Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Revista Colombiana de Ciencias Pecuarias
versão impressa ISSN 0120-0690
Rev Colom Cienc Pecua vol.26 no.1 Medellín jan./mar. 2013
ORIGINALS ARTICLES
Melting ulcer in a colt: clinical management and evolution¤
Úlcera fundente en un potro: manejo clínico y evolución
Úlcera colagenolítica em um potro: manejo clínico e evolução
Rubén D Estrada1, MV; Susana Penagos2, est MV; Elizabeth Viera2, est MV; Paula A Angulo2, est MV; Maria P Arias2*, MV, MS, PhD.
* Corresponding Author: María Patricia Arias Gutiérrez. Faculty of Veterinary Medicine and Animal Science. Universidad CES. Calle 10 No. 22-04. Medellín, Colombia. AA 054591. E-mail: marias@ces.edu.co
1MV Actividad independiente.
2Facultad de Medicina Veterinaria y Zootecnia, Universidad CES, AA 054591, Medellín, Colombia.
(Received: June 1, 2012; accepted: November 19, 2012)
Summary
Anamnesis: a colt showing a whitish coloration accompanied by abundant secretion on the left eye was examined. Clinical and laboratory findings: at ophthalmological examination, signs of melting ulcer were observed. Culture isolation revealed positive growing of Flavobacterium sp. and Gram-negative rods. Treatment approach: several keratectomies and tarsorrhaphies, as well as exhaustive antiproteinases, antiinflammatory, and antibiotic treatments, were conducted. Treatment focused on reducing inflammatory response, eliminating infective organisms, and promoting epithelial healing. Colt showed complete recovery of vision after 3 months. Conclusions: clinical management of melting ulcer implies exhaustive, though unexpensive, treatment.
Key words: cornea, keratectomy, keratomalacia, ophthalmology, tarsorrhaphy.
Resumen
Anamnesis: se examinó un potro que presentó una coloración blanquecina acompañada de abundante secreción en el ojo izquierdo. Hallazgos clínicos y de laboratorio: al examen oftalmológico se observaron signos de ulcera fundente. El aislamiento por cultivo mostró crecimiento de Flavobacterium sp. y cocos Gram negativos. Abordaje terapéutico: se realizaron varias queratectomías y tarsorrafias, además de un tratamiento exhaustivo con antiproteinasas, antiinflamatorios y antibióticos enfocado a reducir la respuesta inflamatoria, eliminar los microorganismos infecciosos y promover la cicatrización epitelial. 3 meses después, el potro mostró recuperación completa de la visión. Conclusiones: el manejo clínico de la úlcera fundente es demandante, pero no es un tratamiento costoso.
Palabras Clave: córnea, keratomalacia, oftalmología, queratectomía, tarsorrafia.
Resumo
Antecedentes: foi examinado um potro que apresentava uma coloração esbranquiçada acompanhada por abundante secreção no olho esquerdo. Achados clínicos e de laboratório: ao exame oftalmológico foram encontrados sinais de úlcera colagenolítica. O isolamento por cultura apresentou crescimento de Flavobacterium sp. e cocos Gram negativos. Abordagem terapêutica: foram realizadas várias ceratectomias e tarsorrafias, além da instauração de um tratamento exaustivo com antiproteinases, anti-inflamatórios e antibióticos voltado para a redução da resposta inflamatória, a remoção de micro-organismos infecciosos e a estimulação da cicatrização epitelial. 3 meses depois o potro apresentou completa recuperação da visão. Conclusões: o manejo clínico da úlcera colagenolítica é exigente, mas não é um tratamento caro.
Palavras chave: ceratectomia, ceratomalacia, córnea, oftalmologia, tarsorrafia.
