Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Colombiana de Ciencias Pecuarias
Print version ISSN 0120-0690
Rev Colom Cienc Pecua vol.25 no.4 Medellín Oct./Dec. 2012
ORIGINAL ARTICLES
Bacillus strains as feed additives: In vitro evaluation of its potential probiotic properties¤
Bacilos utilizados como aditivos para piensos: Evaluación in vitro de sus potenciales propiedades probióticas
Bacilos utilizados como aditivos para concentrados: Avaliação in vitro de suas propriedades probióticas
Jaekoo Lee1,§, MSc; Inkyung Park1,§, PhD; Yunjaie Choi2, PhD; Jaiesoon Cho1,*, PhD.
1Department of Animal Sciences and Environment, College of Animal Bioscience and Technology, Konkuk University, South Korea.
2Department of Agricultural Biotechnology, Seoul National University, South Korea.
* Corresponding author: Jaiesoon Cho. Department of Animal Sciences and Environment, College of Animal Bioscience and Technology, Konkuk University, South Korea. Tel: +82-2-450-3375. Fax: +82-2-455-1044. E-mail: chojs70@konkuk.ac.kr
§ These authors equally contributed to this work.
(Received: 12 january, 2012; accepted: 10 april, 2012)
Summary
Background: much recent attention has been devoted to the genuine value of Bacillus species as multifunctional probiotic products, which produce various extracellular enzymes that enhance feed digestibility as well as many antimicrobial compounds for the purpose of improving animal performance. Objective: to describe novel, in vitro potential probiotic properties such as acid tolerance, bile tolerance, safety, and antimicrobial activity of mesophilic and psychrophilic Bacillus strains in conjunction with their extracellular enzymatic activities. Methods: four Bacillus strains (B. sp. T3, B. sp. T4, B. sp. SM2, and B. sp. JSP1) isolated from different sources were used. Strains were identified according to 16S rDNA sequences. Escherichia coli K88, E. coli O157:H7, Salmonella enteritidis KCCM 12021, Enterococcus faecalis, Listeria monocytogenes, and Staphylococcus aureus were used as indicator bacteria for the antimicrobial activity trial. Strains were activated and cultured in tryptic soy broth (pH 7.0) or broth solidified with 1.5% agar. Results: B. sp. JSP1 was fully resistant to both pH 2 and 3, whereas B. sp. SM2 showed relatively good viability at pH 3. All strains tolerated oxgall (0.3%) bile salt and were not cytotoxic to the HEK 293 human embryonic kidney cells. Three strains, except B. sp. T3, displayed differential production of extracellular enzymes including amylase, xylanase, cellulase, protease, phytase, and α–galactosidase. In particular, B. sp. SM2 inhibited six indicator pathogens: Escherichia coli K88, E. coli O157:H7, Salmonella enteritidis, Enterococcus faecalis, Listeria monocytogenes, and Staphylococcus aureus. Conclusion: the single use of B. sp. SM2 or the mixed use of the strain combined with acid or bile tolerant Bacillus strains secreting extracellular enzymes may be an alternative to antibiotics as a feed additive in farm animal production.
Key words: acid tolerance, animal performance, antimicrobial, bile tolerance, enzymes.
Resumen
Antecedentes: recientemente el valor de las especies de Bacillus como productos probióticos multifuncionales ha recibido bastante atención, debido a que estos producen varias enzimas extracelulares que potencian la digestibilidad de los alimentos, así como también compuestos antimicrobianos que mejoran el desempeño del animal. Objetivo: describir y evaluar potenciales propiedades probióticas ''in vitro'' -tales como acidez, tolerancia a la bilis, seguridad y actividad antimicrobiana- de cuatro cepas de Bacilos (B. sp. T3, B. sp. T4, B. sp. SM2 y B. sp. JSP1) aisladas de diferentes fuentes, en conjunción con sus actividades enzimáticas extracelulares. Métodos: se usaron cuatro cepas de Bacillus (B. sp. T3, B. sp. T4, B. sp. SM2, and B. sp. JSP1) aisladas de diferentes fuentes. Las cepas se identificaron de acuerdo a secuencias 16S rDNA. Escherichia coli K88, E. coli O157:H7, Salmonella enteritidis KCCM 12021, Enterococcus faecalis, Listeria monocytogenes, y Staphylococcus aureus fueron empleadas como bacterias indicadoras para el ensayo de actividad antimicrobiana. Las cepas fueron activadas y cultivadas en caldo soya tripticasa (PH 7.0) o caldo solidificado con 1.5% de agar. Resultados: el B. sp. JSP1 resultó totalmente resistente tanto a pH 2 como a pH 3, mientras que el B. sp. SM2 mostró viabilidad relativamente alta a pH 3. Todas las cepas toleraron oxgall (0.3%) de sales biliares y no resultaron citotóxicas para las células humanas HEK 293 de riñón embrionario. Tres cepas, con excepción de B. sp. T3, presentaron producción diferencial de enzimas extracelulares -incluyendo amilasa, xilanasa, celulasa, proteasa, fitasa y α–galactosidasa. En particular, el B. sp. SM2 inhibió seis indicadores patógenos (Escherichia coli K88, E. coli O157: H7, Salmonella enteritidis, Enterococcus faecalis, Listeria monocytogenes y Staphylococcus aureus). Conclusiones: el uso específico de B. sp. SM2, o el uso combinado de esta cepa junto con cepas secretoras de enzimas extracelulares y tolerantes a ácidos o bilis puede ser una alternativa para reemplazar los antibióticos frecuentemente usados como aditivos en alimentación animal.
