Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Colombiana de Ciencias Pecuarias
Print version ISSN 0120-0690
Rev Colom Cienc Pecua vol.26 no.2 Medellín Apr./June 2013
ORIGINAL ARTICLES
Reducing the incidence of intramammary infection in heifers by using prepartum systemic tylosin therapy: initial results of a single herd pilot study¤
Reducción en la incidencia de infecciones intramamarias en novillas con el uso de tylosina sistémica preparto: resultados iniciales de un estudio piloto en un hato lechero
Redução da incidência de infecção intramamária em novilhas através do uso tilosina sistêmica préparto: resultados iniciais de um único estudo em um rebanho piloto
Genaro Andres Contreras1*, MV, MS, PhD; J David Munoz2, Ing Ag; Phil M Sears1, DVM, PhD.
* Corresponding author: Genaro Andres Contreras. Department of Large Animal Clinical Sciences, Michigan State University, East Lansing, Michigan 48824. 517-353-6869. Fax 517-353-6869. e-mail: contrera@cvm.msu.edu
1 Department of Large Animal Clinical Science, Michigan State University, East Lansing, Michigan, USA.
2 Department of Crop and Soil Sciences, Michigan State University, East Lansing, Michigan, USA.
(Received: June 6, 2012; accepted: January 28, 2013)
Summary
Background: mastitis in heifers is recognized as an important problem that impacts animal health and wellbeing and reduces milk quality and lifetime productivity. Objective: to assess the effectiveness of intramuscular administration of tylosin (20 g) as a method to prevent and treat intramammary infections (IMI) in heifers. Methods: heifers from a commercial farm in Michigan, due to calve within 14 to 18 d, were assigned randomly to one of two treatment groups. The control group (n=108 heifers) received no antibiotic treatment or teat sealants to prevent IMI. The tylosin group (n=112 heifers) was injected intramuscularly with 20 g of tylosin. Quarter milk samples were taken in duplicate for bacterial culture from all functional quarters at 2 to 6 d (sample 1) and 7 to 15 d (sample 2) after calving. Representative isolates from sample 1 were speciated. Somatic cell counts and milk production were recorded. Results: in sample 1, 42% of the heifers, and 16.5% of the quarters were infected. Coagulase-negative staphylococci (CNS) and streptococci infected 10.8% and 3.6% of the quarters, respectively. No antibiotic residues were detected at either sample 1 or sample 2. No differences were observed in Somatic Cell Count (SCC) and milk production between tylosin treated animals and controls, however, uninfected heifers had a lower somatic cell score (SCS). At the heifer level, tylosin did not reduce significantly IMI infection rate caused by Gram-positive bacteria. At the quarter level, tylosin reduced levels of IMI caused by CNS. Conclusion: tylosin administration to primigravid heifers 2 weeks before expected calving should not be advised without first evaluating udder health, management and economic implications on each individual dairy farm should be taken into account.
Key words: coagulase negative staphylococcus, macrolides, mastitis.
Resumen
Antecedentes: la mastitis en novillas es reconocida como un problema importante que afecta la salud y el bienestar animal reduciendo la calidad de la leche y la productividad durante la vida de la vaca. Objetivo: evaluar la efectividad de la administración vía intramuscular de tylosina (20 g) como un método para prevenir y tratar infecciones intramamarias (IMI) en novillas. Métodos: novillas de un hato lechero comercial en Michigan, con fecha tentativa de parto 14 a 18 d después del inicio del estudio, fueron asignadas al azar a uno de dos tratamientos. El grupo control (n=108 novillas) no recibió antibióticos o sellantes de pezón para prevenir IMI. Los animales en el grupo tylosina (n=112) fueron inyectados vía intramuscular con 20 g de tylosina. Muestras de leche fueron tomadas en duplicado de cada uno de los cuartos funcionales a los 2-6 d (muestra 1) y a los 7-15 d (muestra 2) después del parto para cultivo bacteriológico. Las colonias representativas de la muestra 1 fueron clasificadas. Los recuentos de células somáticas y la producción de leche fueron evaluados. Resultados: en la muestra 1, el 42% de las novillas y el 16,5% de los cuartos estaban infectados. Estafilocococoagulasa negativos (CNS) y estreptococos infectaron el 10,8% y el 3,6% de los cuartos, respectivamente. No se encontraron residuos de antibióticos en ninguna de las muestras. No se observaron diferencias en el Recuento de células somáticas (SCC) o producción de leche entre los animales tratados con tylosina y los controles; sin embargo, las novillas no infectadas tuvieron un recuento de células somáticas más bajo. A nivel de novillas, el tratamiento con tylosina no redujo la tasa de IMI causada por bacterias Gram-positivas. A nivel de cuartos, la tylosina redujo los niveles de IMI causados por SCN. Conclusión: la administración de tylosina en novillas primigravidas 2 semanas antes del parto no debe ser formulada sin previa evaluación de la salud de la ubre y de las implicaciones de manejo y económicas pertinentes para cada hato.
