Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Colombiana de Ciencias Pecuarias
Print version ISSN 0120-0690
Rev Colom Cienc Pecua vol.26 no.2 Medellín Apr./June 2013
ORIGINAL ARTICLES
Increase in post-thaw viability by adding seminal plasma proteins to Sanmartinero and Zebu sperm¤
Incremento en la viabilidad espermática post-descongelación por la adición de proteínas del plasma seminal a semen de toros Sanmartinero y Cebú
Aumento da viabilidade espermática pós-descongelamento, com a adição de proteínas do plasma seminal de sêmen de touros das raças Sanmartinero e Zebu
Fabián L Rueda1, Lic Química, MSc; Rocío F Herrera1, Zoot; Luis F Arbeláez2, Med, MSc, PhD; Tatiana Garcés2, Biol; Henry Velasquez3, MVZ, MSc; Miguel A Peña3, MVZ; Jaime A Cardozo1*, MVZ, MSc, PhD.
* Corresponding author: Jaime A Cardozo. Laboratorio de Microbiología Molecular, Centro de Biotecnología y Bioindustria, Corporación Colombiana de Investigación Agropecuaria CORPOICA. Numero de teléfono: 0057 1 4227300 ext 1312, email: jcardozo@corpoica.org.co, jcardozoc@gmail.com
1 Laboratorio de Microbiología Molecular, Centro de Biotecnología Y Bioindustria, Corporación Colombiana de Investigación Agropecuaria CORPOICA, Mosquera, Cundinamarca, Colombia.
2 Laboratorio del Grupo de Investigación en Química, Universidad de Pamplona, Ciudad Universitaria, Pamplona, Norte de Santander, Colombia.
3Centro de Reproducción Integral Animal (CRIA), Centro de Investigación La Libertad, Corporación Colombiana de Investigación Agropecuaria CORPOICA, Villavicencio, Meta, Colombia.
(Received: November 18, 2011; accepted: October 15, 2012)
Summary
Background: cryopreservation decreases sperm viability by approximately 50%. Objective: the objective of this study was to determine the effect of the addition of seminal plasma proteins on post-thawing sperm viability in Sanmartinero and Zebu semen. Methods: semen samples from 10 bulls of each breed were used, and seminal plasma was subjected to two-dimensional electrophoresis to establish the relationship between the relative amount of each protein spot and sperm viability. Then, seminal plasma was subjected to exclusion chromatography to separate the fraction containing these proteins. This fraction was added in doses of 0.5, 1.0, 1.5 and 2.0 mg, to 1 x 106. Sperm was thawed and incubated at 37 °C for 1 h to determine its effect on postthaw viability. Sperm were frozen using two media (citrate-fructose-yolk and Bioxcell®). Results: we found one protein spot (16.20 kDa, PI 5.5) in Sanmartinero seminal plasma that correlated (r = 0.64 p<0.001) with viability. This spot was found in 21-25 chromatography fractions. The percentage of post-thaw viable sperm increased 20% (p<0.05) at 1.0 and 1.5 mg of the fraction when sperm was frozen using citrate-fructose-yolk; it increased 25% (p<0.01) with 0.5 mg when it was frozen with Bioxcell® media. Addition of 0.5 mg of the fraction to semen cryopreserved with Bioxcell® resulted in a greater (p<0.05) percentage increase of viable sperm in Sanmartinero semen (23 ± 8.3%) compared with Zebu semen (6.0 ± 2.0%). Conclusions: these results show that seminal plasma proteins decrease cryopreservation damage in sperm. The effect depends on the cryoprotectant dose as well as the breed of bull.
Key words: creole bulls, cryopreservation, proteins, seminal quality, sperm membrane.