Palabras clave: antimicrobianos, desempeño animal, enzimas, tolerancia a la acidez, tolerancia a la bilis.
Resumo
Antecedentes: o valor das espécies de Bacillus como produtos probióticos multifuncionais tem recebido bastante atenção recentemente, devido a que produzem várias enzimas extracelulares que potenciam a digestibilidade dos alimentos, como também compostos antimicrobianos que melhoram o desempenho do animal. Objetivo: descrever e avaliar as propriedades potenciais ''in vitro'' – como acidez, tolerância à bile, segurança e atividade antimicrobial- de quatro cepas de Bacilos (B. sp. T3, B. sp. T4, B. sp. SM2, and B. sp. JSP1) isoladas de diferentes fontes, em conjunto com as suas atividades enzimáticas extracelulares. Métodos: foram usadas quatro cepas de Bacillus (B. sp. T3, B. sp. T4, B. sp. SM2, and B. sp. JSP1) isoladas de diferentes fontes. As cepas foram identificadas de acordo às sequências 16S rDNA. As seguintes bactérias foram empregadas como indicadoras no teste de atividade antimicrobiana: Escherichia coli K88, E. coli O157:H7, Salmonella enteritidis KCCM 12021, Enterococcus faecalis, Listeria monocytogenes, y Staphylococcus aureus. As cepas foram ativadas e cultivadas em caldo soja tripticase (pH 7.0) ou caldo solidificado com 1.5 de Agar. Resultados: o B. sp. JSP1 foi totalmente resistente tanto no pH 2.0 como no pH 3.0, enquanto que o B. sp. SM2 mostrou viabilidade relativamente alta no pH 3.0. Todas as cepas toleraram oxgall (0.3%) de sais biliares e não foram citotóxicas para as células humanas HEK 293 de rim embrionário. Tres cepas, com exceção de B. sp. T3, apresentaram produção diferenciada de enzimas extracelulares –incluindo amilase, xilanase, celulase, protease, fitase e α–galactosidase. Particularmente, o B. sp, SM2 inibiu seis indicadores patógenos (Escherichia coli K88, E. coli O157: H7, Salmonella enteritidis, Enterococcus faecalis, Listeria monocytogenes y Staphylococcus aureus). Conclusões: O uso específico de B. sp. SM2 ou o uso combinado desta cepa junto com as cepas secretoras de enzimas extracelulares e tolerantes a ácidos ou bile, pode ser uma alternativa para substituir os antibióticos frequentemente usados como aditivos na alimentação animal.
Palavras chave: antimicrobianos, desempenho animal, enzimas, tolerância à acidez, tolerância à bile.
Introduction
Although the use of antibiotics as a feed additive has greatly contributed to improving growth performance and controlling disease in farm animals, their overuse has been an important factor in the development of antibiotic resistance and the persistence of antibiotic residues in animal products (Chen et al., 2009). The resulting health concerns have prompted countries such as South Korea and members of the European Economic Union to ban the use of antibiotics as feed additives (Phillips, 2007; Chen et al., 2009; Lee et al., 2011). An active area of research is the discovery or synthesis of compounds that can have antibiotic-like effects without the undesirable problems caused by antibiotics (Turner et al., 2001).
Probiotics are live microbial feed supplements that can beneficially affect the host animal by improving intestinal balance (Fuller, 1989). Lactic acid, bacteria, and bifidobacteria have been intensively employed as probiotic strains due to their recognition as members of the indigenous microflora of the animals, safety, and the evidence supporting their positive role (Gaggia et al., 2011). However, much recent attention has been devoted to the genuine value of Bacillus species in probiotic products (Cutting, 2011). Bacillus species, which generate dormant spores resistant to heat, radiation, desiccation, enzymatic degradation, and the stomach's acidic conditions (Hong et al., 2005, Leser et al., 2008), produce various extracellular enzymes that enhance feed digestibility, as well as many antimicrobial compounds (Leser et al., 2008; Sutyak et al., 2008). The species also stimulates the immune system of host animals (Schierack et al., 2007; Huang et al., 2008; Sun et al., 2010), thereby improving growth performance, feed conversion ratio, and meat quality in animals (Davis et al., 2008; Vila et al., 2009; Sun et al., 2010; Zhou et al., 2010). However, since all Bacillus strains do not equally possess these probiotic competencies, the selection of appropriate Bacillus strains is essential for the effectiveness of probiotic supplements for use in animal feed (Guo et al., 2006).
