Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Revista Colombiana de Ciencias Pecuarias
versão impressa ISSN 0120-0690
Rev Colom Cienc Pecua vol.26 no.3 Medellín jul./set. 2013
ORIGINAL ARTICLES
Sex-related head size and shape dimorphism in Mapaná snakes (Bothrops asper) kept in captivity¤
Dimorfismo sexual en la forma y tamaño de la cabeza de serpientes Mapaná (Bothrops asper) mantenidas en cautiverio
Dimorfismo sexual no tamanho e na forma da cabeça de serpente de Mapana (Bothrops asper) mantidos sob condições de cativeiro
Ana M Henao-Duque1, MV; Claudia P Ceballos2, MV, MSc, PhD.
* Corresponding author: Claudia P Ceballos. Facultad de Ciencias Agrarias, Universidad de Antioquia A.A. 1226, Medellín, Colombia. E-mail: claudiaceb@gmail.com
1 Programa de Ofidismo/Escorpionismo, Universidad de Antioquia, A.A. 1226, Medellín, Colombia.
2 Grupo de Investigación en Ciencias Veterinarias-CENTAURO, Facultad de Ciencias Agrarias, Universidad de Antioquia A.A. 1226, Medellín, Colombia.
(Received: May 25, 2012; accepted: October 29, 2012)
Summary
Background: sexual size dimorphism in snakes is generally well documented, however, sexual shape dimorphism has been poorly studied. As snakes are considered gape-limited predators, identifying patterns of sexual size and head shape dimorphism can help elucidate the life history of these organisms. Objective: to detect differences between sexes regarding head size and shape dimorphism of Mapaná snakes (Bothrops asper) maintained in captivity under the same diet in order to determine if it has a plastic or genetic origin. Methods: geometric morphometrics were used to quantify the head size and shape of male and female Mapaná snakes. Results: our results suggest that head shape is sexually dimorphic, being relatively wider in females compared to males. In both sexes head shape also varied with snout-vent length (SVL), growing wider as body size increases. Head size was also sexually dimorphic, with female head being larger than that of males of the same body length. Head size also increased with SVL. However, female head size increased disproportionally faster when compared to males. Conclusions: evidence of sexual differences in head size and shape of Mapaná snakes raised under the same diet was found. These findings suggest that sexual head size and shape dimorphism is not a plastic response given that both sexes were maintained under similar conditions,which suggests a strong genetic basis. Sexual shape dimorphism is also being mediated by stronger phenotypic changes of females while males seem to have a more constrained phenotypic head development.
Key words: Colombia, geometric morphometrics, phenotypic plasticity, sexual dimorphism.
Resumen
Antecedentes: el dimorfismo sexual en el tamaño de las serpientes está bien documentado, sin embargo el dimorfismo sexual en la forma ha sido pobremente estudiado. Dado que la dieta de las serpientes está limitada por el ancho de su hocico, identificar patrones de dimorfismo sexual en la forma y tamaño de la cabeza es útil para comprender mejor su historia de vida. Objetivo: detectar evidencias de dimorfismo sexual en el tamaño y forma de la cabeza de serpientes Mapaná (Bothrops asper) mantenidas bajo la misma dieta para determinar si su origen es genético o plástico. Métodos: se utilizó morfometría geométrica para cuantificar el tamaño y la forma de la cabeza de machos y hembras. Resultados: los resultados sugieren que la forma de la cabeza es sexualmente dimórfica, siendo más ancha en las hembras. En ambos sexos, la forma de la cabeza varió positivamente con la longitud hocico-cola (SVL). El tamaño de la cabeza también fue sexualmente dimórfico, siendo más grande en las hembras que en machos de la misma talla. El tamaño de la cabeza también aumentó con la SVL; sin embargo, este aumento fue desproporcionalmente más rápido en las hembras. Conclusiones: se encontraron evidencias de dimorfismo sexual en el tamaño y la forma de la cabeza de serpientes Mapaná alimentadas con la misma dieta. Los hallazgos sugieren que este dimorfismo sexual es de origen genético y no es una respuesta plástica, debido a que ambos sexos fueron mantenidos bajo condiciones homogéneas.Este dimorfismo es además mediado por un ambio fenotípico más fuerte en las hembras, mientras que los machos parecen tener un desarrollo fenotípico más canalizado.