Resumen
Antecedentes: la criopreservación disminuye la viabilidad espermática por debajo del 50%. Objetivo: el objetivo de esta investigación fue determinar el efecto de la adición de proteínas del plasma seminal sobre la viabilidad espermática post-descongelación de semen de toros Sanmartinero y Cebú. Métodos: se colectó semen de 10 toros de cada raza, y el plasma seminal se sometió a electroforesis bidimensional, para establecer la relación entre la cantidad relativa de cada punto de proteína y la viabilidad espermática. Identificados dichos puntos, el plasma seminal se sometió a cromatografía de exclusión para separar la fracción que contenía estas proteínas. Esta se adicionó en dosis de 0,5, 1,0, 1,5 y 2,0 mg, a muestras de 1 x 106 espermatozoides, descongelados e incubados a 37 °C durante 1 hora, para determinar su efecto en la viabilidad post-descongelación. Los espermatozoides se congelaron usando dos medios (citrato-fructosa-yema y Bioxcell®). Resultados: se encontró un punto de proteína (16,20 kDa, punto Isoeléctrico 5,5) en plasma de toros Sanmartinero, que correlacionó (r = 0,64 p<0,001) con la viabilidad. Este punto de proteína se encontró en la fracción 21-25 de la cromatografía. El porcentaje de espermatozoides viables post-descongelación aumentó 20% (p<0,05) con dosis de 1 y 1,5 mg de la fracción, cuando los espermatozoides se congelaron en medio citrato-fructosa-yema; y 25% (p<0,01) con dosis de 0,5 mg cuando se congelaron en medio Bioxcell®. La adición de 0,5 mg de la fracción a semen descongelado previamente criopreservado en medio Bioxcell®, evidenció un incremento mayor (p<0,05) en el porcentaje de espermatozoides viables de semen de toros Sanmartinero (23 ± 8,3 %), que en semen de toros Cebú (6,0 ± 2,0%). Conclusiones: los resultados anteriores demuestran que las proteínas del plasma seminal disminuyen el daño en los espermatozoides por la criopreservación, y que el efecto de estas proteínas depende del medio de congelación, la dosis adicionada y la raza de los toros.
Palabras clave: calidad seminal, criopreservación, membrana espermática, proteínas, toros criollos.
Resumo
Antecedentes: a criopreservação diminui a viabilidade espermática abaixo de um 50%. Objetivo: o objetivo desta pesquisa foi determinar o efeito da adição de proteínas do plasma seminal na viabilidade espermática pós-descongelamento de sêmen de touros das raças Sanmartinero y Zebú. Métodos: coletou-se sêmen de 10 touros de cada raça, as amostras do plasma seminal foram submetidas à eletroforese bidimensional, para estabelecer a relação entre a quantidade relativa de cada ponto de proteína e a viabilidade espermática. Ao serem identificados os pontos, o plasma seminal também foi submetido ao processo de cromatografia por exclusão para separar a fração que continha as proteínas. A fração foi adicionada nas doses de 0,5, 1,0, 1,5 y 2,0 mg, amostras de 1 x 106 espermatozoides, em descongelamento e incubados à temperatura de 37 ° C durante 1 hora, para determinar o efeito na viabilidade pós-descongelamento. Os espermatozoides foram congelados utilizando dois meios (Citrato- frutose-gema e Bioxcell®). Resultados: encontrou-se um ponto de proteína (16,20 kDa, ponto Isoelétrico 5,5) no plasma de touro Sanmartinero, que correlacionou (r=0,64 p<0,001) com a viabilidade. Esse ponto de proteína foi encontrado na fração 21-25 da cromatografia. O percentagem de espermatozoides viáveis pós-descongelamento aumentou em 20% (p<0,05) nas doses de 1 y 1,5 mg da fração, quando os espermatozoides foram congelados em meio de citrato-frutose-gema; e 25% (p<0,01) com doses de 0,5 mg congelados em meio Bioxcell®. A adição de 0,5 mg da fração ao sêmen descongelado e previamente criopreservado em meio Bioxcell®, evidenciou um incremento maior (p<0,05) no percentagem de espermatozoides viáveis do sêmen de touros Sanmartinero (23 ± 8,3 %), do que em sêmen de touros zebu (6,0 ± 2,0%). Conclusões: os anteriores resultados demonstram que as proteínas do plasma seminal diminuem o dano nos espermatozoides pela criopreservação e que o efeito destas proteínas depende do meio de congelação, a dose adicionada e a raça dos touros.
Palavras chave: criopreservação, membrana espermática, proteínas, qualidade seminal, touros mestiços.
Introduction
During the cryopreservation process, distribution of phospholipids and proteins in spermatozoa membrane is altered. It causes premature sperm capacitation and decreased viability (Pursel, 1983). Seminal plasma (SP) is a vehicle to transport spermatozoa, and contains proteins whose functions and structures have allowed their use for increasing post thawing sperm viability (Killian et al., 1993; Barrios et al., 2000; Moura et al., 2006).