Introduction
Keratomalacia, clinically known as ''melting ulcer'', is a progressive destruction of the corneal stroma, leading to liquefactive necrosis with collagen fragmentation and loss of keratocytes (Brooks, 2004). The pathologic stromal collagen and proteoglycans degradation is a consequence of an imbalance in proteinases and proteinase inhibitors activity (Ollivier et al., 2007). Therefore, treatment with antiproteinases is highly recommended to speed healing, minimize corneal scarring, and slow ulcer progression (Ollivier, 2005; Kernacki et al., 1999). In 2011, a colt with corneal opacity arrived to the veterinary clinic at CES University (Medellín, Antioquia province, Colombia). Some keratectomies and tarsorrhaphies were performed, accompanied by an intensive treatment. The colt remained hospitalized for 27 days after which ulcer resolution was observed. 3 months later the colt showed complete recovery. The aim of this report is to describe the exhaustive but successful clinical management of melting ulcer in a colt.
Patient examination
Anamnesis
A 6-day-old colt was examined due to a whitish coloration accompanied by abundant secretion on the left eye. After physical examination, the veterinarian recommended hospitalization for intensive treatment.
Clinical findings
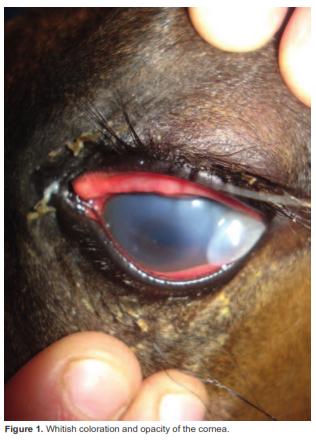
The colt's weight was 42 kg. He was alert and docile. His left eye was whitish with secretion and presented protrusion of the corneal epithelium in the lateral portion (Figure 1). Physical parameters and hydration status were normal.
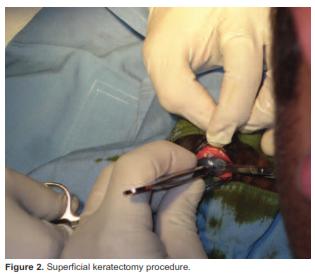
After physical examination, the colt was sedated with 0.5 mg/kg xylazine IV and an auriculopalpebral nerve block was performed with 2 ml (40 mg) lidocaine to evaluate cornea damage, revealing corneal opacity and partial stromal liquefaction. Afterwards, the patient was anesthetized with 2.2 mg/kg ketamine and a surgical excision of a cornea portion was performed to remove the melting tissue (Figure 2).
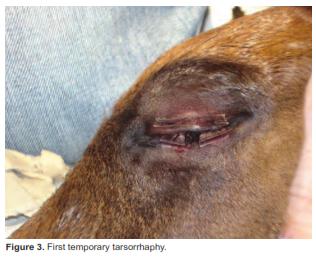
A temporary tarsorrhaphy was performed in order to protect the eye surface from drying and to prevent trauma. Both eyelids were stitched together with the removal of some tissue (Figure 3); this procedure was performed according to the technique described by Ollivier (2005).
Diagnostic aids
Hemogram and blood chemistry were conducted. Ocular secretion samples were cultured for microorganism isolation and antibiogram. Isolation revealed positive Flavobacterium sp. and Gramnegative rods sensitive to all antibiotics. Hemogram parameters and IgG were normal.
Treatment approach
After tarsorrhaphy, a topical antiproteinase, antibiotic, and anti-inflammatory therapy was started. Eyelids were slightly separated in the lateral canthus and Ciprofloxacin, Serum, EDTA, Atropine and Diclofenac drops were instilled holding the head until the drops descended across the ocular surface. Medical management is summarized in table 1.
The colt was alert during the second day of hospitalization. Itraconazole at a dose of 2 mg/kg each 48 hours was administered for 8 days to avoid fungal infections. On day 10 the colt was nursing as always but the affected eye still showed abundant secretion; he was sedated again to make another ophthalmic evaluation. The tarsorrhaphy was removed, an ophthalmic lavage with sodium chloride 0.9% solution was performed and a new keratectomy was done due to persistent stromal liquefaction. A new tarsorrhaphy was performed. As Gram-negative bacilli were isolated, antibiotic treatment continued as previously described.
