Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Colombiana de Ciencias Pecuarias
Print version ISSN 0120-0690
Rev Colom Cienc Pecua vol.26 no.2 Medellín Apr./June 2013
ORIGINAL ARTICLES
Intestinal expression of pro-infiammatory cytokines induced by oral intake of lipopolysaccharide (LPS) from E. coli in weaned pigs¤
Expresión intestinal de citoquinas proinflamatorias inducidas mediante ingestión de lipopolisacáridos de E. coli en lechones recién destetados
Expressão intestinal de citocinas pró-inflamatórias induzida pela ingestão de lipopolissacarídeo de E. coli em leitões recém desmamados
Jaime Parra S1, Zoot, MSc, PhD; Jorge Agudelo T2, Zoot, PhD; David Sanín P3, Ing Biol, PhD (c); Jorge Forero D1, Bact, MSc, PhD(c); Carlos Muskus L4, Bact, MSc, PhD; Albeiro López Herrera1, Zoot, MV, MSc, DrSci.
* Corresponding author: Jaime Parra. Universidad Nacional de Colombia, Facultad de Ciencias Agropecuarias, Departamento de Producción Animal, AA 1779, Medellín, Colombia. e-mail: jeparrasu@unal.edu.co
1 Grupo de investigación BIOGEM, Departamento de Producción Animal, Universidad Nacional de Colombia Sede Medellín, Facultad de Ciencias Agropecuarias, AA 1779, Medellín, Colombia.
2 Grupo de investigación GRICA, Facultad de Ciencias Agrarias, Universidad de Antioquia, AA 1226, Medellín, Colombia.
3 York University, England.
4 Grupo de Investigacion PECET, Facultad de Ciencias Básicas Biomédicas, Universidad de Antioquia, AA 1226, Medellín, Colombia.
(Received: June 14, 2012; accepted: March 14, 2013)
Summary
Background: treatments with lipopolysaccharide (LPS) from E. coli are an accepted way of inducing inflammation in immunological studies since they have the ability to activate a coordinate series of signs through the synthesis of pro-inflammatory cytokines such as interleukin 8 (IL-8), IL-18 and tumor necrosis factor alpha (TNF-α), which can cause significant changes in intestinal structure and functionality. Objective: the aim of this study was to evaluate the effect of adding LPS of E. coli on pro-inflammatory cytokine gene expression IL-8, IL-18 and TNF-α in early-weaned pigs. Methods: fieldwork was conducted at Centro San Pablo, belonging to the Universidad Nacional de Colombia with 32 pigs at 21 days of age, with 6.5 ± 0.5 kg of weight. Animals were fed with a basal diet supplemented with two levels of inclusion of LPS of E. coli serotype 0111:B4 (0 to 0.3 μg/mg of food). Pigs were slaughtered in stages on days 1, 5, 7 and 10 postweaning and complete extraction of small intestine was made. Gene expression was evaluated by qPCR. Blocks at random in a factorial arrangement 2 x 4 were used as statistical design. Results: the basal diet (without addition of LPS) presented increase in mRNA expression (p<0.01) of all the cytokines in jejunum for each post-weaning day, which suggests an inflammatory response and extensive tissue damage in pigs after early weaning. In diet 1 (with consumption of 0.3 μg LPS / mg diet) cytokines TNF-α, IL-8 and IL- 18 showed a significant increase in their levels of expression (p<0.01). All cytokines presented significant increase (p<0.01) in jejunum for each post-weaning day. Conclusion: The increase observed in the expression of TNF-α can be involved in the development of post-weaning diarrhea.
Key words: gut immunology, intestinal villi, pig weaning, RT-PCR, TNF-α.
Resumen
Antecedentes: los tratamientos con lipopolisacáridos (LPS) de E. coli son una forma aceptada para inducción de inflamación en estudios inmunológicos ya que tienen la capacidad de activar una gran variedad de rutas de señalización a través de la síntesis de citoquinas proinflamatorias, tales como interleuquina 8 (IL- 8), IL-18 y factor de necrosis tumoral alfa (TNF-α), las cuales pueden provocar cambios importantes en la estructura y funcionalidad intestinal. Objetivo: el presente estudio tuvo como objetivo evaluar el efecto de la adición de LPS de E. coli sobre la expresión génica de las citoquinas proinflamatorias IL-8, IL-18, y TNF-α en lechones recién destetados. Métodos: este trabajo se realizó con 32 lechones de 21 días de edad, con un peso de 6,5 ± 0,5 kg en el Centro San Pablo, perteneciente a la Universidad Nacional de Colombia. Los animales fueron alimentados con una dieta basal adicionada con dos niveles de inclusión de LPS de E. coli serotipo 0111:B4 (0 y 0,3 μg/mg de alimento). Los animales se sacrificaron escalonadamente los días 1, 5, 7 y 10 posdestete, y se realizó extracción completa del intestino delgado. Se evaluó la expresión génica por qPCR. El diseño estadístico empleado fue de bloques al azar en un arreglo factorial de 2X4. Resultados: la dieta basal (sin adición de LPS) presentó incremento en la expresión de mRNA (p<0,01) de todas las citoquinas en yeyuno para cada día posdestete, lo que sugiere una respuesta inflamatoria y grandes daños tisulares en el lechón luego del destete precoz. En la dieta 1 (con consumo de 0,3 µg LPS /mg dieta) las citoquinas TNF-α, IL-8, e IL-18 presentaron incrementos significativos en sus niveles de expresión (p<0,01). Todas las citoquinas presentaron incremento significativo (p<0,01) en yeyuno para cada uno de los días posdestete. Conclusión: el incremento observado en la expresión de TNF-α puede estar implicado en el desarrollo de diarreas posdestete.