The present study describes novel in vitro potential probiotic properties such as acid tolerance, bile tolerance, safety, and antimicrobial activity of mesophilic and psychrophilic Bacillus strains in conjunction with their extracellular enzymatic activities.
Materials and methods
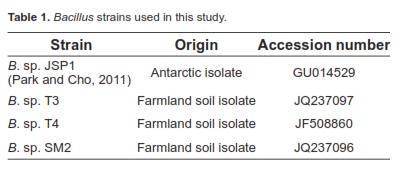
Bacterial strains and maintenance
The Bacillus strains used in this experiment were identified by 16S rDNA sequences. They have been deposited in the NCBI database under the accession numbers listed in table 1. Escherichia coli K88, E. coli O157:H7, Salmonella enteritidis KCCM 12021, Enterococcus faecalis, Listeria monocytogenes, and Staphylococcus aureus, kindly provided by Seoul National University (Seoul, South Korea), were used as indicator bacteria for the antimicrobial activity trial. Unless otherwise stated, all strains were routinely activated and cultured in tryptic soy broth (pH 7.0) (BD Science, Sparks, MD, USA) or broth solidified by the inclusion of 1.5% agar (BD Science). Unless otherwise stated, general chemicals were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Enzyme production
To investigate the production of various extracellular enzymes in each Bacillus strain, the following media were used:
1. Cellulase: 0.5% carboxymethyl cellulose, 0.5% yeast extract (BD Science), 0.45% (NH4)2SO4, 0.01% CaCl2∙2H2O, 0.01% MgSO4∙7H2O, 0.01% NaCl, 0.07% KH2PO4, 0.001% MnSO4∙4H2O, 0.001% FeSO4∙7H2O, pH 6.5.
2. Xylanase: 0.5% xylan, 0.45% (NH4)2SO4, 0.05% yeast extract, 0.01% CaCl2∙2H2O, 0.01% MgSO4∙7H2O, 0.01% NaCl, 0.07% KH2PO4, 0.001% MnSO4∙4H2O, 0.001% FeSO4∙7H2O, pH 6.5.
3. Amylase: 0.5% starch, 0.5% yeast extract, 0.45% (NH4)2SO4, 0.01% CaCl2∙2H2O, 0.01% MgSO4∙7H2O, 0.01% NaCl, 0.07% KH2PO4, 0.001% MnSO4∙4H2O, 0.001% FeSO4∙7H2O, pH 7.4.
4. Protease: 1.0% skim milk, 0.05% yeast extract, 0.45% (NH4)2SO4, 0.01% CaCl2∙2H2O, 0.01% MgSO4∙7H2O, 0.01% NaCl, 0.07% KH2PO4, 0.001% MnSO4∙4H2O, 0.001% FeSO4∙7H2O, pH 6.5.
5. Phytase: 1.0% wheat bran extract, 0.04% (NH4)2SO4, 0.02% MgSO4∙7H2O, 0.05% KH2PO4, 0.04% K2HPO4, 1.0% casein hydrolysate, 0.2% CaCl2, pH 6.5.
6. α-galactosidase: 1% tryptone (BD Science), 0.5% yeast extract, 1% NaCl, 1% galactose, pH 7.2. Each Bacillus strain was aerobically cultivated in 100 mL Erlenmeyer flasks containing the appropriate enzyme production medium (25 mL) at 30 °C for 96 h, culture supernatants were subsequently assessed for enzyme activity.
Enzyme assays
Cellulase, xylanase, and amylase activities were determined at 30 °C by measuring the release of reducing sugar from carboxymethyl cellulose, beech wood xylan, and starch, respectively, in 0.6 mL of 50 mM sodium phosphate (pH 6.0), according to the dinitrosalicylic acid (DNS) method (Miller, 1959). An α-galactosidase activity was determined at 30 °C by measuring the release of p-nitrophenol from p-nitrophenyl-α-D-galactopyranoside (Sigma- Aldrich, St. Louis, MO, USA) in 0.8 mL of 50 mM sodium phosphate (pH 6.0) as previously described (Patil et al., 2010). Protease activity was tested at 30 °C in 0.36 mL of 50 mM sodium phosphate (pH 6.0) using azocasein as substrate as previously described (Hutadilok-Towatana et al., 1999). Phytase activity was measured at 30 °C by testing inorganic phosphate release from phytate dodecasodium salt (Sigma-Aldrich) in 0.8 mL of 50 mM bis-tris (pH 6.0) as previously described (Cho et al., 2006). One unit (U) of each enzyme activity was defined as the release of 1 μmol of product per min under the trial conditions.
