT. S-shaped curved heart. Vitelline veins connected to the vessels of the vitelline membrane. Evident heartbeat. Amniotic membrane visible in the anterior region of the embryo covering the procencephalon and midbrain. Vitelline membrane covering approximately 1/4 of the yolk mass.
Stage 13 (± 32 h incubation): 16 pairs of somites. Cephalic region curved to the right. Telencephalon and mesencephalon more prominent. Mesencephalic flexure distinguishable. r2 r1 more dilated than others. Invaginated lens vesicle, optic cup with distinguishable choroidal fissure. Invaginated otic vesicle visible at the level of r5- r6. Llp ± 7% of the Lt of the embryo. Heart with 4 chambers well defined, expanded laterally to the right. Anterior fold of the amnion extends to the posterior region of the hindbrain. Vitelline membrane similar to the previous stage.
Stage 14 (± 36-40 h incubation): 19-22 pairs of somites. Curvature of the embryo to the right extends to the first somite. Eye and otic vesicle similar to previous stage. First and Second visceral archespresent.Primitivestreakabsent. Well- developed vitelline vascular system. Well-developed vitelline arteries and visible at the level of somite 15-16. Atrial and ventricular more prominent than other cardiac chamber, no obvious septa. Anterior fold of the amnion extends to the level of somite 7.
Stage 15 (± 40-44 h incubation): 25 pairs of somites. Curvature of the embryo to the right more pronounced. Midbrain more prominent than the other brain vesicles. Cervical flexion distinguishable. Eye more prominent with the onset of retinal pigmentation. Auditory vesicle shows dorsal endolymphatic duct. First, second, third, and fourth visceral arches present, the second being the largest. Nasal placodes in lateroventral position visible in the anterior region of the head. Anterior fold of the amnion covers the embryo to the level of somite 14. Vitelline membrane covering about 1/3 of the yolk mass.
Stage 16 (± 44-46 h incubation): 26-28 pairs of somites. Invaginated nasal placode. Anterior amnion fold covers the embryo until the 17 somite; lateral amnion folds visible in the posterior region of the embryo. Distinctive anterior limb bud at the height of 12-14 somites as a lateral expansion. Other structures similar to the previous stage.
Stage 17 (± 48-54 h incubation): 30 to 36 pairs of somites. More pronounced curvature of the embryo. Telencephalon divided into two distinct vesicles visible laterally. Epiphysis visible in the dorsal region of the diencephalon. Midbrain divided into bulky lobes. Four visceral arches present; the second is largest and the fourth is very small. Posterior limb bud visible at the level of somite 25-30. Caudal end is ventrally curved. Anterior fold of the amnion extends to the posterior region of the embryo, lateral folds fused with anterior and posterior, except in the posterior dorsal region. Allantoisvisibleasasmallventralsaccular evagination between the posterior limb buds.
Stage 18 (± 54-63 h incubation): 37-40 pairs of somites. Embryo is C-shaped with large head. Midbrain well developed. Eye prominent with a more pigmented retina. Visceral arch 1 divided into maxillary and mandibular processes, third and fourth visceral arches not visible. Anterior and posterior limb buds equal length. Amnion completely formed. Allantois more prominent than previous stage and laterally visible in the posterior region of the embryo. Vitelline membrane covering about half of the yolk mass with well-developed arterial and venous vessels.
Stage 19 (± 63-69 h incubation): 40-43 pairs of somites. Maxillary process longer than the mandibular process. Second visceral arch visible. Limb buds are as long as wide. Allantois more developed than previous stage with patterns of blood vessels.
Stage 20 (± 69-96 h incubation): 44 pairs of somites. Curvature of the embryo more pronounced. Mandibular process extends to the middle of the maxillary process. Second visceral arch inconspicuous. Heart with ventricle prominently located posteroventral to the right and left atria. Limb buds more developed. Allantois larger and well vascularized.