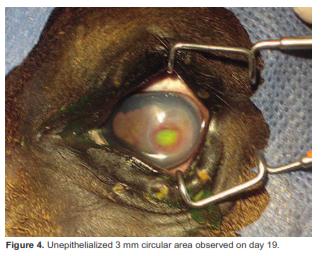
On day 19 the colt was sedated again for a third ophthalmic evaluation, tarsorrhaphy was removed and a fluorescein staining was performed to evaluate complete healing of corneal ulceration. An unepithelialized 3 mm circular area and corneal vascularization were detected (Figure 4). As corneal ulceration remained, the treatment continued and a third tarsorrhaphy was performed.
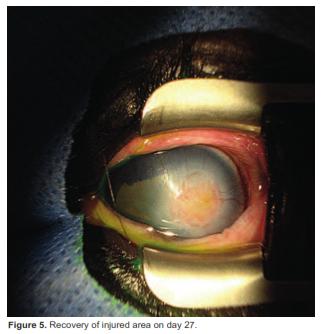
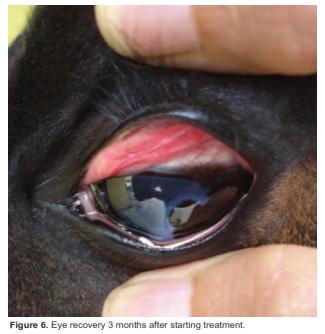
On day 27 another ophthalmic evaluation revealed absence of the ulcer (Figure 5). The patient was discharged with indications of performing ambulatory treatment for three months (Table 2). On March 15, the colt showed complete recovery (Figure 6).
Discussion
Equine melting ulcer is a multi-causal injury that may partially or completely affect corneal stroma. Trauma is the most common cause of corneal damage in horses due to their prominence and lateral position. Trauma usually occurs as a consequence of grazing or physical activity (Ollivier, 2005). Frequently, an initial corneal injury may complicate a melting ulcer, which is described as an imbalance of collagenollysis and repair of the corneal stromal extracellular matrix (Brooks, 1999). The colt was extremely vigorous and housed in a stall with his mother; therefore, it is very possible that a physical trauma caused the initial ulcerative keratitis and melting ulcer worsened the damage. In this case, laceration with severe depth and perforating corneal damage extending to 30% of the corneal surface occurred. Proteinases and proteinase inhibitors play an important role in healing corneal wounds (Sivak and Fini, 2002). Proteinases, naturally expressed in normal tissue, participate in remodeling the corneal stroma, while natural proteinase inhibitors prevent excessive degradation (Twining et al., 1994). Therefore, a predominant proteinases activity causes pathological stromal collagen degradation.
Melting ulcer is an ophthalmic emergency in horses (Couture et al., 2006) and most of the animals that do not receive opportune treatment may lose vision. In general, horses suffer more rapid collagen degradation and a higher degree of collagenolysis than humans and bovines (Twining et al., 1994). Thus, an accurate treatment that focuses on restoring the balance between proteolytic enzymes and proteinase inhibitors should be implemented as soon as possible in order to avoid devastating consequences (Solomon et al., 2000). This colt's injury was discovered on the sixth day of life; his early hospitalization and treatment allowed for complete recovery of corneal integrity.
Invasion of bacteria into the cornea induces host leukocytes to arrive to the damaged tissue and to release proteases. Antiproteinase medications reduce tear proteases produced by leucocytes and accelerate cornea healing (Ollivier et al., 2003). In humans, ophthalmologic antiproteinases are commonly used as part of medical treatment of corneal degeneration since 1990s (Hibbetts et al., 1999). In horses, some antiproteinases such as serum, acetylcysteine, EDTA and doxycycline have been proven in vitro (Ollivier et al., 2003) and in vivo (Baker et al., 2008). In these cases, serum and EDTA were used for preventing destruction of the cornea. EDTA inhibits matrix metalloproteinases and serum contains a1-antitrypsin, which inhibits serine proteinases (Ollivier, 2005; Couture et al., 2006; Haffner et al., 2003). These medications combined altogether helped to enhance the stroma healing in the present case, concluding with a satisfactory evolution, considering the severity of the injury.