Acid tolerance test
The survival rate of each Bacillus strain under simulated gastric conditions was investigated as previously described (Kim et al., 2007) with the following modifications: 0.1 mL of cultures grown in tryptic soy broth for 24 h at 37 °C, representing about 108 colony forming units (CFU)/mL, were transferred to 0.9 mL of synthetic gastric juice [0.5% NaCl, 0.1% peptone (BD Science), 0.3% pepsin] whose pH was adjusted to 2 and 3 using 1 N HCl, then incubated for 0, 30, 60, 90, and 120 min at 37 °C. Viable cells were enumerated by plating 10-fold dilutions of the culture in phosphate buffered saline (PBS; 0.144% Na2HPO4, 0.024% KH2PO4, 0.5% NaCl; pH 7.4) on tryptic soy agar and incubating plates at 37 °C for 24 h.
Bile resistance test
Bile tolerance was determined as previously described (Kheadr et al., 2007) with the following modifications: 10 mL of cultures (about 108 CFU/ mL) of each Bacillus strain grown in tryptic soy broth (pH 7.0) for 24 h at 37 °C, was spun down at 5,000 × g for 20 min at 4 °C. Cell pellets were washed with PBS, collected by centrifugation (5,000 × g, 20 min, 4 °C), and resuspended in tryptic soy broth (pH 7.0) containing 0.3% oxgall (BD Science). Bacterial suspensions were incubated for 0, 1, 2, 3, and 4 h at 37 °C. Viable cells were counted by plating 10-fold dilutions of the culture in PBS on tryptic soy agar and incubating plates at 37 °C for 24 h.
Antimicrobial activity assay
The inhibition test was performed by modified methods of earlier studies (Sugita et al., 1998; Guo et al., 2006). Tested Bacillus strains were amplified at 37 °C for 24 h by streaking each pure colony onto fresh tryptic soy agar. Then, large colonies of tested strains were created on tryptic soy agar by spotting the isolates with sterile toothpicks. After incubating at 37 °C for 24 h, the strains were killed by exposure to chloroform vapor for 30 min. The chloroform was then evaporated for 20 min. Indicator bacteria were incubated for 24 h at 37 °C in tryptic soy broth and diluted until the absorbance at 600 nm (A600) reached 0.5. The cultures were diluted 100-fold and suspended in tryptic soy soft agar (tryptic soy broth + 0.7% agar), which were poured over the tryptic soy agar plates. After incubation at 37 °C for 24 h, the inhibition zones around the spots were measured.
Cell cytotoxicity test
HEK 293 human embryonic kidney cells (cat no. CRL-1573; ATCC, Manassas, VA, USA) were maintained in DMEM medium (Hyclone, Logan, UT, USA) supplemented with 10% fetal bovine serum (FBS; Gibco Invitrogen, Carlsbad, CA, USA). Cultures were incubated at 37 °C in a humidified atmosphere containing 5% CO2. Cell cytotoxicity was examined using the EZ-Cytox cell viability kit (Daeil Lab, Seoul, Korea) according to manufacturer instructions. Initially, the cells were seeded into 96-well culture plates at 1×105 cells/mL and maintained in FBS-supplemented DMEM. The samples included negative control (10 μL of PBS) and cell-free culture supernatant fluids (10 μL) of Bacillus strains obtained at 1, 3, and 5 days (SM2, JSP1, T3, T4, respectively) cultured in the tryptic soy broth. When the HEK293 cells reached 70% confluence, the wells were inoculated aseptically with 10 μL of the samples and the plates were incubated at 37 °C for 48 h. Then, the EZ-Cytox kit reagents were added to the medium before the cells were incubated for 30 min. The optical density was determined at 450 nm using a microplate reader (Bio-Tek, Winooski, VT, USA).
Statistical analysis
Results were presented as the mean ± standard error of three experiments. P values < 0.05 or 0.01 were regarded as statistically different between means using a student's t-test.
Results
Acid tolerance
Generally, all tested Bacillus strains exhibited lower cell viabilities in synthetic gastric fluid at pH 2 than at pH 3 (Table 2). Particularly an Antarctic isolate, Bacillus JSP1, showed the best survival rate of all strains, retaining 65% and 55% of its initial cell numbers during 120 min incubation at pH 2 and pH 3, respectively. Bacillus sp. T3 and Bacillus sp. T4 were the most acid-sensitive strains. In addition, Bacillus sp. SM2 showed relatively good cell viability, retaining 49% of its initial cell numbers during 120 min incubation at pH 3, although viability was drastically reduced during 30 min incubation at pH 2.