Palabras clave: destete, inmunologia intestinal, RT-PCR, TNF-α, vellosidad intestinal.
Resumo
Antecedentes: os tratamentos com lipopolissacarídeos (LPS) de E.coli são uma forma aceita de indução de inflamação para estudos imunológicos quanto que têm a capacidade de ativar uma grande variedade de vias de sinalização através da síntese de citocinas pró-inflamatórias tais como a interleucina 8 (IL-8), IL-18 e fator de necrose tumoral alfa (TNF-α), o que pode causar alterações importantes na estrutura e funcionalidade do intestino. Objetivo: o presente estudo teve como objetivo a adição de LPS de E. coli sobre a expressão génica das citocinas pró-inflamatórias IL-8, IL-18 e TNF-α em leitões desmamados. Métodos: este trabalho foi feito com 32 leitões de 21 dias de idade, com um peso de 6,5 ± 0,5 kg no Centro San Pablo, pertencente à Universidade Nacional de Colômbia. Os animais foram alimentados com uma dieta basal adicionada com dois níveis de inclusão de LPS de E. coli serotipo 0111:B4 (0 y 0,3 μg/mg de alimento). Os animais foram sacrificados nos dias 5, 7 e 10 após o desmame, e foi realizada a remoção completa do intestino delgado. A expressão do gene foi avaliada por qPCR. O desenho estatístico empregado foi de bloco ao acaso num fatorial de 2x4. Resultados: a dieta basal (sem LPS adicionado) mostrou aumento da expressão de mRNA (p<0,01) de todas as citocinas no jejuno para cada dia após o desmame, sugerindo uma resposta inflamatória e grande danos teciduais no porco após o desmame precoce. Na dieta 1 (com um consumo de 0,3 mg de LPS / mg dieta) citocinas TNF-a, IL-8 e IL-18 mostraram aumentos significativos nos níveis de expressão (p<0,01). Todas as citocinas mostraram aumento significativo (p<0,01) no jejuno, para cada um dos dias após o desmame. Conclusão: o aumento observado na expressão de TNF-α pode estar envolvido no desenvolvimento de diarreia pós-desmame.
Palavras chave: desmame, imunologia intestinal, RT-PCR, TNF-α, vilosidades intestinais.
Introduction
The intestinal epithelium plays an active role in defending the body against infections. It serves as a dynamic barrier, regulating the uptake of nutrients and water, and preventing the absorption of bacteria and endotoxins normally found in the intestinal lumen (Pie et al., 2004). Intestinal epithelial cells can activate immune and inflammatory responses by producing cytokines—peptides that are crucial for the recruitment and activation of neutrophils, macrophages, T and B cells and dendritic cells (Pie et al., 2004). Some cytokines -such as interleukin 8 (IL-8), IL-18, and tumor necrosis factor (TNF-α)- are constitutively expressed by intestinal epithelial cells under normal physiological conditions. However, they are abundantly over-expressed in response to microbial infections (Stadnyk, 2002; Gopal et al., 2008) and during various physiological processes triggered by the weaning (Pie et al., 2004; Garriga et al., 2005).
Early weaning is considered to have a negative impact on pig performance (Gomez et al., 2008). Immediately after weaning, pigs enter a fasting period that leads to a reduction of gastrointestinal tract lactobacilli, thus increasing E. coli populations, which in turn release pro-inflammatory lipopolysaccharide (LPS) from their cell walls (Amador et al., 2007). The LPS are recognized by specific receptors on the cell membrane and activate various cellular signaling pathways (Chow et al., 1995).
Once initiated, this cascade of reactions can lead to a septic state and to an unregulated systemic response that may progress to failure of multiple organs. A large number of cytokines have been implicated in such adverse effects, including TNF-α and interleukin (IL) (Asaduzzaman et al., 2008). The release of these pro-inflammatory cytokines causes changes in the structure and functional capacity of the intestine (García-Herrera et al., 2008).
The administration of LPS from E. coli is one of the most used models to study acute infection processes, because LPS response is highly reproducible, without the side effects commonly associated with chronic infections (Albin et al., 2007). This study aimed to evaluate the effect of oral administration of LPS from E. coli on gene expression of pro-inflammatory cytokines IL-8, IL-18 and TNF-α in weaned pigs.
Materials and Methods
Ethical considerations
All experimental procedures were conducted according to the guidelines suggested by the International Guiding Principles for Biomedical Research Involving Animals (CIOMS, 1985). This research was approved by the Ethics Committee for Animal Research of the Universidad Nacional de Colombia, Medellín (CEMED 001 Act of January 26, 2009).
Location
The animal work was conducted at the San Pablo Center of the Universidad Nacional de Colombia, Medellín, located in Rio Negro, Antioquia, at 2,100 meters of altitude, corresponding to tropical lowermontane wet forest (LM-wf) zone, with average annual temperatures between 12 and 18 °C.