A group of acidic low molecular weight proteins (13-16 kDa) have been described to adsorb to plasma membrane, thereby regulating the capacitation process (Manjunath et al., 1987; Gwathmey, 2003; Manjunath et al., 2009). These proteins have been used in research to protect sperm against cold shock and oxidative stress (Calvete et al., 1996).
It has been shown that in vitro sperm incubation with low molecular weight SP proteins may counteract the deleterious effects observed in sperm cells subjected to cold shock or cryopreservation (Cross, 1993; Barrios et al., 2000). Although damage reduction has been attempted by using several diluents such as egg yolk—its LDL content has proved to have a cryoprotective effect (Pace and Graham, 1974; Moussa et al., 2002)—the use of egg yolk may increase the risk of microbiological contamination with the subsequent production of endotoxins.
Barrios (2000) demonstrated through scanning electron microscopy that a fraction of seminal plasma containing low seminal plasma proteins from rams has repairing effects on the membrane of sperm subjected to cold shock. That study also showed that in vitro incubation of ram sperm with low weight seminal plasma proteins increases the percent of sperm viability, compared with sperm incubated without proteins.
Indeed, it is possible that seminal plasma proteins could be used to reverse the damage caused by cryopreservation processes in spermatozoa of Sanmartinero Creole and Zebu bulls. The objective of the present study was to evaluate the addition of low molecular weight seminal plasma proteins to sperm on the percentage of post-thaw sperm viability for both breeds.
Materials and methods
Experimental units and seminal quality
Frozen semen from 10 Sanmartinero bulls and 10 Zebu bulls was used (3 straws from each bull). Animals were aged 3 to 4 years old and remained under Brachiaria decumbens grazing meadows at La Libertad research center of the Colombian Corporation for Agricultural Research (CORPOICA). Semen was collected by electroejaculation and seminal quality parameters were assessed (sperm viability, motility, and concentration). Sperm concentration was determined by spectrometry through a Spermacue (Minitube, Verona WI, USA). Sperm viability was measured through spermatozoa count subjected to Carboxy Fluorescein Diacetate (CFDA) and Propiduim Iodide staining (Harrison and Vickers, 1990) in which, under fluorescence microscopy, spermatozoa with integral membrane turns into a green color and spermatozoa with membrane damage turns into a red color. Sperm motility was determined through optical microscopy, analyzing 8 μl with 40x objective in several optical fields. All experiments including animals were approved by CORPOICA's bioethical committee act of SI-F-16 of February 7, 2005.
Semen cryopreservation
Cryopreservation was performed using two different diluents: citrate-fructose with egg yolk (CFY) and Bioxcell® (IMV Technologies, L'Aigle cedex, France). The CFY diluent was prepared using 300 mM Tris (Sigma, St Louis, MO, USA), 0.17M sodium citrate, 70 mM fructose (Sigma, St Louis, MO, USA), 100 IU/ ml penicillin, 1 mg streptomycin (MP Biomedicals INC, Eschwege, Germany), and 20% egg yolk (A fraction of diluent), while the B fraction was prepared with 86% of the A fraction and 14% glycerol (Mallinckrodt Baker, Xalostoc, México). Temperature was progressively decreased from 37 ºC to 5 ºC (approx. 0.5 ºC per min). When temperature reached 5 ºC the B fraction was added (same amount as the A fraction) and semen was maintained at this temperature during 4 h. Bioxcell® commercial diluent was used in the same way replacing fractions A and B for fractions 1 and 2, according to the manufacturer's instructions. Semen was frozen at 70 x 106 spz per milliliter in 0.5 ml straws sealed by ultrasound, and was carried in liquid nitrogen vapors during 10 min until -120 ºC was reached. Then, straws were introduced into liquid nitrogen until -196 ºC. Only semen with post-thaw progressive motility between 30 to 40% after 48 h was used, in agreement with the minimal frozen semen parameters described by Barth in 1990.
