Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Colombiana de Ciencias Pecuarias
Print version ISSN 0120-0690
Rev Colom Cienc Pecua vol.25 no.4 Medellín Oct./Dec. 2012
ORIGINAL ARTICLES
Ruminant feces used as inoculum for the in vitro gas production technique¤
Heces de rumiantes como fuente de inóculo para la técnica in vitro de producción de gases
Fezes de ruminantes como fonte de inoculo para a técnica in vitro de produção de gases
Sandra L Posada1*, Zoot, PhD; Ricardo R Noguera1, Zoot, PhD; Juan A Segura2, Zoot, MSc.
1GRICA research group, Faculty of Agricultural Sciences, University of Antioquia, AA 1226, Medellín, Colombia.
2Department of Animal Production, Faculty of Agricultural Sciences, National University of Colombia (Medellín, Colombia).
* Corresponding author: Sandra L Posada. Faculty of Agrarian Sciences, University of Antioquia. Tel: (054) 2196524. Email: slposada@agronica.udea.edu.co, slposada@gmail.com
(Received: 16 september, 2011; accepted: 2 february, 2012)
Summary
Background: ruminal feed fermentation can be studied through in vitro gas production. However, this technique requires fistulated animals from which to obtain the inoculum, which limits its use. Objective: the objective of this experiment was to evaluate the usefulness of feces instead of rumen fluid as the inoculum of reference, by determining the precision and accuracy resulting from both methods. Methods: six forage species (Gliricidia sepium, Panicum maximum, Pennisetum clandestinum, Lolium sp., Morus alba and Cynodon nlemfuensis) were incubated with bovine rumen fluid or feces to quantify gas production and dry matter degradation over time. Bacteria, fungi, and protozoa counts were assessed in both inocula. Results: cumulative gas production and gas production rate were higher for the ruminal inoculum during the initial incubation period. Ruminal liquid showed lower variability compared to its own mean. Conclusions: according to the Bland-Altman analysis, inocula are not interchangeable. The difference in gas production kinetics between both inoculum sources reflected a longer time to colonize the substrate and lower microbial concentration in the fecal fluid, which resulted useful solely in determining the extent of dry matter degradation.
Key words: accuracy, fistulation, microbial density, precision , rumen fermentation.
Resumen
Antecedentes: la fermentación ruminal de los alimentos puede ser estudiada a través de la técnica in vitro de producción de gases. No obstante, una de las limitaciones de la técnica es el requerimiento de animales fistulados para la obtención del inóculo. Objetivo: el objetivo de este experimento fue evaluar la utilización de las heces respecto al inóculo de referencia, líquido ruminal, a través de la determinación de la precisión y la exactitud. Métodos: para ello seis especies forrajeras (Gliricidia sepium, Panicum maximum, Pennisetum clandestinum, Lolium sp., Morus alba y Cynodon nlemfuensis) fueron incubadas con líquido ruminal y heces bovinas, cuantificando la producción de gas y la degradación de la materia seca en el tiempo. En los dos inóculos se realizó conteo de bacterias, hongos y protozoos. Resultados: la producción acumulativa de gas y la tasa de producción de gas durante el período inicial de incubación fueron superiores con el inóculo ruminal. En el análisis de repetibilidad, el líquido ruminal exhibió menor variabilidad respecto el valor medio obtenido. Conclusiones: el análisis de Bland-Altman permitió concluir que los dos inóculos no son intercambiables. La diferencia en la cinética de producción de gas entre ambas fuentes de inóculo reflejó el mayor tiempo de colonización del sustrato y la menor concentración de microorganismos en el fluido fecal, resultando sólo de utilidad para determinar la extensión de la degradación de la materia seca.
Palabras clave: densidad microbiológica, exactitud, fermentación ruminal, fistulación, precisión.
Resumo
Antecedentes: a fermentação ruminal dos alimentos no rúmen pode ser estudada através da técnica in vitro de produção de gases. No entanto, uma das limitações da técnica é a exigência de animais fistulados para obter o inóculo. Objetivo: o objetivo deste experimento foi avaliar o uso das fezes em comparação ao inóculo de referência, líquido ruminal, através da determinação da precisão e exatidão. Metodos: seis forragens (Gliricidia sepium, Panicum maximum, Pennisetum clandestinum, Lolium sp., Morus alba e Cynodon nlemfuensis) foram incubadas com líquido ruminal e fezes bovinas, quantificando a produção de gás e a degradação da matéria seca no tempo. Contagem de bactérias, fungos e protozoários foi feita nos dois inóculos. Resultados: a produção acumulativa de gás e a taxa de produção de gás durante o período inicial de incubação foram maiores com o inoculo ruminal. Na análise de repetibilidade, o inoculo ruminal mostrou menor variabilidade ao redor do valor médio obtido. Conclusiones: a análise de Bland-Altman permitiu concluir que os dois inóculos não são intercambiáveis. A diferença na cinética de produção de gás entre as duas fontes de inóculo refletiu o maior tempo de colonização do substrato e a menor concentração de microorganismos no fluido fecal, resultando apenas útil para determinar a extensão da degradação da matéria seca.