Animals and diet
A total of 32 piglets were used; animals were a Duroc x Landrace crossbred and, each was weaned exactly at 21 days of age, weighing 6.5 ± 0.5 kg. The pigs were housed in collective cages (four animals per cage) located in a room with controlled temperature (26 ± 3 °C). The animals were supplied water ad libitum throughout the experiment. The basal diet (BD) offered contained milk and milk derivatives, and was enriched with vitamins, minerals, and lysine-HCL. The diet was balanced to meet all the minimum nutritional requirements proposed by NRC (1998) (Tables 1 and 2). Two experimental diets were evaluated. The control diet (basal) was compared against the same diet added with LPS of E. coli in concentrations of 0.3 µg/mg of feed. Each pen was offered 300 g feed/day. However, extra feed was offered when pigs required it. The experimental diets were offered from days 1 to 10 after weaning. No solid food was offered to the piglets during lactation.
Treatments
Two experimental diets were tested: a basal diet (BD) and the BD added with LPS of E. coli (serotype 0111: B4; Sigma-Aldrich, St. Louis, MO, USA) as follows:
1. BD: Basal Diet (BD; no addition of LPS from E. coli).
2. BD+LPS: BD added with 0.3 μg of LPS from E. coli/mg of feed.
Clinical evaluation
Before starting the experiment, all animals were clinically evaluated and laboratory monitored to avoid including previously sick or diarrheic pigs. Such monitoring was also conducted three times daily throughout the study, keeping records of any health alteration. Rectal temperature was measured daily at 8:00 am using a rectal mercury thermometer. The reference temperature was 38 °C. Consistency of the stools was daily assessed by direct observation, assigning them a number from 0 to 3 according with fecal appearance: A 0 value indicates normal feces, and therefore no diarrhea presence. A value of 1 describes a slightly pasty diarrhea, 2 value indicates a moderate, semiliquid diarrhea, and 3 value severe, very liquid diarrhea. Daily grades during the experimental period were added together and an index of diarrhea severity was estimated (Reis de Souza et al., 2010). This index is expressed by the following equation:
DSI = dFC/Ep
Where:
DSI= Diarrhea severity index.
dFC= Daily fecal consistency grade.
Ep= Experimental period (days).
Sampling of intestinal tissue
The pigs were slaughtered as follows: eight pigs were slaughtered on the weaning day to check the overall health status of the organs before LPS consumption. Two more pigs from each treatment were slaughtered on days 5, 7, and 10 after weaning. All piglets were slaughtered 2.5 hours after their last meal. Pigs were sedated via CO2 inhalation for 3 minutes before being humanely killed by exsanguination through sectioning of the jugular vein. The small intestine was removed from the pyloric junction to the ileocecal valve. The mesentery was longitudinally cut with blunt scissors. Then, the intestine was weighed, measured and lined up without any strain over a table, and divided into three segments (duodenum, jejunum, and ileum) of equal size. 20 cm were cut from the center of each segment, and the digesta was removed by rinsing it with cold saline infusion (Makkink et al., 1994; Reis et al., 2005). Subsamples (1 cm) were then obtained from each segment and were prepared for total RNA extraction. Samples were preserved in liquid nitrogen and RNA was stored in a freezer at -70 oC until laboratory analyses.
RNA extraction
Total RNA was extracted from intestinal samples (25 mg) using a commercial kit (Tissue & Cells ULTRACLEANTM RNA Isolation Kit, MO, USA; BIO Laboratories Inc., San Diego, CA, USA). The tissue was macerated in stone mortars using liquid nitrogen. Work surfaces were previously treated with diethylpyrocarbonate (DEPC) to remove possible RNases. The RNA samples were kept stored at -70 °C throughout the experiment and only aliquots on ice were taken for analysis. Integrity of the extracted material was evaluated running the samples on 1% agarose gel with ethidium bromide staining (100V for 50 min). Ribosomal RNA (rRNA) bands were observed, along with the presence of messenger RNA (mRNA). The extraction was repeated under identical conditions when enough amount of material was not observed, or not clear rRNA bands were detected.
RNA quantification
Purified samples of total RNA were quantified using 1μL of the solution in a Nanodrop 1000 (Thermo Fisher Scientific Inc, Waltham, MA, USA). It was assessed at a rate of 260/230 in order to detect the presence of any potential pollutants. The solution in which RNA was stored during the last extraction step was used as a blank.
Complementary DNA (cDNA) synthesis
The cDNA was synthesized from total RNA using a commercial kit (QuantiTect® Reverse Transcription Kit; QIAGEN, Valencia, CA, USA). This kit uses random hexamer and poly-thymidine primers to maximize the retro-transcription reaction, obtaining both rRNA and mRNA. The same amount of sample was used for all reverse transcription reactions (1μg of RNA in each reaction) based on the quantification results. The remaining genomic DNA was removed followed by the addition of enzyme and triphosphate nucleotides to synthesize cDNA according to manufacturer's recommendations. The obtained cDNA was stored at -20 °C.
Cytokine mRNA quantification
The primers for the genes of interest and constitutive expression of rRNA are shown in table 3. These primers were taken from the literature, verifying that its design had been carried out at the edges intron/exon to avoid amplification from genomic DNA. Additionally, they underwent several bioinformatic analyses to verify their specificity with the mRNA or rRNA fragment studied and the absence of secondary structures, such as the formation of lenses or dimers with themselves or with the other primer. Additionally, primers with Tm above 60 °C were used to amplify fragments with less than 150 base pairs, as several researchers have recommended (Bustin, 2000; Kubista et al., 2006).