Seminal plasma separation and protein quantification
Seminal plasma was obtained through semen centrifugation at to 10000 g during 5 min at 4 ºC. Supernatant was centrifuged twice and the new supernatant was filtered through a 0.22 μm Millipore membrane. Then, 5 μM phenylmetylsoulfonyl fluoride (PMSF) was added (1.0 µl per milliliter of seminal plasma) as a protease inhibitor, and the mixture was stored at -20 ºC. Seminal plasma protein quantification was conducted through a NanoDrop spectrophotometer (Thermo Fisher Scientific, Schwerte, Germany) using molar absorption coefficient (ε1% = 6.67 M-1cm-1) of bovine serum albumin BSA (Pace et al., 1995).
Size exclusion chromatography
Seminal plasma proteins were separated by size exclusion chromatography (SEC) through a low pressure BioLogic LP chromatographic system (Bio-Rad, Hercules, CA, USA), using a 1 x 120 column filled with 79 ml of Sephacryl S-200 HR (Amersham Pharmacia Biotech, Piscataway, NJ, USA), previously washed with 100 ml of 0.1 M NaOH and 2 L water type III. Column was equilibrated with 0.5 M Phosphate Buffer pH 8.0 and the flow was increased from 0.25 ml/min to 1 ml/min. 1 ml of seminal plasma was added to the top of the column and eluted at a 0.5 ml/min flow. 4 ml fractions were collected. The protein amount of each fraction was measured through a NanoDrop spectrometer. Fractions with low weight seminal plasma proteins were concentrated through Amicon ultrafiltration cells (EMS Millipore Corp, Billerica, MA, USA) using cellulose membranes. Concentrated fractions were filtrated with 3 kDa micro filters to eliminate salts, lyophilized during 24 h, and stored at -20 ºC.
Unidimensional electrophoresis (1D SDS PAGE)
Fractions obtained by SEC were subjected to 1D SDS PAGE, according to the Laemmli method (Laemmli, 1970). 5 μg protein from fraction 21-25 were used, diluted 1:1 with denaturing buffer (20% Glycerol, 2% SDS, 0.5 M Tris-HCl pH 6.8, 10% β-mercaptoethanol, and 0.05% bromophenol blue). Samples were incubated at 98 ºC during 5 min and electrophoresis was conducted at 250 V, 0.04 Ω. Bands were visualized with Coomassie Brilliant Blue staining R-250 (Bio-Rad, Hercules, CA, USA).
Two-dimensional electrophoresis (2D SDS PAGE)
Fractions containing low molecular weight proteins from seminal plasma were subjected to 2D SDS PAGE. Separation was made, first by isoelectric point in an IEF protean (Bio-Rad, Hercules, CA, USA) using 8 cm pH immobilized strips with 4-7 pH range. Strips were hydrated overnight with 100 μg protein and hydration buffer (8M Urea Bio-Rad, Hercules, CA, USA; 4% p/v CHAPS Sigma, St. Louis, MO, USA; 50 mM dithiothreitol (DTT), and 2% v/v ampholytes Bio- Rad, Hercules, CA, USA).
Molecular weight separation was carried out in polyacrylamide gels with 10 to 20% gradient concentration. Strips were fixed to gels with 1% agarose containing 2% bromophenol blue. Run was done in a Bio-Rad Mini Protean Cell at 85 V during 180 min. Spots were visualized with Coomassie Brilliant Blue staining.
Gels scanning and image analysis
Images were captured trough a Gel Doc XR Gel Documentation system (Bio Rad, Hercules, CA, USA). Image analysis was performed through Quantity One and PD Quest software.
Protein addition to thawed semen
To estimate the effect of low molecular weight proteins in post-thaw sperm viability, each straw was separated in 1 million sperms per milliliter aliquots and was assigned to one treatment: T1 (no added protein), T2 (1.0 mg total proteins from seminal plasma), T3 (0.5 mg proteins separated by SEC), T4 (1.0 mg proteins), T5 (1.5 mg proteins) and T6 (2.0 mg protein. The fraction (called 21- 25) was re-suspended in Human Tubular Fluid Medium (mHTF). Each aliquot was incubated at 37 ºC during 1 h. At the end of incubation, sperm viability was determined through CFDA staining. Increase in sperm viability was calculated relative to T1 (without protein) and reverse of damage was determined through the equation developed by Barrios in 2000, as follows:
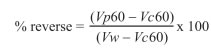
Where:
Vw = sperm viability of fresh sample before freezing process;
Vc60 = sperm viability of sample without proteins added;
Vp60 = sperm viability of sample after 1 h treatment
The percentage represents relation between cells with viability restored and cells theoretically recoverable.