Palabras clave: Colombia, dimorfismo sexual, morfometría geométrica, plasticidad fenotípica.
Resumo
Antecedentes: o dimorfismo sexual no tamanho das serpentes está bem documentado, no entanto o dimorfismo sexual na forma tem sido pobremente estudado. Dado que a dieta das serpentes é limitada pela largura de seu focinho, identificar padrões de dimorfismo sexual no tamanho e forma da cabeça é útil para compreender melhor a sua história de vida. Objetivo: investigar as evidências de dimorfismo sexual no tamanho e forma da cabeça da serpente Jararaca (Bothrops asper), mantidas em condições homogêneas para ambos os sexos com o intuito de esclarecer se a origem deste dimorfismo é plástica ou genética. Métodos: neste estudo utilizamos morfometria geométrica para quantificar o tamanho e o formato da cabeça de machos e fêmeas. Resultados: nossos resultados sugerem que a forma da cabeça é sexualmente dimórfica, sendo mais larga nas fêmeas. Este formato teve uma variação positiva com o comprimento rostro-cauda, este efeito foi observado em ambos os sexos. O tamanho da cabeça também é sexualmente dimórfico, sendo maior nas fêmeas do que nos machos do mesmo tamanho. O tamanho da cabeça também aumentou com o tamanho, no entanto, esse aumento foi desproporcionalmente mais rápido nas fêmeas. Conclusões: neste estudo foram encontradas evidências de dimorfismo sexual no tamanho e na forma da cabeça das serpentes Jararaca alimentadas com a mesma dieta. Sugerimos que este dimorfismo sexual é de origem genética, e não é uma resposta plástica, e é mediado por uma mudança fenotípica mais forte nas fêmeas, enquanto os machos parecem ter um desenvolvimento fenotípico mais canalizado.
Palavras chave: Colômbia, dimorfismo sexual, morfometria geométrica, plasticidade fenotípica.
Introduction
Sexual size dimorphism (SSD) has been well described in vertebrates, including snakes (Shine, 1994; Fairbairn et al., 2007). Snakes are an interesting group to study SSD because they exhibit both patterns: female-biased SSD, in which females are the larger sex (Solorzano and Cerdas, 1989; Rivas and Burghardt, 2001; Krause et al., 2003; Furtado et al., 2006; Pinto et al., 2008) and the less common male-biased SSD, in which males are the larger sex (Taylor and Denardo, 2005; Dubey et al., 2009). Comparatively, there have been fewer empirical studies conducted on sexual shape dimorphism (SShD); some examples include reptiles such as tuataras (Herrel et al. 2010), lizards (Kaliontzopoulou et al., 2008; Kuo et al., 2009b; Ljubisavljevic et al., 2010), and snakes (Vincent et al., 2004b; Smith and Collyer, 2008; Tomovic et al., 2010). In particular, the study of SSD and SShD on snakes' heads is important because they are considered gape-limited predators, and thus studying how head size and shape varies with sex, body size, diet, and environment can direct our understanding of not only the life history of the species, but also of the mechanisms originating and/or maintaining sexual dimorphism itself.