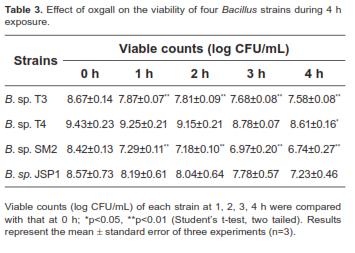
Bile tolerance
All tested Bacillus strains were resistant to oxgall bile salt (Table 3); viability was reduced by only 9%-20%, even at 4 h exposure.
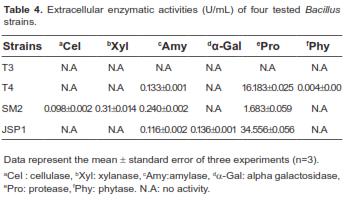
Enzymatic activities
As shown in table 4, no enzymatic activities were detected in Bacillus sp. T3. However, other Bacillus strains commonly possessed amylase and protease activities. Phytase activity was detected only in Bacillus sp. T4. Interestingly, Bacillus sp. JSP1 produced greater concentrations of protease compared to other strains, and α–galactosidase activity, which is involved in the removal of galacto-oligosaccharides such as raffinose and stachyose, acting as an anti-nutritive factor in soybean meal (Anderson and Wolf, 1995), was detected only in strain JSP1. Unlike other strains, Bacillus sp. SM2 also produced additional carbohydrase, such as cellulase and xylanase, that can degrade non-starch polysaccharides.
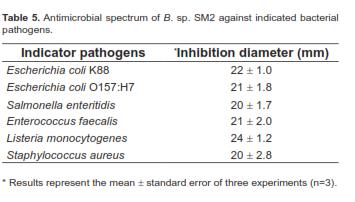
Antimicrobial activity
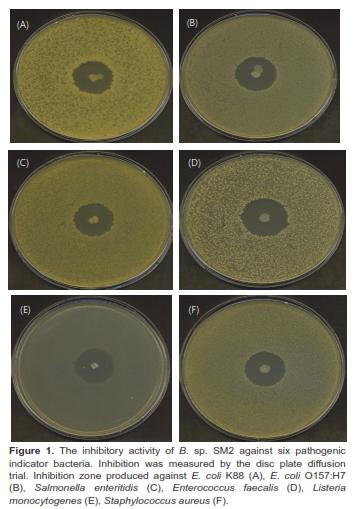
Bacillus sp. SM2 showed inhibitory effects against the six indicator pathogens (Figure 1), producing the largest inhibition zone with Listeria monocytogenes (Table 5). However, the other Bacillus strains did not inhibit the growth of the six pathogens.
Cell cytotoxicity trial
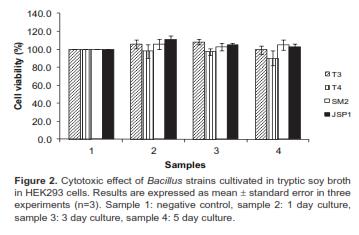
There was no deleterious effect of treatment with cell-free culture supernatants on viability of Bacillus strains (Figure 2), which indicated an inability to produce enterotoxins.
Discussion
Differential survivability of Bacillus strains tested at pH 2 and pH 3 was observed (Table 2), indicating that the acid tolerance is strain-specific, which may be closely associated with previous findings that Bacillus species spores are not equally resistant to simulated gastric fluid (Guo et al., 2006). In addition, Taheri et al. (2009) reported that it is preferable to examine the survivability of the Lactobacillus strains under the retention time of feed and pH (90 min, pH 2.6) in the gizzard when considering the application of probiotics in chickens. Similarly, Bacillus sp. JSP1 and Bacillus sp. SM2 might be promising candidates as chicken probiotics because they maintained high cell viabilities during 90 min incubation at pH 3 (Table 2). Nevertheless, acid tolerance does not appear to be a critical factor for selection of probiotic strains because buffered food or encapsulated delivery systems can improve acid-sensitive strain viabilities during gastric transit (Huang and Adams, 2004).