Statistical analysis
A completely randomized factorial design (4 x 2) was used to analyze the data, according to the following model:
Yij= μ + αi + βj+ (αiβj)+Eij
Where:
Yij = recovery percentage;
µ = total media l;
αi: = doses effect of protein added;
βj = breed effect;
(αiβj) = interaction effect;
Eij = experimental error.
A Tukey comparison test was conducted to evaluate recovery percentage differences between treatments.
Results
Seminal plasma proteins separation by SEC and SDS PAGE
Chromatography showed five protein peaks (Figure 1). We found proteins with different molecular weight to determine separation grade and to identify interest proteins from 10-25 fraction.
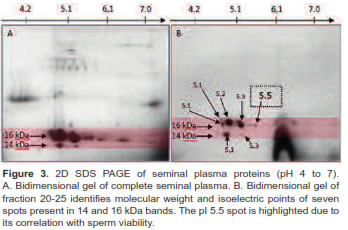
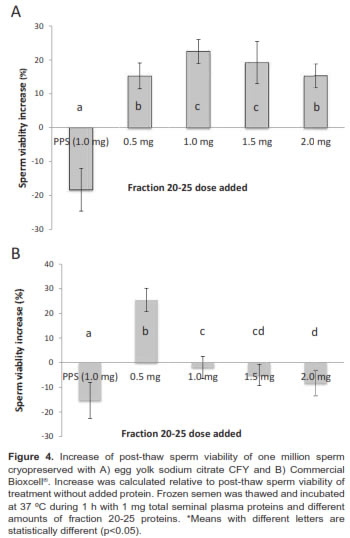
A concentrated sample from 20-25 fractions was identified as the fraction with low molecular weight proteins ranging from 14 to 16 KDa (Figure 2). Separation by isoelectric point demonstrates the presence of seven spots (Figure 3). In this study we used a concentrated from fractions 20-25 containing a protein spot of 15.46 kDa and a 5.5 isoelectric point. This spot has a positive correlation with sperm viability (r = 0.51 p<0.001) in Sanmartinero semen and similar characteristics of BSP proteins (Barajas et al., 2009).
Addition of fraction 21-25
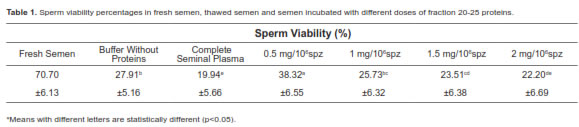
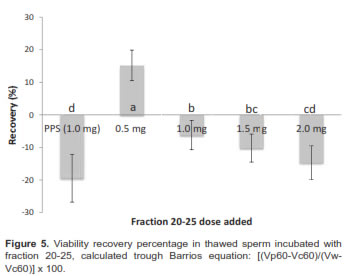
When we evaluated the effect of adding fraction 21-25 in different doses (0.5, 1, 1.5, and 2 mg/ml) to thawed sperm (previously cryopreserved with two different media) and incubated at 37 ºC during 1 h, we observed an increase of post-thaw sperm viability against samples without added proteins. Although dose effect depends on diluent, sperm viability post-thaw significantly decreased when complete seminal plasma was added to sperm previously frozen with CFY and Bioxcell® diluents (Figure 4).
The post-thaw sperm viability of treatment without protein added was 39.8 ± 8.2%. When sperm previously frozen with CFY was incubated with fraction 20-25 in concentrations between 1.0 and 1.5 mg, samples presented a 22.0 ± 6.5% sperm viability increase relative to samples without protein added. Doses greater and lesser than 1.5 mg (0.5 mg and 2.0 mg) were less effective.
Moreover, incubation of thawed samples (previously frozen with Commercial Bioxcell® medium) evidenced different results. 0.5 mg protein from fraction 21-25 was better to obtain a 25.2 ± 5.3% increase. Greater amounts showed a deleterious effect (e.g. when 1.0 mg of complete seminal plasma proteins was added).
We evaluated the reverse effect as the ability of proteins to regenerate sperm viability considering the damage since sample collection as a correction in the equation generated by Barrios (2000). This equation incorporates sperm viability before cryopreservation, which eliminates individual variance between animals.