A commercial kit was used for the qPCR (QuantiFastTM SYBR® Green PCR kit, QIAGEN, Valencia, CA, USA) in a Smart Cycler® (Cepheid Inc., Sunnyvale, CA, USA). All qPCR reactions were run with 0.1 µM of each primer and 1 µL of cDNA, starting with a five-minute activation of the enzyme and then 40 cycles with two stages. The first stage was held at 95 °C for 15 seconds. The second stage was held at 60 °C for 30 seconds (62 °C for 28s rRNA). A denaturation curve was generated at the conclusion of the run to find the Tm of the amplified fragment and confirm its identity.
Standardization strategy, data management and relative quantification
A relative quantification method was used to determine the level of expression of each gene. The method included the use of a constitutively expressed gene (28s ribosomal RNA) following the recommendations of several researchers regarding the choice of gene and overall standardization strategy (Dheda et al., 2005; Huggett et al., 2005; Muller et al., 2002; Vandesompele et al., 2002). The amounts of material from which RNA was extracted, the amount of RNA in reverse transcription reaction, and the amount of cDNA for PCR amplification were identical for all samples. The chosen constitutive expression gene was validated in previous studies under similar experimental conditions (Paulin et al., 2007). The results were analyzed with the assumption-free method, which employs fluorescence data for each qPCR reaction to establish the reaction zone in which there was exponential kinetics (Ramakers et al., 2003). Based on this region, a linear regression was established that fit the data thereby obtaining the reaction efficiency from the slope of the line and the intercept (N0) as an estimate of the initial concentration in terms of fluorescence of SYBR Green. The software recommended by the equipment manufacturer was used for these calculations (LinRegPCR 11.0). The N0 values of each cytokine gene were expressed in arbitrary fluorescence units and were normalized using the corresponding N0 value for the constitutive expression gene in each animal.
Statistical design
The experiment followed a randomized block design (two blocks) in a 2 x 4 factorial arrangement (two experimental diets by four periods after weaning; Steel and Torrie, 1985). Animals were blocked by initial weight. Each animal was assigned one of eight treatments and each treatment had four replicates. Statistical analysis was conducted using the General Linear Model procedure (GLM) of SAS (2006). Differences between treatment means were determined by least squares and analyzed by ANOVA. The Duncan test was used to compare treatment means (p<0.05).
Results
Pigs fed the basal diet maintained normal body temperature, while animals that received LPS had rectal temperatures above 38 °C throughout the experiment. However, pigs fed LPS did not show symptoms of disease, so none required slaughter or exclusion from the experiment. No leftovers were observed during the experiment.
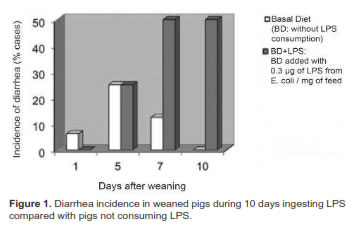
Both treatments had increasing numbers of diarrhea cases during the first 5 days after weaning (Figure 1). Beginning on day 5, diarrhea incidence started to decline in BD, reaching its lowest level on day 10 after weaning. The opposite occurred in pigs exposed to LPS (BD+LPS), in which diarrhea continued to increase from the fifth day, rapidly reaching its peak at day 7, and remaining high until the end of the test (day 10). The incidence of diarrhea was much higher in BD+LPS compared with BD, particularly from day 5 until day 10 after weaning (Figure 1).
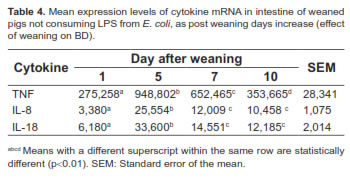
An increase in mRNA expression of cytokines TNF-α, IL-8, and IL-18 was observed from days 1 to 5 after weaning (p<0.01; Table 4). All variables under study showed a partial recovery from days 5 to 10. However, a statistical difference was observed between days 1 and 10 (p<0.01).
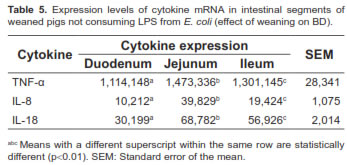
The expression of these cytokines in different parts of the small intestine of pigs not consuming LPS was also assessed. Significant differences (p<0.01) were found in transcription levels of cytokines for selected intestinal segments. The middle part of the intestine (jejunum) showed the highest gene expression for all cytokines (Table 5).
No statistical interaction was observed between LPS concentration and post-weaning period of slaughtering for any of the variables under the present study, therefore it was unnecessary to analyze those two factors independently.
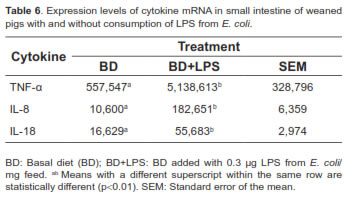
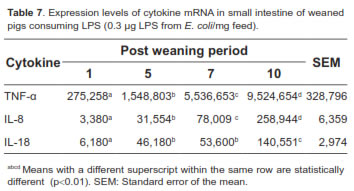
Tables 6, 7 and 8 show the overall mRNA cytokine expression in both treatments, cytokine expression according to the length of exposure to LPS, and the expression in the intestinal segments of pigs consuming LPS, respectively. The expression of cytokines TNF-α, IL-8, and IL-18 was significantly higher for BD+LPS (p<0.01; Table 6). Intestinal production of TNF-α, IL-8 and IL-18, as measured by mRNA expression, significantly raised with increasing exposure to LPS (p<0.01), reaching its peak on day 10 post-weaning (Table 7).