Among the ultimate (evolutionary) mechanisms originating and/or maintaining SSD and SShD are natural selection in the form of niche partitioning via prey divergence (Vincent et al., 2004a), fecundity selection when larger females have larger clutches compared to smaller females (Kuo et al., 2009a), or sexual selection when larger males are more successful in male-male combats to gain access to mating (Shine, 1994, 2000; Ljubisavljevic et al., 2010). Conversely, proximate mechanisms include differential growth rates in which one sex grows faster than the other with both sexes reaching sexual maturity at the same age (Shine and Crews, 1988; Shine, 1994; Lerner and Mason, 2001), or when both sexes grow at the same rate, but one sex reaches sexual maturity at a relatively older age (Andrews, 1982; Parker and Plummer, 1987; Kozlowski, 1989). The environment itself can also have an effect on body and head size and shape in reptiles via phenotypic plasticity (Vincent et al., 2004; Ceballos and Valenzuela, 2011). For example, head SSD and SShD was studied in wild cottonmouth snakes (Agkistrodon piscivorus), a male-biased SSD species. In this study, head SShD was supported with males having longer quadrate bones, which allows the lower jaw to open further and consume larger prey relative to females (Vincent et al., 2004). In a second study, Gaboon vipers (Bitis gabonica) fed in captivity under a low-food diet had narrower heads compared to vipers fed a more abundant diet (Bonnet et al., 2001). In the latter study, however, head shape differences between sexes were not found. Thus, these studies fail to prove whether plasticity is a proximate mechanism of sexual dimorphism for the heads of snakes.
In this study, we used geometric morphometrics to test for differences in head SSD and SShD of Mapaná snakes (Bothrops asper). Because we used snakes of both sexes maintained in captivity under homogeneous conditions and fed the same type of prey (see methods), this study should also help to elucidate if head sexual dimorphism has a plastic origin as has been suggested (Bonnet et al., 2001; Vincent et al., 2004); or if, on the contrary, it has a genetic origin.
Linear measurements have traditionally been used to study head size and shape in reptiles (Bonnet et al., 2001; Vincent et al., 2004b; Kuo et al., 2009a; Tomovic et al., 2010). However, descriptions of shape variation are limited to a function of specific linear measurements, such as size measurements (e.g., head width relative to lateral head length) (Vincent et al., 2004b; Kuo et al., 2009a). Geometric morphometrics (GM) is a more developed technique (Rohlf and Slice, 1990) by which biological shape (size-free) and size are quantified to detect even subtle differences in morphology (Ceballos et al., 2013; Valenzuela et al., 2004). Another advantage of GM is that it allows us to graph average shapes for a better visualization of morphological variation. Only one study has used GM techniques to test for head SSD and SShD in snakes, but only morphological head differences associated to the region of origin were found (Smith and Collyer, 2008). Likewise, because diet shifts occur with ontogeny in snakes (Ford and Hampton, 2009; Sasa et al., 2009) we also tested for any effect of body length (correspondent to age) on head size and shape and any potential interaction between sex and body length.
Bothrops asper (Viperidae), locally known as the Mapaná snake, is a venomous species of special interest because they are responsible for most (50- 70%) ophidian accidents in Colombia (Otero et al., 1992). This species is widely distributed in America, reaching from Mexico to Ecuador (Campbell and Lamar, 2004) and has a large phenotypic variation in its scales and blotches (Saldarriaga et al., 2009). It exhibits a female-biased SSD pattern with females having greater SVL, more dorsal and ventral scale rows, and shorter venttip of tail length when compared to males (Sasa et al., 2009). B. asper is an overall diet generalist consuming mammals, anurans, snakes, lizards, and birds (Martins et al., 2002), however, it exhibits an ontogenetic shift in prey types, from ectotherms as juveniles to endotherms as adults (Campbell and Lamar, 2004). In addition, some studies have found subtle differences in prey in the stomach content between males and females (Sasa et al., 2009), suggesting potential sexual differences in the diet.
Materials and methods
Captivity conditions at the serpentarium
All snakes are measured (total body length and snout-vent length, cm), weighed, and sexed upon arrival to the collection. The sexing method consists of inserting a sexing probe into the vent (Stahl, 2001). If the probe penetrates 1 to 2 ventral scales the individual is classified as a female, but if the probe penetrates 3 or more ventral scales it is classified as a male (Stahl, 2001). Snakes are fed approximately 10% of its body weight with lab albino mice of different ages, which range between newborn 1 to 2 g mice for newborn snakes every 10 d; 2 to 4 g mice for juvenile snakes every 15 d; and up to 26 g mice for adult snakes every 30 d. Water is offered ad libitum. Snakes are housed in wooden boxes of three different sizes (from 28.5 x 33 x 13 cm to 58 x 36 x 22 cm), depending on the size of the snake. Environmental temperature and humidity are not controlled and are expected to be similar to those of the city of Medellín (Antioquia, Colombia): average temperature of 23 °C (range 16 to 28 °C), and relative humidity between 50-65% (IDEAM, 2005).