Stage 21 (4 days of incubation): the embryo becomes white, internal structures not clearly visible. Optic lobes very prominent. Eyes are large. Second visceral arch visible as a ventrolateral expansion posterior to the mandibular process. Limb bud oar-shaped. Allantois completely covers the embryo. Embryo produces muscular torsional movements.
Stage 22 (5 days of incubation): anterior end of the maxillary process close to the nostrils. Anterior end of the mandibular process joint in the midline. Stomodeum plate closed. Limb buds more developed. Allantois larger than previous stage. Smooth integument.
Stage 23 (5 ½ days of incubation): maxillary process exceeds previously to the nostrils. Mandibular process reaches half the length of the maxillary process. Stomodeum plate perforated. Differentiated second visceral arch not visible. Longer limbs with distinguishable digital plates in the autopod region. Vitelline membrane completely covers the yolk mass.
Stage 24 (6 days of incubation): auditive duct visiblebehindthemandibularprocess.Large eyes. Articulation member between stylopod and zeugopod. Forelimb autopod with notch between digits 1 and 2.
Stage 25 (6 ½ days of incubation): limbs longer in length. Autopod of the forelimb and posterior recesses between digits 1 and 2 and 2 and 3.
Stage 26 (7 days of incubation): maxillary process longer and curved down. Forelimb interdigital notches sharper. Interdigital notch between finger 3 and 4 of the hind limb visible. Longer neck.
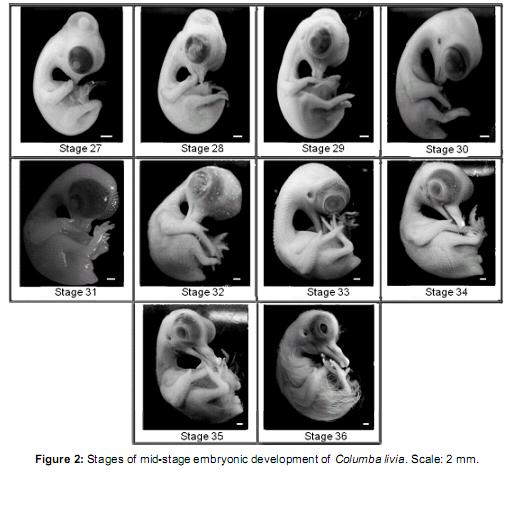
Middle phase (Figure 2)
Stage 27 (7 ½ days of incubation): maxillary process with egg-tooth on the dorsal side. Mandibular process extended to half the length of the maxillary process. Hind limb with digits 2, 3, and 4 dorsally visible; digit 1 in a ventral position. Primordial feather germ placode-shaped distributed in two dorsal rows and two caudal rows.
Stage 28 (8 days of incubation): mandibular and maxillar process form distinguishable beak. Beak: Lpmx: 5.49 mm and Lpm: 4.27 mm. Digit 2 of the forelimb longer than the others. Webbing of the hind limb transparent. Primordial feather germ placode shaped distributed in 4 dorsal rows and 3 caudal rows. 4 scleral papillae visible on the eyeball.
Stage 29 (8 ½ days of incubation): beak: Lpmx: 5.73 mm and Lpm: 4.51 mm. Limbs more developed than in the previous stage. Digit 3 of the hind limb longer than others. Primordial feather germ placode shaped distributed in 4 spinal rows, 5-6 lumbo-sacral rows, 2 femoral rows, 2 scapular rows. Primordial feather germ reticulum-shaped distributed in 4 cuadal rows. 6-7 scleral papillae arranged in a semicircle on the eyeball. Eyelid rim visible around the eyeball.
Stage 30 (9 days of incubation): beak: Lpmx: 5.98 mm and Lpm: 4.76 mm. Forelimb wing-like. Webbing of the limb buds absent. Primordial feather germ placode shaped distributed in 2 marginal spinal rows, 7 lumbo-sacral rows, 4 femoral rows, 4 scapular rows, 1 row around the duct auditive, and dispersed pectoral and capital primordial. Primordial feather germ reticulum shaped distributed in the 2 medial rows and 4 spinal caudal rows.13 scleral papillae around the eyeball.