Bile tolerance is necessary for probiotic strains to colonize the small intestine and is potentially more important in probiotic selection than gastric survival (Huang and Adams, 2004). All tested Bacillus strains displayed high levels of bile tolerance with slight loss of viability at 4 h exposure to 0.3% bile salt (Table 3), the intestinal bile acid concentration in humans, also widely-applied to other monogastrics, such as pigs and chickens (Kim et al., 2007; Taheri et al., 2009). B. subtilis MA139 and Bacillus sp. 634 also tolerate simulated intestinal fluid with 0.3% bile salts, suggesting that their spores should be able to germinate without being inhibited by the presence of bile salts in the small intestine (Guo et al., 2006). Accordingly, low numbers of B. cereus var. toyoi spores were found in the pigs' stomach following oral administration, followed by a rapid increase of spore numbers in the duodenum and jejunum (Jadamus et al., 2001). On the other hand, in probiotic strains such as Lactobacillus and Bifidobacterium, the resistance against bile salt was strongly implicated in the presence of their bile salt hydrolase activity (Tanaka et al., 1999; Kim et al., 2004). However, it is still unclear that Bacillus strains possess bile salt hydrolase activity resistant to bile salts.
Generally, Bacillus species are attractive sources of various extracellular hydrolytic enzymes, which may aid in nutrient digestion and utilization of feed (Davis et al., 2008). Dietary supplementation with B. subtilis, which secretes protease, amylase, and lipase, improves growth performance in broiler chicks (Santoso et al., 2001). Moreover, a great deal of interest has focused on screening Lactobacillus strains that can produce α-amylase, phytase, xylanase, β-glucanase or cellulase for the purpose of improving probiotic efficacy (Taheri et al., 2009), which could improve the digestion or feed conversion ratio in chickens and pigs (Lee et al., 2001; Liu et al., 2007, Yu et al., 2008). In this regard, our results (Table 4) suggest that the combination among Bacillus strains producing different extracellular enzymes may generate a synergistic-mediated improvement of the production performance and nutrient digestibility of monogastric animals.
The main function of probiotics is to inhibit pathogens, which is an important criterion for screening potential probiotic strains (Guo et al., 2006). Bacillus sp. SM2 had a relatively broad range of antibacterial activities because the strain was not only effective against Grampositive bacteria (Enterococcus faecalis, Listeria monocytogenes, and Staphylococcus aureus), but also Gram-negative bacteria (E. coli K88, E. coli O157:H7, and Salmonella enteritidis) (Table 5 and Figure 1). Thus, strain SM2 may be strategically applied to reduce environmental mastitis caused by the opportunistic pathogen Enterococcus faecalis in cattle (Petersson-Wolfe et al., 2008), diarrhea caused by E. coli K88 and E. coli O157:H7 in piglets and calves (Guo et al., 2006; Gaggia et al., 2011), and the prevalence of Salmonella in poultry (Vila et al., 2009). Although the mechanism of SM2 antibacterial activities remains unclear, earlier studies reported that some Bacillus strains could produce bacteriocins or bacteriocin-like substances to kill bacterial pathogens (Sugita et al., 1998; Sutyak et al., 2008). The production of bacteriocins by strain SM2 should be assessed.
The use of Bacillus species as probiotics raises the question of safety because some Bacillus species including B. anthracis, B. cereus, B. thuringiensis, B. pseudomycoides, and B. weihenstephanesis are pathogenic (Hong et al., 2008). In particular, B. cereus is a well-documented food-poisoning bacterium that causes illness due to the production of one or more enterotoxins (Granum and Lund 1997; Granum 2002; Guinebretiere et al., 2002; From et al., 2005). However, pathogenicity is rather strain-specific, as some varieties of B. cereus produce no enterotoxins; indeed, some are presently used as probiotics for both humans and animals (Hong et al., 2008). Thus, all Bacillus strains tested in this study seem to be appropriate for the probable bio-safe utilization as probiotics, based on the strains cytotoxic-free potential (Figure 2).
Today, the use of Bacillus species as probiotic supplements in animal feed is expanding rapidly due to the ease of bulk production and the assurance of stability (Cutting, 2011). Bacillus sp. SM2 can be an alternative to antibiotic growth promoter but the use of the strain SM2 in combination of other Bacillus spp. may offer additional benefits. Further research on additive effects of the combination of Bacillus spp. used in this experiment is needed. This would be a useful future experiment.
Acknowledgement
This research was supported by Technology Development Program for Agriculture and Forestry, Ministry for Food, Agriculture, Forestry, and Fisheries, Republic of Korea (Project No. 610005031SB130).