Data Collection
A total of 114 Bothrops asper are currently maintained under captive conditions at the Serpentarium at the University of Antioquia in Medellín. Of this sample, there were 44 males (37 adults and 7 juveniles) and 70 females (65 adults and 5 juveniles). Male individuals under 99.5 cm, and females under 111.3 cm total body length were considered juveniles (Solorzano and Cerdas, 1989). We evaluated all snakes (n=114) to test for the existence of head SSD and SShD. Most (n=107) of the snakes came from the Magdalena region, while the remainder came from the pacific region (Chocó, n=2), Caribbean region (Maracaibo, n=3), while 2 were of unknown origin.
Because snakes entered the collection at different life stages (12.3% were born in situ, 37.7% entered as juveniles, 19.3% entered as adults, and 30.7% have no records), some of these animals may have reached sexual maturity in the wild. Thus, in order to test if sexual dimorphism also develops under captive conditions with both sexes receiving the same prey type, we used animals that achieved sexual maturity in captivity. These are animals with initial total body length under 50 cm, above 100 cm at present. Only 22 animals, 15 females and 7 males filled this criterion.
Body weight (BW, g), total body length (TBL, cm) and snout-vent length (SVL, cm) were measured, and dorsal views of the heads of all snakes in collection were photographed. To record this information each snake was sedated by introducing it into a 25 gallon container filled with carbon dioxide gas for approximately 2 m. Dorsal views of the snakes' heads were photographed with a digital camera (Olympus SP-500UZ, Center Valley, PA, USA) attached to a tripod. Total body length (cm) and snout-vent linear length (SVL, cm) were recorded using a metric tape. Then, snakes were returned to their cages where they were monitored until their full recovery. The sedation and manipulation process took approximately 5 m per individual. Data on initial body weight and initial total body length were obtained from the Serpentarium records. This protocol was approved by the Animal Experimentation Ethics Committee of the University of Antioquia, Act 72, issued on September 22, 2011.
Geometric-morphometric head size and shape quantification
To quantify head size and head shape of Mapaná snakes we used GM methods (Rohlf and Slice, 1990). A total of 17 landmarks were digitized from photographs of the dorsal side of the snake. Seven of these represented fixed anatomical points, while 10 were sliding semi-landmarks that captured the contour of the head and the supraocular scales (modified from Smith and Collyer, 2008; Figure 1). Landmarks 1 and 17 were located at the head-neck inflexion point, landmarks 5 to 7 and 11 to 13 around the supraocular scales, landmark 9 at the most anterior and medial point of the snout, and the remaining landmarks were located equidistantly at the head contour. Sliding landmarks were allowed to slide along the curve of the head, which improves fitting of the contour. All landmarks were then subjected to a Generalized Procrustes Analysis, which consists of superimposing all landmarks in a coordinate system while holding constant variation due to position, orientation, and size (Rohlf and Slice, 1990). From this analysis we obtained 14 shape variables that provided a multivariate and size-free shape description of the head shape of each animal, and a univariate measure of head size (centroid size; see Bookstein, 1991). Finally, with the objective of visualizing head shape differences among groups, the average shapes were calculated and graphed. Morphometric analyses were performed with tpsDig, tpsRelw, and tpsSplin software (Rohlf, 2001; 2003; 2004).
Data Analysis
We used the full set of animals (n=114) to test for differences in the snake's head shape and size associated to sex, TBL, and any potential interaction (sex x TBL), by performing a permutational multivariate and univariate analyses of variance, respectively (Sokal and Rohlf, 1995). Nonparametric multivariate analyses allowed us to use landmarks in the whole head to fully capture an accurate head shape, as opposed to using landmarks in one half of the head and obtaining a mathematically-calculated head shape which can introduce perceived shape differences not present in the dataset (Pessa et al., 2008). These analyses were implemented using the adonis function in the Vegan package of the R program (Oksanen et al., 2010). To test if head SSD and SShD is a plastic effect or has a genetic basis, analyses were repeated with a subset of animals (n=22) that achieved sexual maturity in captivity (described above). Finally, to test for sexual dimorphism in growth rates (body weight and total body length) associated to captivity we performed analyses of variance at both arrival to collection and after being in captivity using the same subset of animals (n=22).