Sperm viability of fresh samples was 70.7 ± 6.1% higher than post-thawed sperm viability (28.0 ± 5.2% decrease of approx. 40%). The addition of fraction 20-25, using 0.5 mg/million sperm and Bioxcell® as cryoprotector medium presented higher sperm viability compared with other doses (Table 1).
When analysis contemplates sperm viability decrease relative to fresh sample through equation [(Vp60-Vc60)/(Vw-Vc60)] x 100, it showed that the percentage of viability recovery was approximately 15.23% and it was only positive with a 0.5 mg dose (Figure 5).
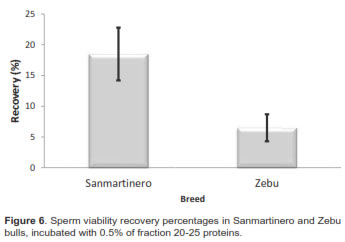
When differences between races were evaluated, the sperm viability decreased, due to the freezing process, from 70. 6 ± 7.4 % (fresh samples) to 29.8 ± 5.3% (thawed samples) in Sanmartinero semen (57.68%), while it decreased in Zebu from 70.8 ± 4.6% to 26.75 ± 4.3% (62.21%), differences being not significant. Comparing post-thaw sperm viability of samples incubated with 0.5 mg protein from fraction 20-25 between breeds, sperm viability from Sanmartinero bulls increased to 38 %, while no significant effect was observed in Zebu semen.
In regard to recovery percentage compared between breeds, when 0.5 mg protein from fraction 20-25 was added, recovery in Sanmartinero bulls was 18.03% while it was 6.8% in Zebu bulls significantly different (p<0.05).
Discussion
Addition of seminal plasma proteins from fraction 20-25 to thawed sperm
A chromatographic fraction of seminal plasma proteins containing one protein spot of 16.20 kDa and pI 5.5 was used in this work, similar with those reported for BSP proteins (Moura et al., 2006). When samples were evaluated without breed discrimination a 20% increase in post-thaw sperm viability was observed when 1.0 to 1.5 mg per million of spermatozoa were used. This is the optimal dose when CTY was used. However, there was a similar effect of viability increase when using a lower dose (0.5 mg per million of spermatozoa) combined with commercial Bioxcell® use. This indicates the possibility that Bioxcell® medium contains some substance that interacts with seminal plasma proteins from fractions 21-25.
Furthermore, the addition of complete seminal plasma proteins seems to have the opposite effect, decreasing sperm viability after thawing. This is in agreement with the report by Baas (1983) which demonstrated that sperm integrity and motility can regenerate only when seminal plasma was subjected to ultrafiltration and protein fraction with molecular weight was lower than 50 kDa. The same work established that an excess of complete seminal plasma has a negative effect on sperm viability and also that the presence of low molecular weight proteins has a stimulating effect on bovine semen viability.
Barrios (2000) established that adding complete seminal plasma proteins did not decrease postthaw sperm viability; it had a lower effect relative to proteins between 14 and 20 kDa. His work also demonstrated that addition of proteins larger than 60 kDa did not have a recovery effect in sperm membrane after a cold shock.
Moreover, low weight seminal plasma proteins prevent deleterious effects when they are added before the cryopreservation process. A work with ram semen demonstrated that protein addition before decreasing sperm temperature to 5 ºC increased semen viability from 26% to 34%, preserving membrane integrity (Perez-Pe et al., 2001) and showing a reverse and protective effect against cold shock. Data showed a 15% percent recovery when 0.5 mg/million sperm was used. Greater doses had no effect or negatively affected post-thaw sperm viability. Recovery studies with ram semen showed the optimal dose to obtain high recovery percentages was 0.5 mg/million sperm. However, greater doses up to 2.5 mg/million sperm did not show negative effects (Barrios, 2000). It has been shown that seminal plasma can repress and revert sperm capacitation, in other words inactivate sperm previously capacitated (Cross, 1993). This may be due to an adsorption effect of some seminal plasma proteins, such as the BSP proteins (possibly contained in fraction 21-25). These proteins bind to membrane phospholipids (Manjunath et al., 2009; Manjunath, 1987; Manjunath et al., 1993; Souza et al., 2008; Moura et al., 2007; Swamy, 2004), which could explain the recovery effect observed when fraction 20-25 protein was added. Since BSP are major seminal plasma proteins (Manjunath et al., 2009; Moura et al., 2007; Manjunath, 1997) their structure, amount in seminal plasma, and interaction with female tract fluids regulate sperm capacitation. It might be possible that a higher dose of these proteins prematurely capacitate instead of inhibiting sperm capacitation, which could explain the negative effect when doses greater than 0.5 ml/ million sperm from fraction 20-25 were added. This indicates that although there is a recovery effect, it depends on the doses added.