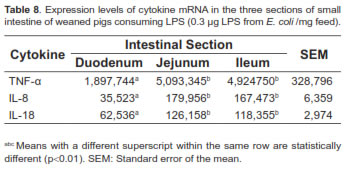
The middle intestine (jejunum) of piglets consuming LPS showed higher cytokines production, particularly TNF-α, whereas duodenum had the lowest production (Table 8). However, there were no statistically significant differences between jejunum and ileum for the different variables studied (p>0.01).
Discussion
Literature is scarce on reports evaluating gene expression of proinflammatory cytokines in weaned piglets. However, in vivo and in vitro studies have shown that after a bacterial infection in pigs, LPS has the ability to activate a variety of signaling pathways through the uncontrolled synthesis of proinflammatory cytokines such as IL-8, IL-18 and TNF-α (Johnson et al., 2005), which can influence the integrity and functional capacity of intestinal cells, permeability to macromolecules and transport of ions and nutrients (Bauer et al., 2006; Liu et al., 2008). Total, efficient, and proper intestinal recovery may take several weeks (Yoo et al., 2000; García-Herrera et al., 2008).
Proinflammatory cytokines play an important role in intestinal physiology and may act on the intestinal barrier increasing epithelial ion secretion, specifically chlorine (Cl-). The increased secretion of Cl- in the crypts and decreased absorption of sodium (Na+) by the intestinal villi promote fluid loss in the small intestine. Both processes depend on the Na+ / K+-ATPase, located in the basolateral membrane and whose functional activity is affected by TNF-α (McKay and Baird, 1999; Pie et al., 2004). Gene expression of TNF-α decreases the Na+ / K+-ATPase activity (Yu and Perdue, 2000) causing fluid accumulation in the small intestine. These factors combined appear to trigger bacterial endotoxin induced diarrhea in rabbits and pigs (García-Herrera et al., 2003, 2008).
The increased expression of TNF-α observed in this study may contribute to the development of post-weaning diarrhea; this factor stimulates Clsecretion in the ileum (Musch et al., 2002; Bertelsen et al., 2004) and colon (Schmitz et al., 1996).
In addition to its role in epithelial ion transport, proinflammatory cytokines are also involved in the integrity of the intestinal tissues (Williams, 2001), altering intestinal permeability through its effect on the structure of tight junctions between epithelial cells (McKay and Baird, 1999; Bruewer et al., 2003; Ma et al., 2004; Wang et al., 2005), mainly at the jejunum (Spreeuwenberg et al., 2001; Pie et al., 2004; Lallès et al., 2004). The LPS released into the gut cause the shortening of actin filaments in the enterocyte, disrupting the structure of tight junctions and causing the internalization of occludin. Under these circumstances, the intestinal barrier may be affected, decreasing transepithelial resistance (Berkes et al., 2003) and thus increasing indiscriminate paracellular transport of molecules, allowing gastrointestinal pathogens to access the systemic circulation (García-Herrera et al., 2003; Pitman and Blumberg, 2000).
At the time of weaning, over-expressed mRNA of proinflammatory cytokines, especially TNF-α, may influence the integrity of intestinal epithelium, favoring the emergence of bacterial infections and diarrhea and increasing the transepithelial transport of microorganisms. In the presence of inflammatory processes, including those caused by bacterial infections, proinflammatory cytokines have been implicated in increasing apoptosis rates in intestinal epithelium (Ramachandran et al., 2000), eliminating and controlling the number of cells in the proliferation area of intestinal crypts (Potten, 1992). Furthermore, TNF-α and IL-8 have mitotic effects on intestinal mucosa (Zachrisson et al., 2001; Schaeffer et al., 2006), causing the conversion of intestinal stem cells into secreting cells, thus decreasing the number of absorptive cells in the intestinal mucosa (Van et al., 2005). This can lead to high malnutrition and mortality of pigs at this stage.
The immune response in the gastrointestinal tract is a constant balance between proinflammatory cytokine (IL-6, IL-8, IL-18, IL-12, TNF-α) and inflammatory cytokine release (IL-1RA, IL-4, IL -10). Under chronic inflammatory conditions, T helper type 1 lymphocytes (LTh1) induce the release of significant levels of proinflammatory cytokines, such as IL-12, IL-18 and TNF-α (Pizarro et al., 1999; Reuter and Pizarro, 2004; Cavani et al., 2000) and, in turn, suppress IL-10 by LTh2 production. Because of this, the activation of antigen-presenting cells, dendritic cell maturation, and intolerance to specific antigens are all increased, and the proliferation of T cells is limited (Enk et al., 1993; Caux et al., 1994). Inhibition of T cells leads to increased chloride secretion, and therefore to the onset of diarrhea (Xu and Verstraete, 2001). The IL- 10 is a key molecule for the induction of T cells and the prevention of intestinal inflammation (León et al., 2006).
The differential expression of proinflammatory cytokines in different parts of the intestinal tract of pigs occurs in response to intestinal disorders caused by stress and intestinal infections associated with the weaning (Oswald et al., 2001; Fournout et al., 2000). These inflammatory responses may reflect, at least in part, anatomical and functional differences between sections of the intestine (Beagley et al., 1995). However, the anatomical changes that occur at weaning can be observed regardless of the weaning age, though its size may vary (Cera et al., 1988; Pluske et al., 2003).