Palavras chave: densidade microbiológica, exatidão, fermentação ruminal, fistulação, precisão.
Introduction
Ruminal feed fermentation can be studied through in vivo, in situ, and in vitro methods. A limitation of the in vitro gas production technique, shared by other bioassay methods (Tilley and Terry, 1963; Orskov et al., 1980), is the need for rumen-fistulated animals from which to obtain the inoculum. Fistulation is an invasive procedure under restrictive legislation in many countries because of ethical considerations related to animal welfare. This technique also has the limitations associated to surgical procedures, including the risk of infections, and the high costs of maintaining the animals. In this context, there is a need to evaluate alternative sources of inoculum to substitute rumen fluid, and one option is the use of feces. Other researchers suggest that feces have potential use as alternative inoculum replacing ruminal liquid for the in vitro gas production technique. Nevertheless, those studies are based on regression and correlation; statistical techniques which give an idea of association, but do not ensure concordance between variables, making it impossible to determine if both inocula can be interchangeable. Thus, the objective of this experiment was to evaluate the reliability of feces as inoculum source for in vitro gas production compared to ruminal fluid, by determining statistical precision and accuracy.
Materials and methods
Evaluation parameters
Two experiments were conducted. The first experiment set precision as the evaluation parameter for both sources of inoculum. Precision represents the approximate matching between a series of measurements obtained from a homogeneous sample, under defined conditions (Godden et al., 2000; Bendicho et al., 2001). Statistical repeatability is one of the most commonly used ways to measure precision. Repeatability is defined as the degree of rapprochement between independent test results obtained by the same method and analyst, on the same sample in a particular laboratory, using the same equipment and within a short time interval (Pinto et al., 2000; Rodriguez et al., 2001). The second experiment was designed to determine accuracy as the evaluation parameter for fecal versus ruminal inocula. Accuracy is determined by comparing the values obtained with the proposed method against the reference values of the standard method (Godden et al., 2000).
Substrates, preparation of culture medium and inoculum
Six forage species were used: Matarratón (Gliricidia sepium), Guinea grass (Panicum maximum), Kikuyu grass (Pennisetum clandestinum), Ryegrass (Lolium sp.), Mulberry (Morera, Morus alba), and Star grass (Estrella, Cynodon nlemfuensis). Dry matter (DM) percentage of the substrates was determined according to the procedures described by AOAC (1990).
The culture medium was prepared in accordance with recommendations by Mauricio et al. (2001). Ruminal fluid and feces were used as sources of inoculum. Both inoculum sources were obtained from three fistulated Holstein cows (mean weight 600 Kg), located at the National University of Colombia (Paysandu facilities) in a tropical moist forest. Animals were grazing on Kikuyo when grass was 45 days old, and had mineralized salt ad libitum. Inocula were collected at 6:30 a.m. Rumen fluid was manually removed and stored in thermal containers pre-warmed with water (40 °C). Ruminal liquid was filtered in the laboratory (Biorum Research Lab, National University of Colombia) through cotton cloths. The solid material remaining in the cloths was then blended with some rumen fluid for 20 seconds, filtered again, and transferred to an Erlenmeyer, where it was continuously saturated with CO2 into a water bath (39 °C).
Feces were collected per rectum and stored in pre-warmed thermal containers. A total of 300 ml of sample was diluted in 150 ml of anaerobic buffer. The resulting suspension was squeezed through cotton cloths and the solids remaining in the cloth were homogenized in a blender with 150 ml of the same buffer. Inocula obtained from the three animals were mixed in the same proportion before being added to the incubation flasks.
Microbial counts
Once prepared, inocula were sampled to determine microbial density. According to protocols established by BIORUM (2004), total bacteria growing on glucose, fungi growing on glucose and cellobiose, and total protozoa were determined in both inocula. Inocula were diluted in liquid medium at a ratio of 10-5 and 10-1 for cultivation of bacteria and fungi, respectively. These dilutions were grown on solid medium, and counting of colony-forming units (CFU) and thallus-forming units (TFU) was conducted at 72 and 96 hours, respectively. For quantification of protozoa, 5 ml of rumen fluid or feces were mixed with 5 ml formalin (10% v/v) in acetic acid (2% v/v). The sample was incubated at room temperature for 12 h and then 9 ml of glycerol 30% v/v were added, obtaining a 5 x 10-2 final dilution. Counts were performed in Neubauer chambers (in nine cells from two chambers, in duplicate).