Results
Head sexual shape dimorphism
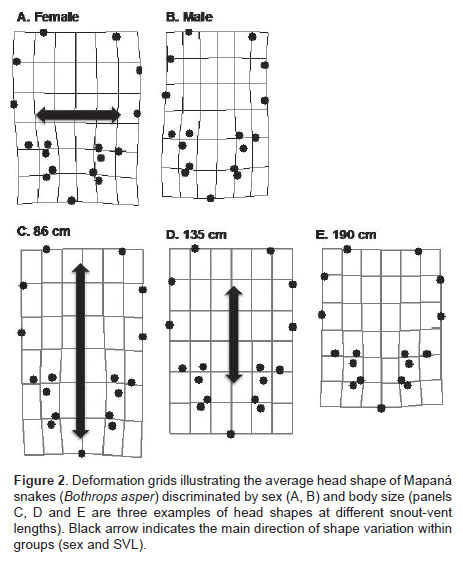
The head shape of B. asper was found to be sexually dimorphic (Table 1), with the head of females relatively wider than the head of males (Figure 2, panels A and B). We also found that head shape covaries with total body length, independent of sex. The interaction sex × SVL was not significant, indicating that allometric patterns of head shape were consistent between males and females. This pattern was generally described by longer individuals having relatively shorter but wider heads, while shorter individuals had relatively longer and thinner heads (Figure 2, panels C to E). Results did not change when the same analyses were repeated with the subset of animals that reached sexual maturity in captivity; head shape remained sexually dimorphic (F=3.279, p=0.012), it covaried with body length (F=2.985, p=0.019), and such sexual shape dimorphism was independent of body length (F=1.824, p=0.103).
Sexual head size dimorphism
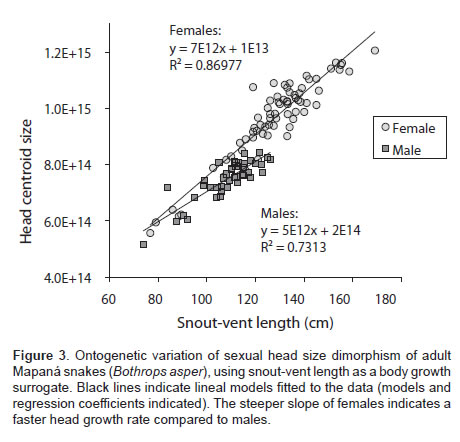
The head size of B. asper was found to be sexually dimorphic (Table 1), with females displaying about 28% larger heads than males on average. Head size was also positively correlated with SVL. The interaction sex × SVL was significant, indicating that head size in one sex increased as a function of SVL disproportionally faster than in the other sex. To better understand this relationship we fitted a linear model of head size and SVL of each sex using the Test of slopes function in the smart library in R software. We found that the slope of females (b=7.44e+12, p<0.0001, R2=0.8684) was significantly steeper (p<0.0001) than the slope of males (b=5.34e+12, p<0.0001, R2=0.7312), confirming that head size in females increased at a faster rate than it did in males (Figure 3).
Using the subset of animals that reached maturity in captivity we found that head size was sexually dimorphic (F=131.004, p=0.0001), and that head size covaried with total body length (F=0.399, p=0.0001). In contrast to the results above, our study shows that SSD was independent of body length (F=4.175, p=0.053). However it should be noted in absolute values the head size of females also increases at a faster rate than that of males (bfemales=6E+12>bmales=3E+12).