Post-thaw sperm viability and recovery of sperm viability in Sanmatinero and Zebu semen
It has been reported that sperm percentage with undamaged membrane dramatically decreases after freeze-thaw processing. This deleterious effect has been demonstrated in ovine (Ashworth and Harrison, 1994; Watson, 2000; Gunay et al., 2006) and bovine semen (Jobim et al., 2004, Rubio et al., 2009). In bull semen, it was found that the decrease of sperm viability can reach between 40% and 60% during evaluation processes between sample collection and post-thaw (Rubio et al., 2009).
When differences in sperm viability decrease after thawing between Sanmartinero and Zebu breeds were evaluated, it was determined that Sanmartinero samples decreased from 70.6 ± 7.4% to 29.8 ± 5.3% after thawing (57.68%). Meanwhile, Zebu semen viability decreased from 70.8 ± 4.6% to 26.7 ± 4.3% (62.2%). No significant differences in viability after thawing were observed in either breed. It was possible to demonstrate that the addition of a low molecular weight protein group was only effective for the recovery of sperm membrane damage when it was added to Sanmartinero semen. Even if there was an effect on Zebu semen, it was not enough to be considered significant.
In conclusion, the use of fractions 20-25 proteins in cryopreserved sperm, thawed and incubated for 1 h at 37°C, increased the percentages of post-thaw sperm viability when cryopreservation media were used. However, the minimal dose required to have a significant effect varies according to the diluent used, indicating that the increase in post-thaw sperm viability as well as the dose required of fraction 20-25 depends on the diluent used for sperm cryopreservation. Furthermore, we were able to demonstrate that when using a commercial freezing medium, post-thaw viability of samples subjected to doses greater than 0.5 mg per million sperm, decreased significantly, probably due to interactions between proteins and commercial additives.
Although there is a reversion effect on the damage caused by cryopreservation on sperm membrane, Sanmartinero sperm was more capable of improving its viability when incubated with fraction 20-25, in comparison with Zebu sperm. This may be because decrease in post freezing sperm viability in Zebu bulls is higher than Sanmartinero.
Notes
¤ To cite this article: Rueda FL, Herrera RF, Arbeláez LF, Garcés T, Velasquez H, Peña MA, Cardozo JA. Increase in post-thaw viability by adding seminal plasma proteins to Sanmartinero and Zebu sperm. Rev Colomb Cienc Pecu 2013; 26:98-107.
Acknowledgements
The authors give special thanks to Samuel Correa, Gustavo Quijano, and Eliana Neira. This work was funded by the Ministry of Agriculture and Rural Development (MARD, agreement 517- 2007), the Colombian Corporation of Agricultural Research, the National Livestock Federation (FEDEGAN), and the Inter-American Institute for Cooperation on Agriculture (IICA).