The data from this study suggest that weaning is associated with increased intestinal inflammation, mainly caused by the expression of proinflammatory cytokines. An increase in proinflammatory cytokine expression was observed until day 5 after weaning. However, a decrease in the expression of these cytokines was noted after the fifth postweaning day, without returning to the values found on day 1 after weaning. Therefore, we found a clear relationship between the expression of proinflammatory cytokines (TNF-α) and the incidence of diarrhea during the weaning stage.
Animals consuming BD+LPS presented an excessive inflammatory response due to the overexpression of proinflammatory cytokines. The mRNA expression of TNF-α, IL-8, and IL-18 increased by 922%, 1723%, and 335%, respectively, compared to BD, which coincides with the high rates of diarrhea observed during the postweaning period. Considering the advantages of this inflammation model, it is necessary to conduct more studies in order to confirm the validity of the results observed.
Notes
¤ To cite this article: Parra J, Agudelo J, Sanín D, Forero J, Muskus C, López A. Intestinal expression of pro-inflammatory cytokines induced by oral intake of lipopolysaccharide (LPS) from E. coli in weaned pigs. Rev Colomb Cienc Pecu 2013; 26:108-118.
References
Albin DM, Wubben JE, Rowlett JM, Tappenden KA, Nowak RA. Changes in small intestinal nutrient transport and barrier function after lipopolysaccharide exposure in two pig breeds J Anim Sci 2007; 85:2517-2523. [ Links ]
Allen DG, Rivier, JE, Monteiro-Riviere NA. Cytokine induction as a measure of cutaneous toxicity in primary and immortalized porcine keratinocytes exposed to jet fuels, and their relationship to normal human epidermal keratinocytes. Toxicol Lett 2001; 119:209-217. [ Links ]
Amador P, García-Herrera J, Marca MC, de la Osada J, Acin S, Navarro MA, Salvador MT, Lostao MP, Rodriguez-Yoldi MJ. Intestinal D-galactose transport in an endotoxemia model in the rabbit. J Membr Biol 2007; 215:125-133. [ Links ]
Asaduzzaman M, Zhang S, Lavasani S, Wang Y, Thorlacius H. Lfa-1 and Mac-1 Mediate Pulmonary Recruitment of Neutrophils and Tissue Damage in Abdominal Sepsis. Shock 2008; 30:254-259. [ Links ]
Bauer E, Williams BA, Martin HS, Verstegen WA, Mosenthin R. Influence of the gastrointestinal microbiota on development of the immune system in young animals. Curr Issues Intestinal Microbiol 2006; 7:35-52. [ Links ]
Beagley K, Fujihashi K, Lagoo AS, Lagoo-Deenadaylan S, Black CA, Murray AM, Sharmanov AT, Yamamoto M, McGhee JR, Elson CO. Differences in intraepithelial lymphocyte T cell subsets isolated from murine small versus large intestine. J Immunol 1995; 154:5611-5619. [ Links ]
Berkes J, Viswanathan VK, Savkovic SD, Hecht G. Intestinal epithelial responses to enteric pathogens: effects on tight junction barrier, ion transport, and inflammation. Gut 2003; 52:439-451. [ Links ]
Bertelsen LS, Eckmann L, Barrett KE. Prolonged interferon-γ exposure decreases ion transport, NKCC1, and Na+-K+-ATPase expression in human intestinal xenografts in vivo. Am J Physiol Gastrointest Liver Physiol 2004; 286:G157-G165. [ Links ]
Bruewer M, Luegering A, Kucharzik T, Parkos CA, Madara JL, Hopkins AM, Nusrat A. Proinflammatory cytokines disrupt epithelial barrier function by apoptosis-independent mechanisms. J Immunol 2003; 6164-6172. [ Links ]
Bustin S.A. Absolute quantification of mRNA using real-time reverse transcription polymerase chain reaction assays. J Mol Endocrinol 2000; 25:169-193. [ Links ]
Cavani A, Nasorri F, Prezzi C, Sebastiani S, Albanesi C, Girolomoni G. Human CD4+ T lymphocytes with remarkable regulatory functions on dendritic cells and nickel-specific Th1 immune responses. J Investig Dermatol 2000; 114:295-302. [ Links ]
Caux C, Massacrier C, Vanbervliet B, Dubois B, Van Kooten C, Durand I, Banchereau J. Activation of human dendritic cells through CD40 cross-linking. J Exp Med 1994; 180:1263-1272. [ Links ]
Cera KR, Mahan DC, Cross RF, Reinhart GA, Whitmoyer RE. Effect of age, weaning and postweaning diet on small intestinal growth and jejunal morphology in young swine. J Anim Sci 1988; 66:574-584. [ Links ]
Chow CW, Grinstein S, Rotstein OD. Signaling events in monocytes and macrophages. New Horiz 1995; 3:342-35. [ Links ]
Council for International Organizations of Medical Sciences (CIOMS). International guiding principles for biomedical research involving animals. 1985. Geneva. 28pp. [ Links ]