Inoculation and reading of pressure
Incubation was conducted in 100 ml glass flasks, adding each with 0.5 g of substrate (ground to 1 mm), 45 ml of medium, and 5 ml of rumen fluid or feces. Then flasks were transferred to a forced-air oven at 39 °C (time zero). Simultaneously, a series of blank flasks were used to correct for the gas production caused by fermentation of inoculum and medium. These flasks contained culture medium and inoculum, but no substrate. A sample from each of the six forage species was incubated in Experiment one, replicating six times per inoculum, and using six blanks per inoculum, for a total of 84 incubation flasks. In Experiment two, a total of 648 flasks were incubated, using 96 flasks per forage (4 samples/ forage x 12 replicates/sample x 2 inocula) and 72 flasks for the blanks (2 inocula x 36 replications/ inoculum).
Gas pressure (psi) was measured with a T443A transducer connected to a digital meter (Bailey and Mackey, England). Readings were taken at 2, 4, 6, 8, 10, 12, 15, 19, 24, 30, 36, 48, 72, and 96 h. The volume of gas produced (V, ml) was obtained by replacing the pressure data (P) from the regression equation V = -0.1375 + (5.1385 * P) + (0.0777 * P2), previously determined by Posada et al. (2006).
Dry matter (DM) degradation and partitioning factor (PF)
Gas production kinetics was evaluated simultaneously with the DM degradation process. To do this, the content of each bottle was filtered (filter paper porosity: 20 μm) at 6, 12, 24, 48, 72, and 96 h using a vacuum pump. Degraded DM was determined at 110 °C at constant weight. In order to study variations in microbial biomass production, the partition factor (PF) was obtained for both inocula at the same times established for the filtration. The PF is the relationship between substrate degraded (mg) and volume of gas produced (ml).
Statistical analysis
Experiment one. The evaluation of both inoculum sources included a repeatability analysis based on data dispersion. Mean, standard deviation (SD), variance, and variation coefficient (CV) were calculated from gas production measurements at different times for each inoculum (ISO 5725, 1994). A t-test and F-test were conducted to determine mean gas production differences and homogeneity of variances between both inoculum sources, respectively.
Experiment two. The evaluation of fecal inoculum with respect to the reference inoculum (rumen fluid) included an accuracy analysis. Regression and correlation analysis of gas production and DM degradation for both inocula were conducted. Interchangeability between both inocula was assessed using the Bland-Altman method (Altman and Bland, 1983; Bland and Altman, 1986).
Cumulative gas production and gas production rate were analyzed with a mixed-model of repeated measures, where inoculum source and incubation time represented fixed effects, while substrate was considered a random effect. Dry matter digestibility and PF were analyzed under a completely randomized design using a general linear model and the Tukey comparison test. All statistical procedures were conducted using the SAS program (SAS, 2001) with a 5% significance level.
Results
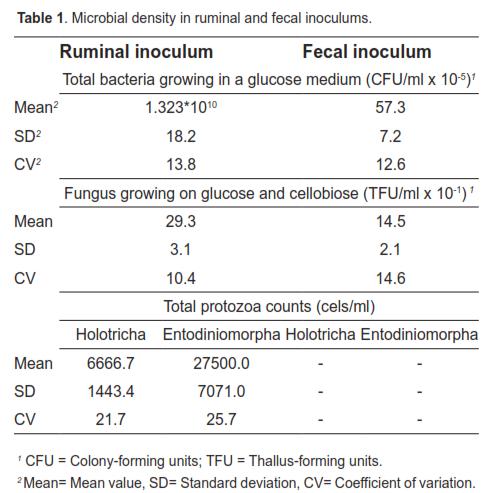
Microbial density
Microbial density data for each inoculum are presented in table 1. Protozoa were not found in the fecal inoculum. Holotricha counts considerably exceeded entodinomorphs in the rumen inocula.
Determination of precision
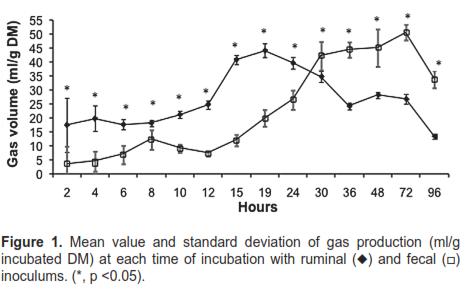
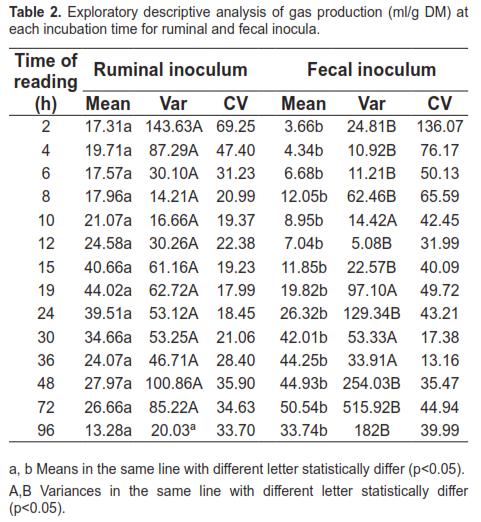
Figure 1 and table 2 show average gas production values (ml/g incubated DM) of all substrates at each reading time for both inoculum sources. The gas volume recorded for ruminal inoculum was higher up until 24 h. After 30 h, fecal inoculum values were higher. Statistical differences were found at all times measured (p <0.05), according to the t-test.