The use of topical non-steroidal anti-inflammatory drugs (NSAIDs) in ophthalmology is controversial. Diclofenac stabilizes blood-aqueous barrier decreasing uveal exudation and inhibiting miosis occurring after some surgeries; it also relieves post-operative ocular pain, photophobia and inflammation (Dwyer, 2012); however some ophthalmologists have noticed delayed cornea epithelialization due to its use (Abelson and Lilyestrom, 2007). Others describe stinging and conjunctival hyperemia as their most common side effects (Abelson and Lilyestrom, 2007). As long-term corticosteroid administration may be deleterious in foals, some ophthalmologists recommend the use of NSAIDs with subconjunctival corticosteroids (Severin, 1998). In this case, diclofenac reduced pain and inflammation, and cornea delayed epithelization was not observed because of its use.
Harmless bacteria, fungi, viruses or parasites may become opportunistic pathogens in the ulcerated cornea. Gram-positive bacteria predominate as normal flora in cornea and conjunctiva, while Gram-negative bacteria and fungi are the most frequently isolated pathogens from ulcerated cornea in horses (Whitley and Moore, 1984; Moore et al., 1983). In concordance with the aforementioned findings, Flavobacterium sp. and Gram-negative rods were isolated from cultures. Topical chloramphenicol, gentamicin, ciprofloxacin and amikacin are recommended for the treatment of bacterial ulcers (Brooks et al., 2000; Davidson, 1991; Sweeney and Irby, 1996). Mills (2003) reported the use of systemic and topic antibiotics in stromal keratomalacia as useful, but Brooks (2004) reports that topical antibiotics according with sensitivity testing are sufficient to control ophthalmic infections. In this patient, ophthalmic Ciprofloxacin every 2 hours was sufficient to control the infection. This treatment functions very well if the horse is hospitalized. If hospitalization is not possible, a person responsible for administering treatment must be present.
In this colt, melting ulcer was treated as an ophthalmic emergency; therefore, the prompt diagnosis and treatment were clue factors for preventing serious complications and devastating consequences that could lead to vision loss.
Conclusions
Because of the rapid corneal tissue destruction, the patient should be intensively treated with antibiotics—depending on the results of corneal culture—, anti-inflammatory drugs, and topical antiproteases, which are crucial for recovery. Frequent administration of medication required for keratitis therapy can often fatigue the owners. Therefore, hospitalization of these patients is recommended. Although clinical melting ulcer management is exhaustive and requires intensive care, it is not an expensive treatment.
Notas al pie
¤ To cite this article: Estrada R, Penagos S, Viera E, Angulo P, Arias MP. Melting ulcer in a colt: clinical management and evolution. Rev Colomb Cienc Pecu 2013; 26:31-36.
Ackonowledgemnts
Special thanks to Nelson Pinto for his valuable assistance.