Growth rates
Using only individuals that reached maturity in captivity (n=22) we found no differences in body weight (F=7.5183, p>0.06), or total body length (F=2.4358, p>0.1) between the sexes upon arrival to the collection. However, these same animals exhibited sexual differences in body weight (F=7.5183, p=0.0125) and total body length (F=10.273, p=0.004). This indicates that females obtained greater length (TBLfemales=148.99 cm, TBLmales=124.18 cm) and weight (BWfemales=1045 g, BWmales=582 g) than males in captive conditions (Table 2). As previously mentioned, body growth was achieved by males and females fed the same type of diet (mice) while varying only the amount of food (number of mice offered) according to the body weight of the snake. Conversion rates were not calculated because food weight (mouse weight) offered to each snake is not regularly recorded.
Discussion
This study found that the head of B. asper is sexually dimorphic in terms of shape and size, with females having relatively wider and larger heads compared to males. We also found that head size and shape increases as body length increases. While other forms of sexual dimorphism on body size, numbers of scales, and tail coloration have been reported for B. asper (Solorzano and Cerdas, 1989; Sasa 2002; Hoyos et al. 2003; Sasa et al. 2009), this is the first report on sexual shape dimorphism in the head of this species. In addition, because we found that head sexual dimorphism developed in snakes maintained under the same feeding conditions, the sole variation being the amount of food offered to snakes with prey type remaining constant, we reject a plasticity origin of head SSD and SShD (Bonnet et al., 2001; Vincent et al., 2004). We suggest that head SSD and SShD in B. asper is more likely to be caused physiologically, thus indicating underlying genetic differences between sexes.
Finding head sexual differences in B. asper is of particular importance given that they are gapelimited predators (Forsman, 1991), and suggests that the type of prey that each sex of B. asper consumes in the wild may be mediated by its head size and shape. Indeed, wild females in Costa Rica have been reported to consume mainly rodents, while males consume rodents in addition to birds and lizards (Sasa et al., 2009). Sexual differences in the venom composition and volume have also been reported in Bothrops jararaca, which suggests it may also be possible in B. asper. Females of B. jararaca produce five times more venom than males, and that of females has a higher hemorrhagic and lethal activity than that of males (Furtado et al., 2006). Other studies found that some peptides are found only in the female venom of B. jararaca (Pimenta et al., 2007). Further field studies on stomach content discriminated by sex are needed to confirm if such sexual differences in the diet are general in this species, as it would support the niche partitioning hypothesis (Furtado et al., 2006) as an ultimate mechanism maintaining head SSD and SShD in B. asper.
Likewise, a larger head and a wider gape may not only facilitate the ingestion of larger prey by females, but also facilitate the ingestion of more mammals given larger prey items are generally mammals compared to insects, reptiles and birds. A recent study on the nutritional value of snake prey found that mice are richer in energy and lipids than lizards and crickets (Zuffi et al., 2010), which may favor lipid reserves needed by females during vitellogenesis (Solorzano and Cerdas, 1989). Larger B. asper females have been correlated with larger clutches (Sasa et al., 2009), thus, it seems there is a direct relationship between a larger and wider head, an energy-rich diet, and a fecundity advantage in females.
In addition to sex, we also found that head size and shape also varied in terms of body length, and thus age (under normal conditions). Indeed, an ontogenetic shift in the diet and the venom composition of B. asper has been reported. Juveniles of B. asper consume mainly ectotherms, such as frogs and lizards, while adults consume mainly endotherms, including birds and mammals (Sasa et al., 2009). Accordingly, an ontogenetic variability in the venom composition has also been reported in B. asper (Saldarriaga et al., 2003; Alape-Giron et al., 2008), and its congeners B. atrox (Saldarriaga et al., 2003; Guercio et al., 2006; Salazar et al., 2007) and B. jararaca (Pimenta et al., 2007; Zelanis et al., 2010). Thus, observing these sexual and ontogenetic dietary shifts we suggest that juveniles of both sexes of B. asper may prey mainly on ectotherms and that only adult females may switch to endotherms while adult males may maintain the same diet of juveniles. It would be interesting to test for diet differences between juveniles and adults while taking into account the sex as suggested above. Such information would also help to explain the large variability of the predator-prey mass ratio (0.002–0.889) observed for Bothrops spp. in general (Martins et al., 2002).