Table 2 also shows CVs and variances of gas production obtained with both inocula for the various incubation times. The fecal inoculum showed higher CVs in 11 of the 14 analyzed hours. Homogeneity of variances was found only at 10, 19, 30, and 36 h of incubation.
Determination of accuracy
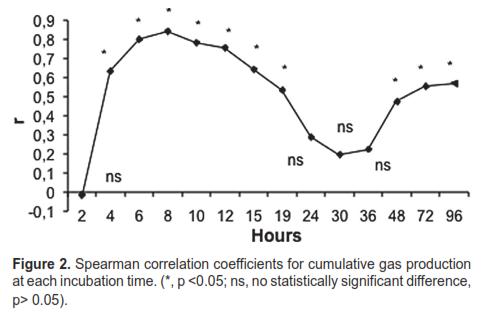
The correlation coefficient for cumulative gas production observed for both inocula is shown in figure 2. The level of association was low and not significant (p> 0.05) at 2, 24, 30, and 36 h. Correlations were significant (p <0.05) and positive in the remaining hours.
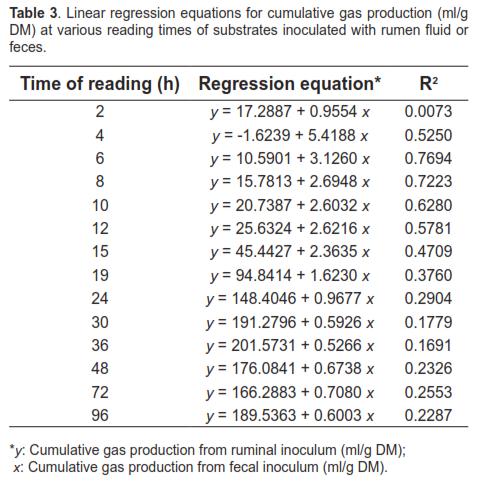
Linear regression analysis between cumulative gas production with ruminal inoculum (y) and cumulative gas production with fecal inoculum (x) is presented in table 3. The equations obtained were characterized by low coefficients of determination (R2), with values above 50% only between 4 and 12 h.
Table 4 shows the average cumulative gas production and gas production rate observed with rumen fluid and feces at several incubation times. Cumulative gas production for ruminal liquid was greater than for fecal fluid throughout and the differences increased as incubation time advanced, up to 24 h (p <0.05). Gas production rates for feces were higher than those for rumen fluid only after 30 h (p <0.05).
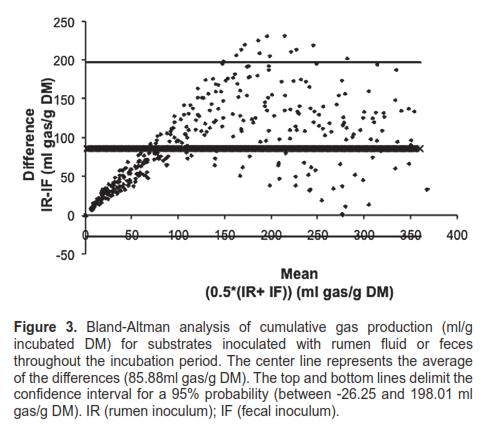
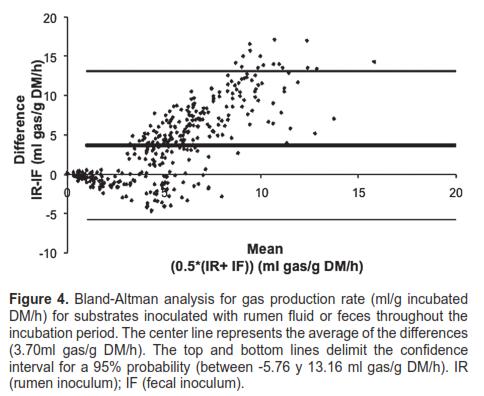
The level of agreement between inocula, measured through the Bland-Altman interchangeability test, is shown in figures 3 and 4. When this test was applied to cumulative gas production (Figure 3) and gas production rate (Figure 4), 96.67 and 95.56% of the data, respectively, were included within the 95% confidence interval (degree of agreement). The Spearman correlation coefficient (r; cumulative gas production= 0.7137; gas production rate = 0.7383) and regression slope (b) were statistically significant (p <0.0001) in the regression and correlation analysis for the differences (y) and average values (x) between the two inocula.
Regarding cumulative gas production (Figure 3), the observed differences were all positive, favoring the rumen inoculum—all measurements were above zero at the y-axis. Likewise, the data show high dispersion toward the upper limit of the mean differences, which illustrates the variability of the differences as fermentation progresses. In figure 4, the x-axis is not directly related with time, as in figure 3. The smallest differences in gas production rates were observed at an advanced stage of the fermentation process.