References
Abelson MB, Lilyestrom L. Melting away the myths of NSAIDs: An in-depth look at NSAIDs and the risk of side effects associated with their use. Rev Ophthalmol 2007; 14:124-127. [ Links ]
Baker A, Plummer CE, Szabo NJ, Barrie KP, Brooks DE. Doxycycline levels in preocular tear film of horses following oral administration. Vet Ophthalmol 2008; 11:381-385. [ Links ]
Brooks DE. Equine ophthalmology. In: Gelatt KN, editor. Veterinary Ophthalmology. 3rd ed. Philadelphia: Lippincott Williams & Wilkins; 1999. p.1053-1116. [ Links ]
Brooks DE. Inflammatory stromal keratopathies: medical management of stromal keratomalacia, stromal abscesses, eosinophilic keratitis, and band keratopathy in the horse. Vet Clin Equine 2004; 20:345-360. [ Links ]
Brooks DE, Andrew SD, Biros DJ, Denis HM, Cutler TJ, Strubbe DT, Gelatt KN. Ulcerative keratitis caused by betahemolytic Streptococcus equi in 11 horses. Vet Ophthalmol 2000; 3:121-125. [ Links ]
Couture S, Doucet M, Moreau M, Carrier M. Topical effect of various agents on gelatinase activity in the tear film of normal dogs. Vet Ophthalmol 2006; 9:157-164. [ Links ]Davidson MG. Equine ophthalmology. In: Gelatt KN, editor. Veterinary Ophthalmology. 2nd ed. Philadelphia: Lea & Febiger; 1991. p.576-610. [ Links ]
Dwyer AE. Ophthalmology in equine ambulatory practice. Vet Clin North Am Equine Pract 2012; 28:155-174. [ Links ]
Haffner JC, Fecteau KA, Eiler H. Inhibition of collagenase breakdown of equine corneas by tetanus antitoxin, equine serum and acetylcysteine. Vet Ophthalmol 2003; 6:67-72. [ Links ]
Hibbetts K, Hines B, Williams D. An overview of proteinase inhibitors. J Vet Intern Med 1999; 13:302-308. [ Links ]
Kernacki KA, Barrett R, Hazlett LD. Evidence for TIMP-1 protection against P. aeruginosa induced corneal ulceration and perforation. IOVS 1999; 40:3168-3176. [ Links ]
Mills R. View 1. Corneal scraping and combination antibiotic therapy is indicated. Br J Ophthalmol 2003; 87:1167-1169. [ Links ]
Moore CP, Fales WH, Whittington P, Bauer L. Bacterial and fungal isolates form Equidae with ulcerative keratitis. J Am Vet Med Assoc 1983; 182:600-603. [ Links ]
Ollivier FJ. Medical and surgical management of melting corneal ulcers exhibiting hyperproteinase activity in the horse. In: Clinical Techniques in Equine Practice. Philadelphia: Elsevier; 2005. p. 50-71. [ Links ]
Ollivier FJ, Brooks DE, Kallberg ME, Komaromy AM, Lassaline ME, Andrew SE, Gelatt KN, Stevens GR, Blalock TD, Setten GV, Schultz GS. Evaluation of various compounds to inhibit activity of matrix metalloproteinases in the tear film of horses with ulcerative keratitis. Am J Vet Res 2003; 64:1081- 1087. [ Links ]
Ollivier FJ, Gilger BC, Barrie KP, Kallberg ME, Plummer CE, O'Reilly S, Gelatt KN, Brooks DE. Proteinases of the cornea and preocular tear film. Vet Ophthalmol 2007; 10:199-206. [ Links ]
Severin GA. Equine Ophthalmology. Proceedings of the Annual Convention of the AAEP 1998; 24:105-124. [ Links ]
Sivak JM, Fini ME. MMPs in the eye: emerging roles for matrix metalloproteinases in ocular physiology. Progress in Retina and Eye Research 2002; 21:1-14. [ Links ]
Solomon A, Rosenblatt M, Li DQ, Liu Z, Monroy D, Ji Z, Lokeshwar BL, Pflugfelder SC. Doxycycline inhibition of interleukin 1 in the corneal epithelium. IOVS 2000; 41:2544-2557. [ Links ]
Sweeney CR, Irby NL. Topical treatment of Pseudomonas spinfected corneal ulcers in horses: 70 cases (1977–1994). J Am Vet Med Assoc 1996; 209:954-957. [ Links ]
Twining SS, Fukuchi T, Yue BY, Wilson PM, Zhou X, Loushin G. Alpha 2-macroglobulin is present in and synthesized by the cornea. IOVS 1994; 35:3226-3233. [ Links ]
Whitley RD, Moore CP. Microbiology of the equine eye health and disease. Vet Clin North Am-Large Anim Pract 1984; 6;451-466. [ Links ]