References
1. Anderson RL, Wolf WJ. Compositional changes in trypsin inhibitors, phytic acid, saponins and isoflavones related to soybean processing. J Nutr 1995; 125:581S-588S. [ Links ]
2. Chen KL, Kho WL, You SH, Yeh RH, Tang SW, Hsieh CW. Effects of Bacillus subtilis var. natto and Saccharomyces cerevisiae mixed fermented feed on the enhanced growth performance of broilers. Poult Sci 2009; 88:309-315. [ Links ]
3. Cho J, Choi K, Darden T, Reynolds PR, Petitte JN, Shears SB. Avian multiple inositol polyphosphate phosphatase is an active phytase that can be engineered to help ameliorate the planet's phosphate crisis. J Biotechnol 2006; 126:248-259. [ Links ]
4. Cutting SM. Bacillus probiotics. Food Microbiol 2011; 28:214- 220. [ Links ]
5. Davis ME, Parrott T, Brown DC, de Rodas BZ, Johnson ZB, Maxwell CV, Rehberger T. Effect of a Bacillus-based directfed microbial feed supplement on growth performance and pen cleaning characteristics of growing-finishing pigs. J Anim Sci 2008; 86:1459-1467. [ Links ]
6. From C, Pukall R, Schumann P, Hormazabal V, Granum PE. Toxin producing ability among Bacillus spp. outside the Bacillus cereus group. Appl Environ Microbiol 2005; 71:1178- 1183. [ Links ]
7. Fuller R. Probiotics in man and animals. J Appl Bacteriol 1989; 66:365-378. [ Links ]
8. Gaggia F, Gioia DD, Baffoni L, Biavati B. The role of protective and probiotic cultures in food and feed and their impact in food safety. Trends Food Sci Tech 2011; 22:S58-S66. [ Links ]
9. Granum PE. Bacillus cereus and food poisoning. In: Berkeley R, Heyndrickx M, Logan NA, de Vos P, editors. In Applications and Systematices of Bacillus and Relatives. Oxford:Blackwell Science; 2002. p.37-46. [ Links ]
10. Granum PE, Lund T. Bacillus cereus and its food poisoning toxins. FEMS Microbiol Lett 1997; 157:223-228. [ Links ]
11. Guinebretiere MH, Broussolle V, Nguyen-The C. Enterotoxigenic profiles of food poisoning and food borne Bacillus cereus strains. J Clin Microbiol 2002; 40:3053-3056. [ Links ]
12. Guo X, Li D, Lu W, Piao X, Chen X. Screening of Bacillus strains as potential probiotics and subsequent confirmation of the in vivo effectiveness of Bacillus subtilis MA139 in pigs. Antonie van Leeuwenhoek 2006; 90:139-146. [ Links ]
13. Hong HA, Duc LH, Cutting SM. The use of bacterial spore formers as probiotics. FEMS Microbiol Rev 2005; 29:813-835. [ Links ]
14. Hong HA, Huang JM, Khaneja R, Hiep LV, Urdaci MC, Cutting SM. The safety of Bacillus subtilis and Bacillus indicus as food probiotics. J Appl Microbiol 2008; 105:510-520. [ Links ]
15. Huang JM, La Ragione RM, Nunez A, Cutting SM. Immunostimulatory activity of Bacillus spores. FEMS Immunol Med Microbiol 2008; 53:195-203. [ Links ]
16. Huang Y, Adams MC. In vitro assessment of upper gastrointestinal tolerance of potential probiotic dairy propionibacteria. Int J Food Microbiol 2004; 91:253-260. [ Links ]
17. Hutadilok-Towatana N, Painupong A, Suntinanalert P. Purification and characterization of an extracellular protease from alkaliphilic and thermophilic Bacillus sp. PS 719. J Biosci Bioeng 1999; 87:581-587. [ Links ]
18. Jadamus A, Vahjen W, Simon O. Growth behavior of a spore forming probiotic strain in the gastrointestinal tract of broiler chickens and piglets. Arch Anim Nutr 2001; 54:1-17. [ Links ]
19. Kheadr E, Dabour N, Le Lay C, Lacroix C, Fliss I. Antibiotic susceptibility profile of bifidobacteria as affected by oxgall, acid, and hydrogen peroxide stress. Antimicrob Agents Chemother 2007; 51:169-174. [ Links ]
20. Kim GB, Miyamoto CM, Meighen EA, Lee BH. Cloning and characterization of the bile salt hydrolase genes (bsh) from Bifidobacterium bifidum strains. Appl Environ Microbiol 2004; 70:5603-5612. [ Links ]
21. Kim PI, Jung MY, Chang YH, Kim S, Kim SJ, Park YH. Probiotic properties of Lactobacillus and Bifidobacterium strains isolated from porcine gastrointestinal tract. Appl Microbiol Biotechnol 2007; 74:1103-1111. [ Links ]