Description of DM degradation
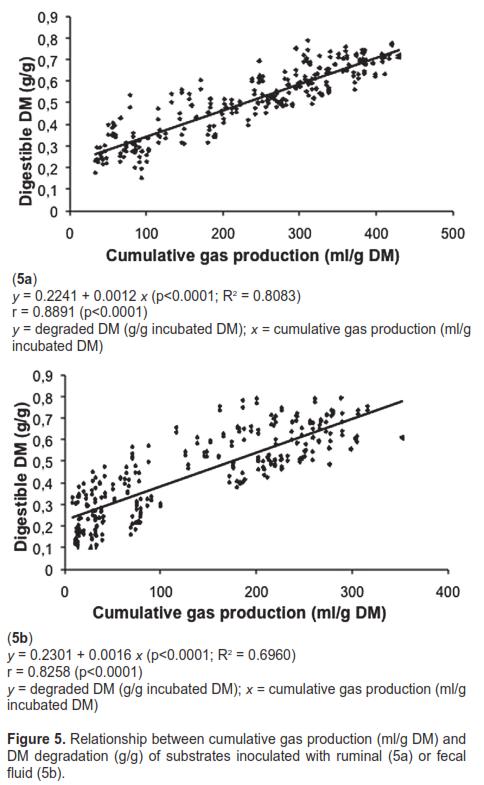
The relationship between cumulative gas production (ml/g incubated DM) (x) and DM degradation (g/g incubated DM) (y) at different filtration times (6, 12, 24, 48, 72, and 96 h) is shown in figure 5 for the 24 substrates tested. The ruminal inoculum had a higher R2 (0.8083 vs. 0.6960).
Using linear regression and correlation analysis between DM degraded with ruminal (y) and fecal inoculum (x), expressed in g/g incubated DM for the 24 substrates, the equation obtained was: Y = 0.1755 + 0.7483 X (p <0.0001, R2 = 0.8118) and r was: 0.9160 (p <0.0001).
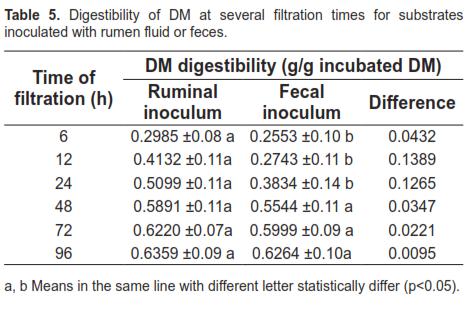
When ruminal fluid and feces were compared for their ability to degrade DM in all substrates (Table 5), it was found that mean values were different (p <0.05) only at 6, 12, and 24 h, but not at the end of the fermentation process (p> 0.05), meaning that the extent of degradation was similar between both inocula.
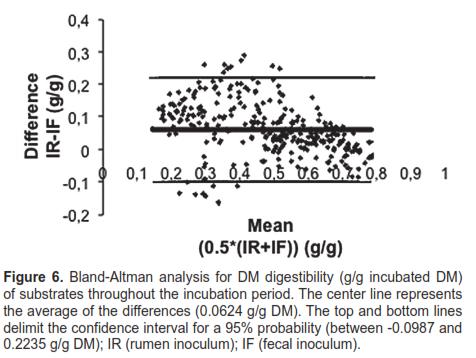
The agreement for DM digestibility with both inocula, assessed by Bland-Altman analysis, is shown in figure 6. 94.1% of the data were included within a 95% confidence interval, and 80.2% of the differences were positive, favoring the ruminal inoculum, with a tendency to decrease as incubation time progressed.
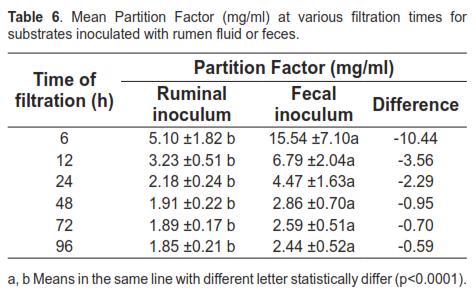
Table 6 shows the average PF with both inoculum sources at different filtration times for the 24 substrates tested. Increased PF was associated with the use of fecal fluid and the initial incubation times.