Palabras clave: staphylococcus coagulasa negativo, macrólidos, mastitis.
Resumo
Antecedentes: a mastite em novilhas é reconhecida como um dos principais problemas que afetam a saúde e o bem-estar animal e reduz a qualidade do leite e a produtividade ao longo da vida da vaca. Objetivo: avaliar a efetividade da administração de tilosina (20 g) via intramuscular como um método para prevenir e tratar as infecções intramamárias (IMI) em novilhas. Métodos: novilhas de um rebanho leiteiro comercial de Michigan, com a data de parto prevista para 14 a 18 dias após o início do estudo, foram escolhidas ao azar para um dos tratamentos. O grupo controle (n=108 novilhas) não recebeu antibióticos ou desinfetantes de mamilos para prevenir a IMI. Os animais do grupo tilosina (n=112) receberam 20 g de tilosina via intramuscular. As amostras de leite de cada um dos quartos para o cultivo bacteriológico foram obtidas em duplicado de todos os quartos funcionais 2-6 d (amostra 1) e a 7-15 d (amostra 2) depois do parto. As colônias representativas da amostra 1 foram classificadas. As contagens de células somáticas e a produção de leite foram avaliadas. Resultados: na amostra 1, 42% das novilhas e 16,5% dos quartos estavam infectados. Staphylococos coagulase negativos (CNS) e estreptococos infectaram 10,8% e 3,6% dos quartos, respectivamente. Não se encontraram resíduos de antibióticos em nenhuma das amostras. Não se observaram diferenças na contagem de células somáticas (SCC) ou na produção de leite entre os animais tratados com tilosina e os controles, no entanto, as novilhas não infectadas apresentaram uma contagem de células somáticas mais baixa. Enquanto a novilhas, o tratamento com a tilosina não reduziu a taxa de IMI causada por bactérias Gram-positivas. No nível de quartos, a tilosina reduziu os níveis de IMI causadas por CNS. Conclusão: a administração de tilosina às novilhas de primeira gestação, duas semanas antes do parto, não deve ser indicada sem uma avaliação prévia da saúde da glândula mamária e as implicações no manejo e na economia pertinente a cada rebanho.
Palavras chave: Staphylococcus coagulase negativo, macrolídeos, mastite.
Introduction
While mastitis in heifers is recognized as a problem, most dairymen often view heifers at parturition as free of intramammary infections (IMI). Mastitis in heifers is highly prevalent in dairy herds in North America. In a study that included farms in six states and one province (Borm et al., 2006) as many as 63% of the heifers and 34% of their quarters had IMI at calving. IMI during lactogenesis can impair mammary development and cause a decrease in milk yield during the productive life at the heifer level (Contreras, 2009). At the herd level, IMI could cause an increase in somatic cell counts (SCC) and clinical mastitis cases (Trinidad et al., 1990a).
The predominant pathogen causing IMI in heifers from breeding age to first parturition are the coagulase negative staphylococci (CNS), although different factors such as season, geographical location and the herd-status prevalence regarding contagious agents that cause mastitis modify the etiology of the disease (Contreras and Rodríguez, 2011).