Our findings show that females not only have wider and larger heads, but that head size increases at a relatively faster rate than males (Figure 3). In addition, the head shape of females is similar to that of larger individuals (Figure 2, panels A and E), and the head shape of males resembles that of smaller individuals (Figure 2, panels B and C). These observations have important developmental implications (i.e., it suggests that head SSD and SShD is driven by stronger phenotypic development of females, while males seem to have a more constrained development of the head phenotype). Previous studies have shown that the sexual hormone testosterone has a differential growth role in male-biased or female-biased SSD species (Shine and Crews, 1988; Lerner and Mason, 2001; John- Alder and Cox, 2007). In the case of B. asper, a female-biased SSD species, testosterone may inhibit the head growth of males. Further experimental studies manipulating testosterone availability may be useful to test this hypothesis.
Notas
¤ To cite this article: Henao-Duque AM, Ceballos CP. Sex-related head size and shape dimorphism in Mapaná snakes (Bothrops asper) kept in captivity. Rev Colomb Cienc Pecu 2013; 26:201-210.
Acknowledgments
We thank J. Asprilla at the Serpentarium of the University of Antioquia for his observations on the snakes that inspired this study and his logistic support during data collection. We thank D.C. Adams for valuable comments to an earlier version of this manuscript. This study was logistically supported by the Ophidism/Scorpionism program and economically sponsored by the 2013-2014 Sustainability Program of the University of Antioquia.
References
Alape-Giron A, Sanz L, Escolano J, Flores-Diaz M, Madrigal M, Sasa M, Calvete JJ. Snake venomics of the lancehead pitviper Bothrops asper: geographic, individual, and ontogenetic variations. J Proteome Res 2008; 7:3556-3571. [ Links ]
Andrews RM. Patterns of growth in reptiles. In: Gans C, Pough FH, editors. Biology of the reptilia. New York: Academic Press; 1982. [ Links ]
Bonnet X, Shine R, Naulleau G, Thiburce C. Plastic vipers: influence of food intake on the size and shape of Gaboon vipers (Bitisgabonica). J Zool Lond 2001; 255:341-351. [ Links ]
Bookstein FL. Morphometric tools for landmark data: Geometry and Biology. New York: Cambridge Press; 1991. [ Links ]
Campbell JA, Lamar WW. The venomous reptiles of the western hemisphere. Ithaca: Cornell University Press; 2004. [ Links ]
Ceballos CP, Valenzuela N. The role of sex-specific plasticity in shaping sexual dimorphism in a long-lived vertebrate, the snapping turtle Chelydraserpentina. Evol Biol 2011; 38:163- 181. [ Links ]
Ceballos CP, Hernández O, Valenzuela N. Divergent sexspeci fic plasticity in long-lived vertebrates with contrasting sexual dimorphism. Evol Biol 2013 (DOI) 10.1007/s11692-013- 9249-0 [ Links ]
Dubey S, Brown GP, Madsen T, Shine R. Sexual selection favours large body size in males of a tropical snake (Stegonotuscucullatus, Colubridae). Anim Behav 2009; 77:177- 182. [ Links ]
Fairbairn DJ, Blanckenhorn WU, Szekely T. Sex, size and gender roles. Evolutionary studies of sexual size dimorphism. New York: Oxford University Press; 2007. [ Links ]
Ford NB, Hampton PM. Ontogenetic and sexual differences in diet in an actively foraging snake, Thamnophisproximus. Can J Zool/Rev Can Zool 2009; 87:254-261. [ Links ]
Forsman A. Variation in sexual size dimorphism and maximum body size among adder populations: Effects of prey size. J Anim Ecol 1991; 60:253-267. [ Links ]