Discussion
Microbial density
The period during which bacteria adhere to the substrate with little or no digestion is referred to as colonization time. Results shown in figure 1 indicate a longer colonization (lag phase) of the insoluble substrate when fecal inoculum was used. The longer colonization time required for this inoculum is explained by its lower microbial density (Table 1). The low bacterial concentration in feces may be due to the type of substrate available for fermentation in the lower intestine. According to Hoover and Stokes (1991), the digestion-rate of carbohydrates (which is directly related to the proportion of starches, pectins, and sugars) is the main factor controlling the available energy for microbial growth. Given that most ingested carbohydrates are fermented in rumen and reticulum, fermentation in cecum and colon is restricted to slowly digested residues, mainly fiber, resulting in reduced nutrient availability for microbial growth. Mauricio et al. (1998, 2001) argue that the reduced fermentation capacity in cecum and colon, relative to that occurring in the rumen, may be due to a combination of several factors including substrates of lower nutritional value and shorter retention time, which result in a lower microbial population. In this context, according to McAllister et al. (1994), fungi and protozoa require longer digesta retention, since their generation times range from 5 to 14 and 24 to 30 h, respectively. While in this study the low microbial concentration in fecal inoculum reflected a longer colonization time, this can also be the result of lower enzymatic activity associated with the use of feces. This was evidenced by Michlet-Doreau et al. (2002) who compared cellulolytic microbial ecosystems in rumen and cecum, reporting increased ruminal enzyme activity (polysaccharidase, glycosylase) without a parallel difference in RNA for the three main cellulolytic bacterial species (Fibrobacter succinogenes, Ruminococcus flavefaciens, and Ruminococcus albus). The discrepancy between cellulolytic activity of rumen and cecum microbes was explained by the hypothetical differences in physical and chemical conditions in each compartment, including pH. Martin (1994) points out that those changes in environmental conditions can affect the transport of carbon, nitrogen, and energy by microorganisms.
Considering that protozoa are sensitive to low pH (Hungate, 1966; Mould et al., 2005), the passage of digesta through the abomasum may explain the absence of these microorganisms in the fecal inoculum. The findings of this study coincide with those of Kern et al. (1974), who compared the microorganisms in rumen and cecum of cattle and found no protozoa in the latter compartment. The presence of fungi in feces can be explained by: a) their ability to survive under extreme conditions (Mauricio, 1999), and b) the absence of protozoa in the fecal inoculum, considering that protozoa consume fungal zoospores (Akin and Borneman, 1990).
Determination of precision
Homogeneity of variances for 10, 19, 30 and 36 hours incubation time indicates that gas production SD was similar for these incubation periods. However, as the means were different (p<0.05), it is concluded that CV is the most appropriate criterion to assess repeatability (Table 2).
As the substrate was the same for both inocula, the higher CV obtained for feces (Table 2) can be solely explained by the characteristics of the inocula. Due to the fact that microbial density is higher in ruminal inoculum at the time of inoculation, fermentation is more uniform and the rate of microbial growth and gas production is more constant. The lower CV values obtained for the ruminal inoculum make it the inoculum of choice under similar experimental conditions.
The high CV obtained during the first hours may be based on two premises: a) the ability of microbes to colonize the substrate, which is determined by the microbial population size and the chemical, anatomical, and morphological composition of the forage; b) gas production is proportional to the amount of metabolic products generated during microbial growth and this, in turn, is affected by the amount of substrate that has been degraded. As incubation time elapses, it is likely that conditions for microbial growth stabilize, ensuring a more homogeneous colonization and degradation of materials.
Determination of accuracy
According to the correlation coefficient (criterion for assessing the degree of association between cumulative gas production for both inocula, Figure 2), there was no correspondence or definite tendencies among continuous values at the measured times. The values for the coefficient of determination (R2) obtained from the linear regression analysis between cumulative gas production with both inocula (Table 3) correspond with the trend shown in figure 2, so it can be concluded that the obtained models only provide good estimates at the start of fermentation.
Goncalves and Borba (1996) compared sheep's ruminal fluid and feces in their ability to ferment different materials, and reported that total gas production from ruminal fluid was higher than that from fecal fluid, which is consistent with the results shown in table 4. The difference in gas production kinetics with both inocula ratifies the longer time required to colonize insoluble substrate by microbes and their low concentration in fecal inoculum.
Since regression and correlation analysis discussed so far measure the degree of relationship, but not the degree of matching, a third statistical approach was used to determine the level of agreement between inocula: the Bland-Altman interchangeability test (Figures 3 and 4). In this test—based on the regression and correlation analysis for the differences (y) and means (x) between both inocula—the Spearman correlation coefficient (r) and the regression slope (b) were statistically significant. The statistical significance of r and b indicates that discrepancy between ruminal and fecal inocula was not constant throughout the range or distribution interval, but varied with the increase of the mean values. Therefore, it can be concluded that ruminal and fecal inocula are not interchangeable.
Description of DM degradation
After evaluating the potential of feces to ferment tropical leguminous shrubs using the technique by Tilley and Terry (1963), Jones and Barnes (1996) concluded that DM digestibility was 3.5% units lower than that using ruminal fluid. These authors found a high r-value (0.98) for DM degradability between both inocula, which was close to the result obtained in the present study (0.9160). However, figure 5 shows that the percentage of variability in DM digestibility attributable to cumulative gas production was higher with ruminal inoculum, which is confirmed by the smaller dispersion of the data in the regression curve.