Stage 31 (9 ½ days of incubation): beak: Lpmx: 6.1 mm and Lpm: 4.88 mm. Limbs developed. Eyelid covers half of the eyeball. Primordial feather germ reticulum shaped distributed throughout the body except the ventral region of the neck and abdomen.
Stage 32 (10 days of incubation): beak: Lpmx: 6.1 mm and Lpm: 5.49 mm. Phalanges of the hind limb distinguishable. Primordial feather germ reticulum shaped in the ventral region of the neck and entire body except in the abdominal region. Upper and lower eyelids cover the eye scleral papillae. Nictitating membrane visible in the vertice of the eye. Chorioallantoideal membrane closely attached to the eggshell.
Stage 33 (10 ½ days of incubation): beak: Lpmx: 6.8 mm and Lpm: 6.34 mm. Primordial feather germ conical-shaped in the dorsal part of the body; the rest form a lattice. Filiform feather germ in the wing and femoral tract. Footpads and claws not yet cornified.
Stage 34 (11 days of incubation): beak: Lpmx: 7.93 mm and Lpm: 9.37 mm with prominent egg tooth. Contour eyeling oval-shaped. Patterns of scales slightly visible on the front of the hind limb. Footpads well differentiated onset cornification in claws. Feathers similar to the previous stage.
Stage 35 (11 ½ days of incubation): beak: Lpmx: 8.54 mm and Lpm: 10.98 mm. Filiform markers throughout the body except in the circumorbital region and around the auditive duct where they remain reticulum-shaped. Leg scales arranged in imbricate form.
Stage 36 (12 days of incubation): beak: Lpmx: 10.04 mm and Lpm: 11.83 mm. Long thread-like feathers in trunk region, short feathers on the head region, neck, and wing. Footpads with pattern of scales visible as papillae. Claws well cornified. Eyelid covers most of the eye.
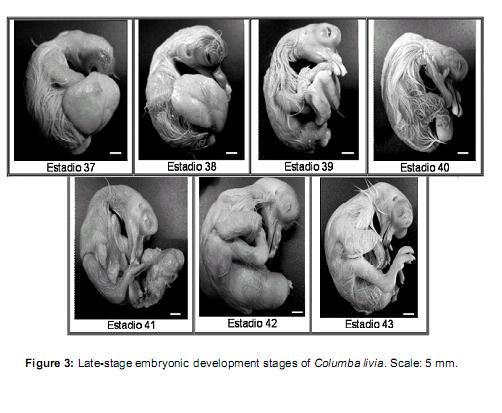
Stage 37 (12 ½ days of incubation): Vv = 50- 40% / Vt. Beak: Lpmx: 10.37 mm and Lpm: 12.2 mm. Feathers similar to the previous stage, but corneal sheath.
Stage 38 (13 days of incubation): Vv = 40-25% / Vt. Beak: Lpmx: 10.49 and Lpm: 12.4 mm, well cornified. Filiform feathers longer than previous stage.Footpads coveredwithgranular scales. Eyelids cover the lenses.
Stage 39 (13 ½ days of incubation): Vv = 25- 20% / Vt. Beak: Lpm Lpmx idem to previous stage. Feathers are longer.
Stage 40 (14 days of incubation): Vv = 20-10% / Vt. Closed eyelids, not fused. All others similar to the previous stage.
Stage 41 (15 days of incubation): Vv = <10% / Vt. Beak: Lpmx: 12.2mm and Lpm: 15.25mm. Feathers have lost the sheath cornea. Eyelids same as previous stage.
Stage 42 (16 days of incubation): Yolk completely incorporated into the abdominal cavity of the embryo. Feathers acquired yellow color. Beak: Lpm Lpmx idem to previous stage. Eyelid same as previous stage. Onset of egg shells breaking (time ± 24 h).