Dheda K, Huggett JF, Chang JS, Kim LU, Bustin SA, Johnson MA, Rook GA, Zumla A. The implications of using an inappropriate reference gene for real-time reverse transcription PCR data normalization. Anal Biochem 2005; 344:141-143. [ Links ]
Enk AH, Angeloni VL, Udey MC, Katz SI. Inhibition of Langerhans cell antigen-presenting function by IL-10. A role for IL-10 in induction of tolerance. J Immunol 1993; 151:2390- 2398. [ Links ]
Fournout S, Dozois C.M, Odin M, Desautels C, Peres S, Herault F, Daigle F, Segafredo C, Laffitte J, Oswald E, Fairbrother JM, Oswald IP. Lack of a role of cytotoxic necrotizing factor 1 toxin from Escherichia coli in bacterial pathogenicity and host cytokine response in infected germfree piglets. Infect Immun 2000; 68:839-847. [ Links ]
García-Herrera J, Abad B, Rodríguez-Yoldi MJ. Effect of lipopolysaccharide on D-fructose transport across rabbit jejunum. Inflamm Res 2003; 52:177-184. [ Links ]
García-Herrera J, Marca MC, Brot-Laroche EGuillen, N, Acin S, Navarro MA, de la Osada J, Rodriguez-Yoldi MJ. Protein kinases, TNF-α and proteasome contribute in the inhibition of fructose intestinal transport by sepsis in vivo. Am J Physiol Gastrointest Liver Physiol 2008; 294:G155-G164. [ Links ]
Garriga C, Perez-Bosque A, Amat C, Campbell JM, Russell L, Polo J, Planas JM, Moreto M. Spray-dried porcine plasma reduces the effects of staphylococcal enterotoxin B on glucose transport in rat intestine. J Nutr 2005; 135:1653-1658. [ Links ]
Gómez IAS, Vergara D, Argote F. Efecto de la dieta y edad del destete sobre la fisiología digestiva del lechón. Revista Biotecnología en el Sector Agropecuario y Agroindustrial 2008; 6:32-41. [ Links ]
Gopal R, Birdsell D, Monroy FP. Regulation of toll-like receptors in intestinal epithelial cells by stress and Toxoplasma gondii infection. Parasite Immunol 2008; 11-12:63-576. [ Links ]
Huggett J, Dheda K, Bustin S, Zumla A. Real-time RT-PCR normalization; strategies and considerations. Gennes Immun. 2005; 6:279-284. [ Links ]
Johnson GB, Brunn GJ, Samstein B. New insight into the pathogenesis of sepsis and the sepsis syndrome. Surgery 2005; 137:393-395. [ Links ]
Kubista M, Andrade JM, Bengtsson M, Forootan A, Jonák J, Lind K, Sindelka R, Sjöback R, Sjögreen B, Strömbom L, Stahlberg A, Zoric N. The real-time polymerase chain reaction. Mol Aspects Med 2006; 27:95-125. [ Links ]
Lallès JPl, Boudry G, Favier C, Le Floc'h N, Luron I, Montagne L, Oswald IP, Pié S, Piel C, Sève B. Gut function and dysfunction in young pigs: physiology. Anim Res 2004; 53:301- 316. [ Links ]
Liu Y, Huang J, Hou Y, Zhu H, Zhao S, Ding B, Yin Y, Yi G, Shi J, Fan W. Dietary arginine supplementation alleviates intestinal mucosal disruption induced by Escherichia coli lipopolysaccharide in weaned pigs. Brit J Nutr 2008; 100:552- 560. [ Links ]
León AJ, Garrote JA, Arranza E. Citocinas en la patogenia de la enfermedad inflamatoria intestinal. Med Clin (Barc) 2006; 127:145-152. [ Links ]
Ma TY, Iwamoto GK, Hoa NT, Akotia V, Pedram A, Boivin MA Said HM. TNF-alpha-induced increase in intestinal epithelial tight junction permeability requires NF-kappa B activation. Am J Physiol 2004; 286:G367-G376. [ Links ]
Makkink CA, Berntsen PJM, op den Kamp BML, Kemp B, Verstegen WA. Gastric protein breakdown and pancreatic enzyme activities in response to two different dietary protein sources in newly weaned pigs. J Anim Sci 1994; 72:2843-2850. [ Links ]
McKay DM, Baird AW. Cytokine regulation of epithelial permeability and ion transport. Gut 1999; 44:283-289. [ Links ]
Muller PY, Janovjak H, Miserez AR, Dobbie Z. Processing of gene expression data generated by quantitative real time RTPCR. Biotechniques 2002; 32:1372-1379. [ Links ]
Musch MW, Clarke LL, Mamah D, Gawenis LR, Zhang Z, Ellsworth W, Shalowitz D, Mittal N, Efthimiou P, Alnadjim Z, Hurst SD, Chang EB, Barrett TA. T cell activation causes diarrhea by increasing intestinal permeability and inhibiting epithelial Na+ /K+ -ATPase. J Clin Invest 2002; 110:1739-1747. [ Links ]
National Research Council (NRC). Nutrient Requirements of Swine. 10th ed. Washington: National Academy Press,1998. [ Links ]
Oswald IP, Dozois CM, Barlagne R, Fournout S, Johansen MV, Bogh HO. Cytokine mRNA expression in pigs infected with Schistosoma japonicum. Parasitology 2001; 122:299-307. [ Links ]
Paulin SM, Jagannathan A, Campbell J, Wallis TS, Stevens MP. Net replication of Salmonella enterica serovars Typhimurium and Choleraesuis in porcine intestinal mucosa and nodes is associated with their differential virulence. Infect Immun 2007; 75:3950-3960. [ Links ]