Once regression and correlation analysis for the differences (y) and means (x) in DM digestibility between both inocula was conducted, the Spearman correlation coefficient (r) (-0.5011) and the regression slope (b) were highly significant (p <0.0001). The statistical significance of r and b indicates that discrepancy between ruminal and fecal inocula was not maintained constant throughout the range or distribution interval, and therefore inocula are not interchangeable to determine DM digestibility in time (degradation kinetics).
The relationship between substrate degraded (mg) and gas produced (ml) may reflect variations in microbial biomass production. This ratio is defined as the partitioning factor (PF) (Posada and Noguera, 2005; Lopez et al., 1998). As gas production was higher with ruminal inoculum while DM degradation remained similar for both inocula, the PF for fecal fluid is higher (Table 6). Increased PF indicates higher biomass production per ATP generated (YATP) (Blümmel et al., 1997) and higher efficiency of microbial protein synthesis (Makkar, 2001; Getachew et al., 1998). These results could be associated with the lower competition occurring among microbial populations in the fecal inoculum, given their lower density. Conversely, the higher microbial density and diversity in ruminal inoculum leads to rapid depletion of available substrates for microbial growth. Because microbial growth efficiency depends on the availability of fermentable substrate—which, in turn, is used up during the incubation period— the highest PF were recorded in the initial stages, in agreement with reports by Lopez et al. (1998). The higher microbial growth efficiency of fecal inoculum during early fermentation leads to a closer relationship between microbial biomass and the substrate available for degradation, which would explain the lack of statistical difference in DM digestibility after 48 h of incubation (Table 5). According to this study, it can be concluded that fecal inoculum is useful only to determine endpoint DM digestibility (or the extent of degradation) and cannot be used to describe degradation kinetics.
While other researchers report that feces are potential alternative inoculum to ruminal fluid for in vitro gas production, our results do not validate this claim. Their statistical approach is based on regression and correlation analysis, which gives an idea of the degree of association between variables, but does not measure matching or concordance between them, making it impossible to determine whether both inocula can be considered as equivalent. Method comparison studies should rely on those statistical techniques that allow direct comparison of the results, determining to what extent one method can be replaced by another with sufficient accuracy. The Bland-Altman method applied in this study allows for the conclusion that feces were not comparable to ruminal fluid for DM degradation kinetics evaluation.
Precision, defined as the degree of rapprochement between values obtained from repeated measurements, is not equivalent to accuracy, which refers to how close together are reference and measured values. The higher CVs observed for fecal inoculum (Experiment 1) could have negatively affected the accuracy results in Experiment two. This can be explained by the fact that a set of values showing high repeatability (precision) may or may not be accurate, but a set of non-repeatable data can never be exact, thus increasing the absolute error or the difference between experimental and reference values.
As the differences in colonization time and fermentation dynamics are due to the smaller size and –possibly- activity of fecal microbial populations, an increase in fecal fermentation activity through a pre-incubation process in a nitrogen and energy enriched medium merits further investigation. This pre-incubation procedure could be used to increase microbial density of the fecal inoculum, allowing us to replace the ruminal fluid, given the current limitations for using surgically fitted animals.
Acknowledgements
The authors thank the Sustainability Project 2011-2012 (Estrategia de Sostenibilidad CODI 2011-2012, University of Antioquia), the faculty members at the University of Antioquia and the National University of Colombia (Medellín, Colombia) for providing resources and spaces required to conduct this study.
References
1. Akin DE, Borneman WS. Role of rumen fungi in fiber degradation. J Dairy Sci 1990; 73:3023-3032. [ Links ]
2. Altman DG, Bland JM. Measurement in medicine: The analysis of method comparison studies. The Statistician 1983; 32:307- 317. [ Links ]
3. Association of Official Analytical Chemist. Official Methods of Analysis. 15 th. ed. Arlington: AOAC; 1990. [ Links ]
4. Bendicho S, Trigueros MC, Hernández T, Martín O. Validation and comparison of analytical methods based on the release of p-nitrophenol to determine lipase activity in milk. J Dairy Sci 2001; 84:1590-1596. [ Links ]