Stage 43 (± 17 days): Neonate with closed eyelids and low mobility.
Discussion
Hendrickx and Hanzlik (1965) described the ontogeny of Colinus virginianus pointing at a gradual delay in the development of this species compared to what was described by Hamburger and Hamilton (1952). Yamasaki and Tonosaki (1988) found differences between Lonchura striatavar domestica and Gallus gallus domesticus in the following: the development and duration of the primitive streak, neural folds, brain, retinal pigmentation, and feathers. Kˆppl et al. (2005) reported the normal developmental sequence of Tyto alba and exposed differences found with respect to the ontogeny of the chicken concerning the development of the eyelids and feather germs. Likewise, Ainswort et al. (2009) compared the morphogenesis and feather germ pigmentation, the development of the beak and toes between Coturnix coturnix japonica and Gallus gallus domesticus, and identified variations in the developmental time, mainly during late ontogenetic stages.
Regard ing the observations of the present study, Columbaliviadomesticus showedsimilarities with Gallus gallus domesticus in the ontogenetic sequence of events during the early stages of development. These include the formation of the primitive streak, the appearance of the neural plate and consequent formation of the neural folds and neural tube, the formation of the primary circulatory system, and the organization of extra- embryonic membranes. However, consistent with what has been reported by other authors, we observed differences in middle and late stages corresponding mainly to the timing of appearance of limb buds, the growth of the beak, the development of feathers and the organization and cornification of legs, scales, and claws. Considering the stages and developmental time, these structures appear earlier in Columba livia than in Gallus gallus domesticus. These differences could be explained bythe morphological characteristics of neonates and the incubation of eggs, which are 17 and 21 days, respectively. Upon hatching, the chicks of these species differ in the degree of functional maturity of some organ systems, such as muscular, and in behavioral aspects, such as nest permanence and feeding by parents, which are characteristics associated with semialtricial-2 andprecocial-2developmentalmodels(Starck and Ricklefs, 1998; Pough et al., 2003). However, the external morphology of hatchlings is similar in the development of feathers, scales, claws, beak, and eyes. In this regard, we believe that the advancement in time of these characteristically morphogenetic events allows Columba livia to reach a degree of morphological development similar to that of Gallus gallus domesticus in a shorter time period and represents a clear example of heterochrony in the ontogeny of these species.
Blom and Lilja (2005) described differences in developmental time and maturation of the digestive system, muscular somite formation and size of the brain, eyes, and locomotive organs among precocial and altricial species with different patterns of postnatal growth. In conclusion, they suggest that the assessment of embryonic growth rate is essential to characterize the patterns of development and also offers an excellent opportunity to analyze heterochonyc events. Based on the results of the present study we propose that the analysis and comparison of ontogenetic sequences of varying speciesof birdswith differentdevelopmental models is also important not only to obtain a wider knowledge of embryonic characteristics in each species, but also to identify ontogenetic patterns related to altricial and precocial models.
Acknowledgements
To Lic. Alejandra Hernando and Lic. Blanca Beatriz Alvarez de Avanza for their critical reading of the manuscript and their comments as well as the General Secretariat of Science and Technology of the Universidad Nacional del Nordeste for funding this work (Res. 740/07 CS). We also thank the suggestions and corrections made by this paperís reviewers astheysubstantiallycontributedto improve the quality of the manuscript.