To prevent and treat IMI in heifers, infusion of antibiotics in the mammary gland before calving was proposed (Borm et al., 2006; Oliver et al., 2003). Intramammary antibiotics are usually effective in reducing IMI, but economic benefits can differ among farms depending on the prevalence (Borm et al., 2006). Furthermore, intramammary antibiotic infusions in heifers result in additional labor. In an effort to diminish the losses by disease, antibiotic administration immediately after calving, either intramammary (Kreiger et al., 2007; Oliver et al., 2007) or systemic (Kreiger et al., 2007) has been attempted, but issues regarding antibiotic residues in milk were reported.
In contrast, systemic administration to heifers before calving could offer advantages related to its relative lower cost and easier to administer when compared to intramammary infusion. Tylosin has been used in the treatment of clinical mastitis in mature cows (McDougall et al., 2007). The major advantage of this antimicrobial is its excellent diffusion into the mammary gland, which is related to its basic pK (pH at which concentrations of dissociated and undissociated antibiotic are equal), resulting in a very high milk-to-plasma concentration ratio of 5:1 (Omura, 1984; Riviere, 1999). In this study, we analyzed the effectiveness of tylosin injected intramuscularly at a dose of 20 g to prepartum heifers 14 to 18 d before the expected calving for reducing IMI.
Materials and methods
Animals
Heifers from a commercial farm in Michigan, due to calve within 12 to 18 d, were selected every week during 8 weeks in the spring of 2007. Animals were housed in a free-stall barn with sand bedding. The Michigan State University Animal Care and Use Committee approved animal procedures. A completely randomized design that included a systemic antibiotic treatment group and a negative control group was used. Before randomization, animals were properly identified and their gestational stage was confirmed. Heifers then were assigned randomly to one of two treatment groups. The control group (n=108 heifers), reflecting standard practice on the farm, received no antibiotic treatment or teat sealants to prevent IMI. The tylosin group (n=112 heifers) received 20 g of tylosin (Elanco, Greenfield, IN). Injections were administered intramuscularly in the neck and the 20 g dose was divided among three different sites.
Sampling
Quarter milk samples were taken in duplicate following National Mastitis Council guidelines (Hogan et al., 1999) from all functional quarters at 2 to 6 d (sample 1) and 7 to 15 d (sample 2) after calving. Samples were stored in a container at 5 °C and processed within 3 h after collected. A 10 mL aliquot from each sample was cultured on blood agar containing 0.1% esculin at 37 °C for 24h. Colonies were tentatively identified at 24 h and 48 h as CNS, streptococci, Staphylococcus aureus, coliform, or others. A presumptive diagnosis was made based on colony growth, morphology and appearance, pattern of hemolysis, and catalase reaction.
Staphylococcal isolates were tested for coagulase production using the tube coagulase test. Gramnegative bacteria were plated on MacConkey agar to facilitate identification. For speciation, biochemical tests were performed on representative positive cultures from sample 1 of streptococci and staphylococci isolates by using the API20 Strep SYSTEM and the API-Staph SYSTEM, (BioMerieux, Hazelwood, MO, USA). A heifer was considered infected if at least one quarter was infected at sample 1; considered newly infected if it was negative for all quarters at sample 1 but infected at sample 2; and was spontaneously cured if it was infected at sample 1 but negative at sample 2. Quarters were considered infected if at sample 1, five or more Colony Forming Units (CFU) of the same kind were identified, and were considered contaminated if three or more different colony types were present on both duplicate samples. A new infection was considered when a quarter was negative at sample 1 but was positive at sample 2. A quarter was considered spontaneously cured if it was infected at sample 1 but negative at sample 2.
Two composite milk (3 ml) samples were collected during sampling 1 and 2. One of the samples was used to perform antibiotic residue test with the Delvotest® (DMS food specialties, Parsippany, NJ, USA) and the second sample was used to determine SCC at the regional Dairy Herd Improvement (DHI) laboratory. Dairy Herd Improvement tests were used to monitor SCC and milk production during lactation. Milk production records were collected by DHI personnel and total production was calculated as projected to 305 d mature equivalent (ME) by a herd management software based on milk production at DHI test taken between 180 and 200 d in milk (DIM) after calving.