22. Lee CY, Lim JW, Ko YH, Kang SY, Park MJ, Ko T, Lee JH, Hyun Y, Jeong KS, Jang IS. Intestinal growth and development of weaning pigs in response to dietary supplementation of antibiotics, phytogenic products and brewer's yeast plus Bacillus spores. J Anim Sci Tech (Kor) 2011; 53:227-235. [ Links ]
23. Lee HS, Gilliland SE, Carter S. Amylolytic cultures of LactoBacillus acidophilus: Potential probiotics to improve dietary starch utilization. J Food Sci 2001; 66:338-344. [ Links ]
24. Leser TD, Knarreborg A, Worm J. Germination and outgrowth of Bacillus subtilis and Bacillus licheniformis spores in the gastrointestinal tract of pigs. J Appl Microbiol 2008; 104:1025- 1033. [ Links ]
25. Liu JR, Lai SF, Yu B. Evaluation of an intestinal Lactobacillus reuteri strain expressing rumen fungal xylanase as a probiotic for broiler chickens fed on a wheat-based diet. Br Poult Sci 2007; 48:507-514. [ Links ]
26. Miller GL. Use of dinitrosalicyclic acid reagent for determination of reducing sugar. Anal Chem 1959; 31:426-428. [ Links ]
27. Park I, Cho J. Production of an extracellular protease by an Antarctic bacterial isolate (Bacillus sp. JSP1) as a potential feed additive. Rev Colomb Cienc Pecu 2011; 24:3-10. [ Links ]
28. Patil AG, Praveen Kumar SK, Mulimani VH, Veeranagouda Y, Lee K. Α-galactosidase from Bacillus megaterium VHM1 and its application in removal of flatulence-causing factors from soymilk. J Microbiol Biotechnol 2010; 20:1546-1554. [ Links ]
29. Petersson-Wolfe CS, Adams S, Wolf SL, Hogan JS. Genomic typing of enterococci isolated from bovine mammary glands and environmental sources. J Dairy Sci 2008; 91:615-619. [ Links ]
30. Phillips I. Withdrawal of growth-promoting antibiotics in Europe and its effects in relation to human health. Int J Antimicrob Agents 2007; 30:101-107. [ Links ]
31. Santoso U, Tanaka K, Ohtania S. Effect of fermented product from Bacillus subtilis on feed efficiency, lipid accumulation and ammonia production in broiler chicks. Asian-Aust J Anim Sci 2001; 14:333-337. [ Links ]
32. Schierack P, Wieler LH, Taras D, Herwig V, Tachu B, Hlinak A, Schmidt MFG, Scharek L. Bacillus cereus var. toyoi enhanced systemic immune response in piglets. Vet Immunol Immunopathol 2007; 118:1-11. [ Links ]
33. Sugita H, Hirose Y, Matsuo N, Deguchi Y. Production of the antibacterial substance by Bacillus sp. strain NM12, an intestinal bacterium of Japanese coastal fish. Aquaculture 1998; 165:269-280. [ Links ]
34. Sun P, Wang JQ, Zhang HT. Effects of Bacillus subtilis natto on performance and immune function of preweaning calves. J Dairy Sci 2010; 93:5851-5855. [ Links ]
35. Sutyak KE, Wirawan RE, Aroutcheva AA, Chikindas ML. Isolation of the Bacillus subtilis antimicrobial peptide subtilosin from the dairy product-derived Bacillus amyloliquefaciens. J Appl Microbiol 2008; 104:1067-1074. [ Links ]
36. Taheri HR, Moravej H, Tabandeh F, Zaghari M, Shivazad M. Screening of lactic acid bacteria toward their selection as a source of chicken probiotic. Poult Sci 2009; 88:1586-1593. [ Links ]
37. Tanaka H, Doesburg K, Iwasaki T, Mierau I. Screening of lactic acid bacteria for bile salt hydrolase activity. J Dairy Sci 1999; 82:2530-2535. [ Links ]
38. Turner JL, Dritz SS, Minton JE. Review: alternatives to conventional antimicrobials in swine diets. Prof Anim Sci 2001; 17:217-226. [ Links ]
39. Vila B, Fontgibell A, Badiola I, Esteve-Garcia E, Jimenez G, Castilo M, Brufau J. Reduction of Salmonella enterica var. Enteritidis colonization and invasion by Bacillus cereus var. toyoi inclusion in poultry feeds. Poult Sci 2009; 88:975-979. [ Links ]
40. Yu B, Liu JR, Hsiao FS, Chiou PWS. Evaluation of Lactobacillus reuteri Pg4 strain expressing heterologous Β– glucanase as a probiotic in a barley-base poultry diet. Anim Feed Sci Technol 2008; 141:82-91. [ Links ]
41. Zhou X, Wang Y, Gu Q, Li W. Effect of dietary probiotic, Bacillus coagulans, on growth performance, chemical composition, and meat quality of Guangxi Yellow chicken. Poult Sci 2010; 89:588-593. [ Links ]
Notas al pie
¤ To cite this article: Lee J, Park I, Choi Y, Cho J. Bacillus strains as feed additives: In vitro evaluation of its potential probiotic properties. Rev Colomb Cienc Pecu 2012; 25:577-585.