5. BIORUM. Grupo Interinstitucional de Biotecnología Ruminal. Manual de Procedimientos, Parte I: Microbiota. Universidad Nacional de Colombia, sede Medellín. Mayo de 2004, 29 p. [ Links ]
6. Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. The Lancet 1986; 1:307-310. [ Links ]
7. Blümmel M, Makkar HPS, Becker K. In vitro gas production: a technique revisited. J Anim Physiol Anim Nutr 1997; 77:24-34. [ Links ]
8. Getachew G, Blümmel M, Makkar HPS, Becker K. In vitro measuring techniques for assessment of nutritional quality of feeds: a review. Anim Feed Sci Tech 1998; 72:261-281. [ Links ]
9. Godden SM, Lissemore KD, Kelton DF, Lumsden JH, Leslie KE, Walton JS. Analytic validation of an infrared milk urea assay and effects of sample acquisition factors on milk urea results. J Dairy Sci 2000; 83:435-442. [ Links ]
10. Gonçalves LMBO, Borba AES. Study of gas production capacity by three sources of inocula. J Agr Sci 1996; 127:511- 515. [ Links ]
11. Hoover WH, Stokes SR. Balancing carbohydrates and proteins for optimum rumen microbial yield. J Dairy Sci 1991; 74:3630- 3644. [ Links ]
12. Hungate RE. The Rumen and its Microbes. Academic Press, New York. 1966. 533 p. [ Links ]
13. ISO 5725-2, 1994. Accuracy (trueness and precision) of measurement methods and results-Part 2: Basic methods for determination of repeatability and reproducibility of a standard measurement method. Int. Org. Stand. Genève, Switzerland. [ Links ]
14. Jones RJ, Barnes P. In vitro digestibility assessment of tropical shrub legumes using rumen fluid or fecal fluid as the inoculum source. Trop Grasslands 1996; 30:374-377. [ Links ]
15. Kern DL, Slyter LL, Leffel EC, Weaver JM, Oltjen RR. Ponies vs. steers: microbial and chemical characteristics of internal ingesta. J Anim Sci 1974; 38:559-564. [ Links ]
16. López S, Carro MD, González JS, Ovejero FJ. Comparison of different in vitro and in situ methods to estimate the extent and rate of degradation of hays in the rumen. Anim Feed Sci Tech 1998; 73:99-113. [ Links ]
17. Makkar H. Recent advances in in vitro gas method for evaluation of nutritional quality of feed resources. 2001; [October 20, 2012] URL: http://www.fao.org/DOCREP/ARTICLE/AGRIPPA/570_EN_toc.htm [ Links ]
18. Martin SA. Nutrient transport by ruminal bacteria: A review. J Anim Sci 1994; 72:3019-3031. [ Links ]
19. Mauricio RM. Comparison of bovine rumen liquor and bovine faeces as inoculum for an In Vitro gas production technique for evaluating forage. Ph.D. Thesis. The University of Reading, Reading, UK, pp. 281, 1999. [ Links ]
20. Mauricio RM, Mould F, Owen E. Comparison of rumen liquor and faeces from cows as sources of microorganisms for the in vitro gas production technique. Arq Bras Med Zootec 1998; 50:569-572. [ Links ]
21. Mauricio RM, Owen E, Mould FL, Givens I, Theodorou MK, France J, Davies DR, Dhanoa MS. Comparison of bovine rumen liquor and bovine faeces as inoculum for an in vitro gas production technique for evaluating forages. Anim Feed Sci Tech 2001; 89:33-48. [ Links ]
22. McAllister TA, Bae HD, Jones GA, Cheng KJ. Microbial attachment and feed digestion in the rumen. J Anim Sci 1994; 72:3004-3018. [ Links ]
23. Michalet-Doreau B, Fernandez I, Fonty G. A comparison of enzymatic and molecular approaches to characterize the cellulolytic microbial ecosystems of the rumen and the cecum. J Anim Sci 2002; 80:790-796. [ Links ]
24. Mould FL, Kliem KE, Morgan R, Mauricio RM. In vitro microbial inoculum: A review of its function and properties. Anim Feed Sci Tech 2005; 123:31-50. [ Links ]
25. Orskov ER, DeB Hovell FD, Mould F. Uso de la técnica de la bolsa de nylon para la evaluación de alimentos. Prod Anim Trop 1980; 5:213-223. [ Links ]
26. Pinto M, Carrasco E, Fraser B, Barriga C. Validación del método butirométrico de Gerber por comparación con el método de referencia de RöseGottlieb para la determinación de la materia grasa en leche. Agro sur 2000; 28. [ Links ]
27. Posada SL, Noguera RR. Técnica in vitro de producción de gases: Una herramienta para la evaluación de alimentos para rumiantes. Livest Res Rural Development 2005; 17. [ Links ]
28. Posada SL, Noguera R, Bolívar D. Relación entre presión y volumen para la implementación de la técnica in Vitro de producción de gases en Medellín, Colombia. Rev Colomb Cienc Pecu 2006; 19:407-414. [ Links ]
29. Rodríguez N, Lorente A, Velásquez Y, González E. Confiabilidad del método slott modificado por laboratorios Heiga para la determinación directa de la creatinina. Revista de la Facultad de Farmacia 2001; 42: 67-71. [ Links ]
30. Statistical Analysis Systems. SAS®, versión 8.2 para Windows,. User's Guide. Statistics. Statistical Analysis Systems Institute. Inc., Cary, NC. 2001. [ Links ]
31. Tilley JMA, Terry RA. A two stage technique for the in vitro digestion of forage crops. J Br Grassl Soc 1963; 18:104-111. [ Links ]
Notas al pie
¤ To cite this article: Posada SL, Noguera RR, Segura JA. Ruminant feces used as inoculum for the in vitro gas production technique. Rev Colomb Cienc Pecu 2012; 25:592-602.