Minimal inhibition concentration (MIC) values for the following representative isolates were determined using the Sensititre® system (Trek® diagnostics, Cleveland, OH, USA): 6 Streptococcus uberis, 6 Enterococcus faecalis, 10 Staphylococcus chromogenes, and 10 Staphylococcus simulans. The following antimicrobials were included in the Sensititre® plate format: ampicillin, ceftiofur, chlortetracycline, clindamycin, danofloxacin, enrofloxacin, gentamicin, neomycin, oxytetracycline, penicillin, spectinomycin, sulphadimethoxime, trimethropin/sulphamethoxazole, and tylosin.
Statistical Analysis
Culture results were analyzed under a generalized linear mixed model (PROC GLIMMIXf). The response variable was expressed as the number of cases assuming a binary distribution using a logit transform as the link function. Treatment was included as a fixed effect. Clustering among quarters within the same cow was included as random effect. SCC scores were analyzed using a linear mixed model with a repeated measures structure (PROC MIXED; SAS Inst. Inc., Cary NC) following the equation:
Yijk = μ+Gi +IJ+Gi *Ij+Sk+Sk*Gi+Sk *Ij+Sk *Gi *Ij+Eijk
Where Yijk is the dependent variable SCC score for a heifer in group Gi, with infection status Ij, at sample Sk as repeated measure, and Eijk is the random error assumed to be correlated. ''Compound symmetry'' or ''Autoregressive'' heterogeneous or homogeneous was chosen as the best variance/ covariance structure based on the lower Akaike information criteria (AIC), indicating equal or unequal variances across time. The Satterthwaite's method for approximate degrees of freedom was used. Significance level was set at alpha = 0.05.
Results
A total of 92 (42%) heifers had an IMI at sample 1. 50 (46%) heifers were infected in the control group, and 42 (38%) in the tylosin group (p=0.18). In sample 2, 76 (34.5%) heifers were infected: 43 (40%) in the control group and 33 (29%) in the tylosin group (p=0.10). Spontaneous cures were observed in 19 heifers (38%) from the control group and 21 animals (50%) from the tylosin group. Furthermore, in both groups, 11% of the heifers acquired a new IMI in the first week after calving.
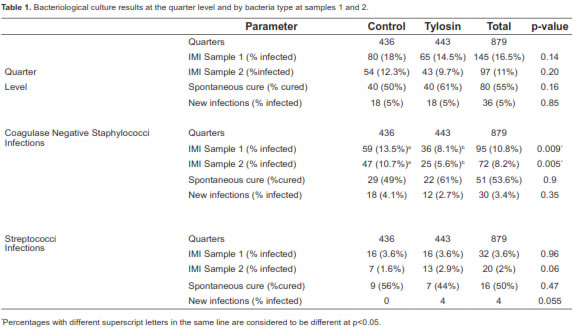
At sample 1, 145/879 (16.5%) quarters were infected. At sample 2, 97/879 (11%) quarters had an IMI. The quarter spontaneous cure rate for tylosin group was 61%, and 50% for control group. New infections accounted for 5% of the quarters and no statistical difference was found between groups (Table 1). Remarkably, the tylosin group had fewer CNS infections in sample 1 (36 quarters; 8.1%) than the control group (59 quarters; 13.5%) and this difference was significant (p<0.01; Table 1). At sample 2, CNS infected 25 quarters (5.6%) in the tylosin group and 47 quarters (10.7%) in the controls. This difference in infection rate was significant (p<0.01), but could be related to the initial lower prevalence of CNS IMI in the tylosin group at sample 1. Spontaneous cure for CNS was 61% for tylosin and 49% for the controls.
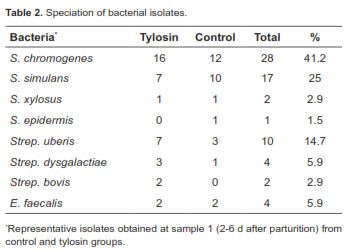
Bacterial speciation results are shown in table 2. Staphylococcus chromogenes was the most prevalent species among CNS in this study, representing 41.2% of CNS infections. In contrast, S. simulans affected 25% of the quarters in this trial.