References
Ashworth PJC, Harrison RAP, Miller NGA, Plummer JM, Watson PF. Survival of ram spermatozoa at high dilution: protective effect of simple constituents of culture media as compared with seminal plasma. Reprod Fert Develop 1994; 6:173-80. [ Links ]
Baas JW, Molan PC, Shannon P. Factors in seminal plasma of bulls that affect the viability and motility of spermatozoa. J Reprod Fertil 1983; 68:275-80. [ Links ]
Barajas DP, Coy P, Velásquez JG, Rueda F, Herrera R, Cardozo JA. Proteínas del plasma seminal asociadas con la fertilidad de toros criollos Sanmartinero y Cebú en condiciones del trópico colombiano. Memorias Simposio de recursos genéticos para América Latina y el Caribe SIRGELAC 2009; 153-154. [ Links ]
Barrios B, Perez-Pe R, Gallego M. Seminal plasma proteins revert the cold-shock damage on ram sperm membrane. Biol Reprod 2000; 63:1531-1537. [ Links ]
Barth AD. Evaluación de semen bovino congelado. CABIA 1990; 21:28-36. [ Links ]
Calvete J, Varela P, Sanz L, Romero A. A procedure for the large-scale isolation of major bovine Seminal plasma proteins. Protein Expres Purif 1996; 8:48-56. [ Links ]
Cross NL. Multiple effects of seminal plasma on the acrosome reaction of human sperm. Mol Reprod Dev 1993; 35:316-323. [ Links ]
Gunay U, Dogan I, Nur Z. Influence of bull seminal plasma on post-thaw ram semen parameters and fertility. B Vet I Pulawy 2006; 50:503-507. [ Links ]
Gwathmey TM. PDC-109 (BSP-A1/A2) promotes bull sperm binding to oviductal epithelium in vitro and may be involved in forming the oviductal sperm reservoir. Biol Reprod 2003; 69:809-815. [ Links ]
Harrison RA, Vickers S. Use of fluorescent probes to assess membrane integrity in mammalian spermatozoa. J Reprod Ferti 1990; 88:342-353. [ Links ]
Jobim M, Oberst E. Two-dimensional polyacrylamide gel electrophoresis of bovine seminal plasma proteins and their relation with semen freezability. Theriogenology 2004; 61:255- 266. [ Links ]
Killian J, Chapman D, Rogowsky L. Fertility-Associated Proteins in Holstein Bull Seminal Plasma. Biol Reprod 1993; 49:1202-1207. [ Links ]
Manjunath P, Sairam M. Purification and Biochemical characterization of three major acidic proteins (BSP A1, A2 y A3) in bovine seminal plasma. J Biochem 1987; 241:685-692. [ Links ]
Manjunath P. Purification of four gelatin-binding proteins from bovine seminal plasma by affinity chromatography. Biosciences Rep 1987; 7:231-238. [ Links ]
Manjunath P, Chadonet E. Major proteins of bovine seminal vesicles bind to spermatozoa. Biol Reprod 1993; 49:27-37. [ Links ]
Manjunath P, Therien I. Role of seminal plasma phospholipids binding proteins in seperm membrane lipids modification that occurs during capacitation. J reprod immunol 2002; 53:109-19. [ Links ]
Manjunath P, Lefebvre J, Wright W. New Nomenclature for Mammalian BSP Genes. Biol reprod 2009; 80:394-397. [ Links ]
Moura A, Koc H. Identification of proteíns in accessory Sex Gland Fluid associated with fertility indexes of dairy bulls. J Androl 2006; 27:201-212. [ Links ]
Moura A, Chapman D, Koc H, Killian G.A. Comprehensive proteomic analysis of the accessory sex gland fluid from mature Holstein bulls. Anim Reprod Sci 2007; 98:169-188. [ Links ]
Moussa M, Matinet V, Trimeche A, Tainturier D, Anton M. Low density lipoproteins extracted from hen egg yolk by an easy method: cryoprotective effect on frozen-thawed bull semen. Theriogenology 2002; 57:1695-706. [ Links ]
Pace MM, Graham EF.Components in egg yolk which protect bovine sperm during freezing. J Anim Sci 1974; 39:1144-1149. [ Links ]
Pérez-PeR, Cebrián-Pérez JA, Muiño-Blanco T. Semen plasma prevent cold shock membrane damage to ram spermatozoa. Theriogenology 2001; 56:425-435. [ Links ]
Pursel VG. Effect of processing of semen on capacitation time of fresh and frozen-thawed boar spermatozoa. J Anim Sci 1983; 56:1161-1166. [ Links ]
Rubio J, Quintero A, Gonzales D. Effect of Cryopreservation on Integrity of Plasmatic and Acrosomal Membrane of Bulls Sperm. Rev Cient Fac Cien V 2009; 29:382-389. [ Links ]
Sanchez S. Interaction of PDC 109, the major secretory proteins from bull seminal vesicles, with bovine sperm membrane. J Androl 2004; 25:234-244. [ Links ]
Swamy M. Interaction of bovine seminal plasma proteins with model membranes and sperm plasma membranes. Curr Sci India 2004; 87:203-211. [ Links ]
Watson PF. The causes of reduced fertility with cryopreserved semen. Anim Reprod Sci 2000; 60-61:481-492. [ Links ]