Pié S, Lallès JP, Blazy F, Laffitte J, Sève B, Oswald IP. Weaning is sssociated with an upregulation of expression of inflammatory cytokines in the intestine of piglets. J Nutr 2004; 134:641-647. [ Links ]
Pitman RS, Blumberg RS. First line of defense: the role of the intestinal epithelium as an active component of the mucosal immune system. J Gastroenterol 2000; 35:805-814. [ Links ]
Pizarro TT, Michie MH, Bentz M, Woraratanadharm J, Smith MF. Jr, Foley E, Moskaluk CA, Bickston SJ, Cominelli F. IL-18, a novel immunoregulatory cytokine, is upregulated in Crohn's disease: expression and localization in intestinal mucosal cells. J Immunol 1999; 162:6829-6835. [ Links ]
Pluske JR, Kerton DJ, Cranwell PD, Campbell RG, Mullan BP, King RH, Power GN, Pierzynowski SG, Westrom B, Rippe C, Peulen O, Dunshea FR. Age, sex, and weight at weaning influence organ weight and gastrointestinal development of weanling pigs. Aust J Agric Res 2003; 54:515-527. [ Links ]
Potten CS. The significance of spontaneous and induced apoptosis in the gastrointestinal tract of mice. Cancer Metastasis Rev 1992; 11:179-195. [ Links ]
Ramachandran A, Madesh M, Balasubramanian KA. Apoptosis in the intestinal epithelium: its relevance in normal and pathophysiological conditions. J Gastroenterol Hepatol 2000; 15:109-120. [ Links ]
Ramakers C, Ruijter JM, Deprez RH, Moorman AF. Assumption-free analysis of quantitative real-time polymerase chain reaction (PCR) data. Neurosci Lett 2003; 339:62-66. [ Links ]
Reis STC, Guerrero CMJ, Aguilera BA, Mariscal LG. Effect of different cereals on intestinal morphology of weaned piglets. Tec Pecu Mex 2005; 43:309-321. [ Links ]
Reis STC, Mariscal LG, Escobar GK. Algunos factores fisiológicos y nutricionales que afectan la incidencia de diarreas posdestete en lechones. Vet Méx 2010; 41:275-288. [ Links ]
Reuter BK, Pizarro TT. Commentary: the role of the IL-18 system and other members of the IL-1R/TLR superfamily in innate mucosal immunity and the pathogenesis of inflammatory bowel disease: friend or foe? Eur J Immunol 2004; 34:2347- 2355. [ Links ]
SAS®. SAS/STAT User's Guide. Institute Inc. Statistical Analysis Systems Institute. Version 9.1th Ed. Cary, NC: SAS Institute Inc. 2006. [ Links ]
Schaeffer C, Habold C, Martin E, Lignot JH, Kedinger M, Foltzer-Jourdainne C. Cytokine expression in rat colon during postnatal development: regulation by glucocorticoids. J Pediatr Gastroenterol Nutr 2006; 43:439-450. [ Links ]
Schmitz H, Fromm M, Bode H, Scholz P, Riecken EO, Schulzke JD. Tumor necrosis factor-alpha induces Cl- and K+ secretion in human colon driven by prostaglandin E2. Am J Physiol 1996; 271:G669-G674. [ Links ]
Spreeuwenberg MAA, Verdonk JMAJ, Gaskins HR, Verstegen MWA. Small intestinal epithelial barrier function is compromised in pigs with low feed intake at weaning. J Nutr 2001; 131:1520-1527. [ Links ]
Stadnyk AW. Intestinal epithelial cells as a source of inflammatory cytokines and chemokines. Can J Gastroenterol 2002; 16:241-246. [ Links ]
Steel RG, Torrie JH. Principles and Procedures of Statistics: a biometrical approach. 2nd Ed. New York: McGraw-Hill; 1985. [ Links ]
Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 2002; 3: research 0034.1- 0034.11 [ Links ]
Van JH, Jay P, Gregorieff A, van Gijn ME, Jonkheer S, Hatzis P, Thiele A, Van den Born M, Begthel H, Brabletz T, Taketo MM, Clevers H. WNT signalling induces maturation of Paneth cells in intestinal crypts. Nat Cell Biol 2005; 7:381-386. [ Links ]
Wang F, Graham WV, Wang Y, Witkowski ED, Schwarz BT, Turner J. Interferon-γ and tumor necrosis factor-α synergize to induce intestinal epithelial barrier dysfunction by up-regulating myosin light chain kinase expression. Am J Pathol 2005; 166:409-419. [ Links ]
Williams DA. Inflammatory cytokines and mucosal injury. J Natl Cancer Inst Monogr 2001; 29:26-30. [ Links ]
Xu J, Verstraete W. Evaluation of nitric oxide production by lactobacilli. Appl Microbiol Biotechnol 2001; 56:504-507. [ Links ]
Yoo D, Lo W, Goodman S, Ali W, Semrad C, Field M. Interferon-gamma down regulates ion transport in murine small intestine cultured in vitro. Am J Physiol 2000; 279:G1323-G1332. [ Links ]
Yu LC, Perdue MH. Immunologically mediated transport of ions and macromolecules. Ann NY Acad Sci 2000; 915:247- 259. [ Links ]
Zachrisson K, Neopikhanov V, Wretlind B, Uribe A. Mitogenic action of tumour necrosis factor-alpha and interleukin-8 on explants of human duodenal mucosa. Cytokine 2001; 15:148- 155. [ Links ]