Streptococci infections accounted for 32/145 (22%) of all IMI after calving. Over all, streptococci infected 3.6% of the quarters at sample 1 and 2% at sample 2 (Table 1). The streptococcal infection rate was equal for both groups (3.6%) at sample 1, but was slightly higher at sample 2 for tylosin (2.9%) than for control (1.6%). Streptococcus uberis was the most prevalent streptococcal infection, followed by Strep. dysgalactiae. Spontaneous cures for streptococcal infection accounted for 44% of the quarters in the tylosin group and 56% in the control group. Four new infections were found in the tylosin group, and none in the control group.
Contagious pathogens, Streptococcus agalactiae and Staphylococci aureus were absent from the study animals. Coliform bacteria infected a total of 18/879 quarters at sample 1, eight in the control group and 10 in tylosin treated heifers (results not included in Table 1). Seven quarters were infected at sample 2, three in the control group, and four in the tylosin group.
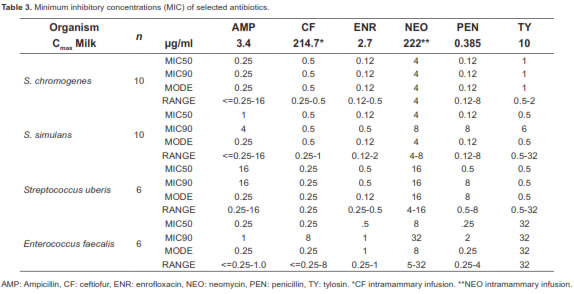
MICs are reported on table 3. Tylosin was effective in inhibiting CNS growth. However, this macrolide's activity against enterococci was poor, and although it had a MIC90 0.5 mg/ml, for Strep. uberis, the enterococci isolates were resistant (MIC90 32 mg/ml) to this macrolide (Table 3).
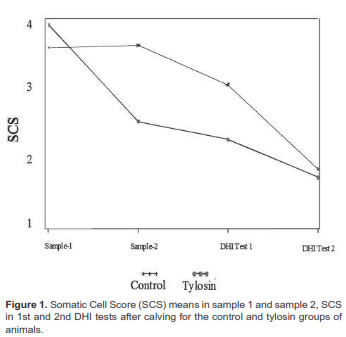
The Log scores for SCC results are shown in figure 1. Antibiotic treatment was not a significant (p=0.17) source of SCS variation. The tylosin group had a higher score (4.2 ± 3) than the control group (3.7 ± 2.3) in sample 1. In contrast, the 1st and 2nd DHI tests for both groups had similar means for the somatic cell scores in sample 2. The tylosin group had an average milk projection at 305 d of 9515 ± 1847 kg in the first test and 10,556 ± 1790 kg in the second test. Similarly, the control group had 9495 ± 1658 kg in the first, and 10346± kg in the second test. No difference in milk yield was found between the two groups.
Discussion
At the heifer level, the prevalence of IMI observed in this study is similar to previous reports with heifers housed under different environments (Borm et al., 2006; Trinidad et al., 1990b). Results in the present study indicate that, at the heifer level, tylosin administration to prepartum heifers did not significantly reduce IMI caused by gram-positive bacteria.
Similar to previous studies (Fox et al., 1995; Oliver et al., 2003; Trinidad et al., 1990b), CNS was the principal agent causing IMI in heifers. Consistent with other reports (Aarestrup and Jensen, 1997; Middleton et al., 2005; Oliver et al., 2007), spontaneous cure for CNS was observed in more than half of the group (61% for tylosin and 49% for controls). Remarkably, tylosin had high efficacy against CNS and decreased the infection rate due to these microbes in the treated heifers. Further studies are needed to evaluate economic and physiological implications of transient infections of CNS during the first week of lactation in heifers, since at least half of the CNS infections will clear without antibiotic intervention.
Bacterial speciation results (Table 2) are similar to those reported by Aarestrup and Jensen (1997). In their study, there was a higher prevalence of S. chromogenes (15% of the quarters) in the first 2 weeks after calving, probably reflecting the normal presence of this species on the skin. In the present study, S. simulans affected 25% of the quarters and only 3% in Aarestrup and Jensen's trials (1997) but were persistent several weeks after calving. Both CNS are reported to be highly prevalent around parturition in mature dairy cows as well (Taponen et al., 2007).