References
1. Ainsworth SJ, Stanley RL, Evans DJR. Developmental stage japanece quail. J Anat 2009; 216:3-16. [ Links ]
2. Bellairs R. The conversion of yolk into cytoplasm in the chick blastoderm as shown by electron microscopy.Embryol Exp Morph 1958; 6:149-61. [ Links ]
3. Blom J, Lilja CA. Comparative study of embryonic development of some bird species with different patterns of postnatal growth. Zoology 2005; 108:81-95. [ Links ]
4. Brush A, Scott A. Development of protein polymorphisms in redwing blackbirds. J Embryol Exp Morph 1972; 28:481-489. [ Links ]
5. Caprioli A, Jaffredo T, Gautier R, Dubourg C, Dieterlen-Lievre F. Blood-borne seeding by hematopoietic and endothelial precursors from the allantois. Proc Natl Acad Sci 1998; 95:1641-1646. [ Links ]
6. Chevallier A. Origine des ceintures scapulaires et pelviennes chez líembryon díoiseau. J Embryol Exp Morph 1977; 42:275- 292. [ Links ]
7. Couly GF, Coltey PM, Le Douarin NM. The developmental fate of the cephalic mesoderm in quail-chick chimeras. Development 1992; 114:1-15. [ Links ]
8. Del Conte E, Sirlin L. The first stages of Bufo arenarum development. Acta Zool Lilloana 1951; 12:495-499. [ Links ]
9. Echeverría DD, Fiorito De López LE. Estadios de la metamorfosis en Bufo arenarum (Anura) Physis Secc B. 1981; 40:15-23. [ Links ]
10. Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. J Morphol 1952; 88:49-92. [ Links ]
11. Hendrickx AG, Hanzlik R. Developmental stages of the bobwhite quail embryo, Colinus virginianus. Biol Bull1965; 129:523-531. [ Links ]
12. Keller R. Early embryonic development of Xenopus laevis. Methods Cell Biol 1991; 36:61-113. [ Links ]
13. Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Develop Dynam 1995; 203:253-310. [ Links ]
14. Koecke HU. Normalstadien der Embryonalentwicklung bei der Hausente,(Anas boschas domestica). Embryologia 1958; 4:55- 78. [ Links ]
15. Köppl C, Futterer E, Nieder B, Sistermann R,Wagner H. Embryonic and Posthatching Development of the Barn Owl (Tyto alba): Reference Data for Age Determination. Develop Dynam 2005; 233:1248-1260. [ Links ]
16. Kuroda O, Matsunaga C, Whittow G, Tazawa H. Comparative metabolic responses to prolonged cooling in precocial duck (Anas domestica) and altricial pigeons (Columba domestica) embryos. Comparative Biochemistry and Physiology Part A: Physiology 1990; 95:407-410. [ Links ]
17. Mun AM, Kosin IL. Developmental stages of the broad breasted bronze turkey embryo. Biol Bull 1960; 119:90-97. [ Links ]
18. Pardanaud L, Dieterlen-LièvreF. Manipulation of the angiopoietic/hemangiopoietic commitment in the avian embryo. Development 1999; 126:617-627. [ Links ]
19. Petitte JN, Liu G, Yang Z. Avian pluripotent stem cells. Mech Dev.2004; 121:1159-1168. [ Links ]
20. Petitte JN. Avian germplasm preservation: embryonic stem cells or primordial germ cells? Poult Sci 2006; 85:237-242. [ Links ]
21. Pough HF, Janis CM, Heiser JB. Vertebrate Life. Sixth edition. Ed. Prentice Hall. New Jersey; 2003. [ Links ]
22. Starck JM, Ricklefs RE. Avian growth and development: evolution within the altricial precocial spectrum. Ed. Oxford University Press, New York; 1998. [ Links ]
23. Schultheiss TM, Xydas S, Lassar AB. Induction of avian cardiac myogenesis by anterior endoderm. Development 1995; 121:4203-4214. [ Links ]
24. Tam PPL, Beddington RSP. The formation of mesodermal tissues in the mouse embryo during gastrulation and early organogenesis. Development 1987; 99:109-126. [ Links ]
25. Teillet MA. Evolution of the lumbo-sacral neural crest in the avian embryo: origin and differentiation of the ganglionated nerve of Remak studied in interspecific quail-chick. Wilhelm Rouxís Arch Dev Biol 1978; 184:251-268. [ Links ]
26. Yamasaki M, Tonosaki A. Developmental stages of the society finch, Lonchura striata var. domestica. Dev Growth Diff 1988; 30:515-542. [ Links ]