In contrast to the results from this clinical trial, studies with pasture-grazed heifers in New Zealand (Compton et al., 2007) revealed a higher infection rate by Strep. uberis (10% of the quarters) during the first week after calving, reflecting changes in IMI prevalence due to the housing system. Thus, it is necessary to evaluate tylosin use in animals housed in different conditions such as pasture and dry-lots to determine its efficiency against streptococcal and CNS infections.
Regarding MIC (Table 3), it is presumable that peak milk concentration in tylosin-treated animals was higher than previously reported, therefore achieving bactericidal activity, which was observed for macrolides when used in high concentrations (Diarra et al., 1999). Effectiveness of systemic treatment with tylosin varies depending on the dose and time interval between administration and calving. Parker et al. (2008) compared a commercial teat sealant, tylosin (5 g of tylosin base IM for 3 d at 24 h intervals) or a combination of both in pasture-grazed heifers in New Zealand. The teat sealant reduced the risk of new IMI by 74% and reduced the risk on new IMI with Strep uberis by 70%. Tylosin did not have any effect in reducing risks.
Differences in response to tylosin treatment between the present study and Parker et al. (2008) could have been related to the relative low dose used in New Zealand and the timing of treatment (27 d before expected calving). A higher dose with a single injection was used in this trial, and the treatment was administered close to the scheduled calving date (14 d). Tylosin's peak concentration in milk was reported to be 10 mg/ml after a single injection at a dose of 20 mg/kg BW (Ziv and Sulman, 1973), and 18 mg/ml after three repeated injections at a dose of 10 mg/kg BW (el-Sayed et al., 1986). In the present work, tylosin dose was based on the average calving weight of heifers (600 kg) at 33 mg/kg. Despite of the relative high dose used, antibiotic residues were not found in the composite samples taken from sample 1 and sample 2. It is unknown if the use of tylosin as a systemic treatment to eliminate or prevent IMI in heifers at calving is dose and time sensitive, thus further studies are needed to evaluate the extended therapy at different doses with this macrolide to improve its response. Because of logistic limitations of the study, multiple administrations and dosage based on individual weight was not possible.
Systemic administration of tylosin was effective in treating heifers for IMI caused by CNS at the quarter level, but its routine use in this farm as a prophylactic may not be economically sound and could promote the development of bacterial resistance to macrolide antibiotics. Transient IMI occurs early in lactation with at least half of the infections cleared without antibiotic intervention. Therefore tylosin administration to primigravid heifers 2 wk before parturition should not be advised without a previous analysis of udder health, management, and economic implications in each individual farm. Furthermore, in this study 10% of the heifers acquired IMI within the first week after calving. Therefore, special attention has to be given to environmental and housing conditions. Antibiotic therapy will not replace poor management in a close-up, calving or fresh groups.
Notes
¤ To cite this article: Contreras GA, Munoz JD, Sears PM. Reducing the incidence of intramammary infection in heifers by using prepartum systemic tylosin therapy: initial results of a single herd pilot study. Rev Colomb Cienc Pecu 2013; 26:119-126.
Acknowledgments
The American Association of Bovine Practitioners (AABP) funded this study through its research assistantship for graduate students.
References
Aarestrup FM, Jensen NE. Prevalence and Duration of Intramammary Infection in Danish Heifers During the Peripartum Period. J Dairy Sci 1997; 80:307-312. [ Links ]
Borm AA, Fox LK, Leslie KE, Hogan JS, Andrew SM, Moyes KM, Oliver SP, Schukken YH, Hancock DD, Gaskins CT, Owens WE, Norman C. Effects of prepartum intramammary antibiotic therapy on udder health, milk production, and reproductive performance in dairy heifers. J Dairy Sci 2006; 89:2090-2098. [ Links ]
Compton CWR, Heuer C, Parker K, McDougall S. Epidemiology of mastitis in pasture grazed peripartum dairy heifers and its effects on productivity. J Dairy Sci 2007; 90:4157-4170. [ Links ]
Contreras G, Rodríguez J. Mastitis: Comparative etiology and epidemiology. J Mammary Gland Biol 2011; 16: 339-356. [ Links ]
Contreras GA. Alternative strategies in the management of heifers' mastitis. Alternativas en el manejo de la mastitis en Novillas. Revista MVZ Cordoba 2009; 14:1642-1653. [ Links ]
Diarra MS, Malouin F, Jacques M. Postantibiotic and physiological effects of tilmicosin, tylosin, and apramycin at subminimal and suprainhibitory concentrations on some swine and bovine respiratory tract pathogens. Int J Antimicrob Ag 1999; 12: 229-237. [ Links ]
el-Sayed M, el-Attar H, Atef M, Yousif M. Pharmacokinetic profile of tylosin in mastitic cows. Dtsch Tierarztl Wochenschr 1986; 93:326-328. [ Links ]
Fox LK, Chester ST, Hallberg JW, Nickerson SC, Pankey JW, Weaver LD. Survey of intramammary infections in dairy heifers at breeding age and first parturition. J Dairy Sci 1995; 78:1619- 1628. [ Links ]
Hogan J, Gonzalez R, Harmon R, Nickerson SC, Oliver S, Pankey J. Laboratory handbook on bovine mastitis. The National Mastitis Council, Inc, Madison, WI. 1999. [ Links ]
Kreiger M, Friton GM, Hofer J, Fuchs K, Winter P. Effects of periparturient systemic treatment with penethamate hydriodide on udder health and milk yield of dairy heifers. J Dairy Res 2007; 74:392-398. [ Links ]
McDougall S, Agnew KE, Cursons R, Hou XX, Compton CRW. Parenteral treatment of clinical mastitis with tylosin base or penethamate hydriodide in dairy cattle. J Dairy Sci 2007; 90:779-789. [ Links ]
Middleton JR, Timms LL, Bader GR, Lakritz J, Luby CD, Steevens BJ. Effect of prepartum intramammary treatment with pirlimycin hydrochloride on prevalence of early first-lactation mastitis in dairy heifers. J Am Vet Med Assoc 2005; 227:1969- 1974. [ Links ]
Oliver SP, Headrick SI, Gillespie BE, Lewis MJ, Johnson DL, Lamar KC, Moorehead H, Dowlen HH, Hallberg JW. Intramammary infections in heifers during early lactation following intramammary infusion of pirlimycin hydrochloride or penicillin-novobiocin at the first milking after parturition. J Dairy Res 2007; 74:211-217. [ Links ]
Oliver SP, Lewis MJ, Gillespie BE, Dowlen HH, Jaenicke EC, Roberts RK. Prepartum antibiotic treatment of heifers: milk production, milk quality and economic benefit. J Dairy Sci 2003; 86:1187-1193. [ Links ]
Omura S. Macrolide antibiotics macrolide antibiotics, chemistry, biology and practice. Orlando: Academic Press; 1984. p 302-338. [ Links ]
Parker KI, Compton CWR, Anniss FM, Heuer C, McDougall S. Quarter-level analysis of subclinical and clinical mastitis in primiparous heifers following the use of a teat sealant or an injectable antibiotic, or both, precalving. J Dairy Sci 2008; 91:169-181. [ Links ]
Riviere J. Comparative pharmacokinetics: principles, techniques, and applications. Ames: Iowa State University Press; 1999. [ Links ]
Taponen S, Koort J, Bjorkroth J, Saloniemi H, Pyorala S. Bovine intramammary infections caused by coagulase-negative Staphylococci may persist throughout lactation according to amplified fragment length polymorphism-based analysis. J Dairy Sci 2007; 90:3301-3307. [ Links ]
Trinidad P, Nickerson SC, Adkinson RW. Histopathology of Staphylococcal mastitis in unbreed dairy heifers. J Dairy Sci 1990a; 73:639-647. [ Links ]
Trinidad P, Nickerson SC, Alley TK. Prevalence of intramammary infection and teat canal colonization in unbreed and primigravid dairy heifers. J Dairy Sci 1990b; 73:107-114. [ Links ]
Ziv G and Sulman F. Serum and milk concentrations of spectinomycin and tylosin in cows and ewes. Am J Vet Res 1973; 34:329-333. [ Links ]