Furtado MFD, Travaglia-Cardoso SR, Rocha MMT. Sexual dimorphism in venom of Bothrops jararaca (Serpentes: Viperidae). Toxicon 2006; 48:401-410. [ Links ]
Guercio RAP, Shevchenko A, Lopez-Lozano JL, Paba J, Sousa MV, Ricart CAO. Ontogenetic variations in the venom proteome of the Amazonian snake Bothrops atrox. Proteome Sci 2006; 4:1-14. [ Links ]
Herrel A, Moore JA, Bredeweg EM, Nelson NJ. Sexual dimorphism, body size, bite force and male mating success in tuatara. Biol J Linn Soc 2010; 100:287-292. [ Links ]
Hoyos MA, Otero R, Saldarriaga M, Jaramillo N. Divergencia morfométrica entre Bothrops atrox y Bothrops asper (Serpentes: Viperidae). Actual Biol 2003; 25:157-165. [ Links ]
IDEAM. Atlas climatológico de Colombia. Colombia: Imprenta Nacional de Colombia; 2005. [ Links ]
John-Alder H, Cox RM. Development of sexual size dimorphism in lizards: testosterone as a bipotential growth regulator. In: Fairbairn DJ, Blackenhorn WU, Székely T, editors. Sex, size and gender roles: evolutionary studies of sexual size dimorphism. New York:Oxford University Press; 2007. [ Links ]
Kaliontzopoulou A, Carretero MA, Llorente GA. Head shape allometry and proximate causes of head sexual dimorphism in Podarcis lizards: joining linear and geometric morphometrics. Biol J Linn Soc 2008; 93:111-124. [ Links ]
Kozlowski J. Sexual size dimorphism: A life history perspective. Oikos 1989; 54:253-255. [ Links ]
Krause MA, Burghardt GM, Gillingham JC.Body size plasticity and local variation of relative head and body size sexual dimorphism in garter snakes (Thamnophissirtalis). J Zool 2003; 261:399-407. [ Links ]
Kuo C-Y, Lin Y-T, Lin Y-S. Sexual size and shape dimorphism in an agamid lizard, Japaluraswinhonis (Squamata: Lacertilia: Agamidae). Zool Stud 2009; 48:351-361. [ Links ]
Lerner DT, Mason RT. The influence of sex steroids on the sexual size dimorphism in the red-spotted garter snake, Thamnophissirtalisconcinnus. Gen Comp Endocr 2001; 124:218-225. [ Links ]
Ljubisavljevic K, Urosevic A, Aleksic I, Ivanovic A. Sexual dimorphism of skull shape in a lacertid lizard species (Podarcis spp., Dalmatolacerta sp., Dinarolacerta sp.) revealed by geometric morphometrics. Zoology 2010; 113:168-174. [ Links ]
Martins M, Marques OAV, Sazima I. Ecological and phylogenetic correlates of feeding habits in Neotropicalpitvipers (genus Bothrops). In: Schuett GW, Höggren M, Douglas ME, Greene HW, editors. Biology of the vipers. Eagle Mountain: Eagle Mountain Publishing; 2002. p. 307-328. [ Links ]
Oksanen J, Blanchet FG, Kindt R, Legendre P, O'Hara RB, Simpson GL, Solymos P, Stevens M, Wagner H. Vegan: community ecology package. R package version 1.17-4.2010. [ Links ]
Otero R, Tobón GS, Gómez LF, Osorio R, Valderrama R, Hoyos D, Urreta E, Molina S, Arboleda JJ. Accidente ofídico en Antioquia y Chocó. Aspectos clínicos y epidemiológicos. Acta Med Colomb 1992; 17:229-249. [ Links ]
Parker WS, Plummer MV. Population ecology. In: Seigel RA, Collins JT, Novak SS, editors. Snakes: ecology and evolutionary biology. New York: McGraw–Hill; 1987. p. 253- 301. [ Links ]
Pessa JE, Slice DE, Hanz KR, Broadbent TH, Rohrich RJ. Aging and the shape of the mandible.Plas Reconstr Surg 2008; 121:196-200. [ Links ]
Pimenta DC, Prezoto BC, Konno K, Melo RL, Furtado MF, Camargo ACM, Serrano SMT. Mass spectrometric analysis of the individual variability of Bothrops jararaca venom fraction. Evidence for sex-based variation among the bradykininpotentiating peptides. Rapid Commun Mass Spectrom 2007; 21:1034-1042. [ Links ]
Pinto RR, Fernandes R, Marques OAV. Morphology and diet of two sympatric colubrid snakes, Chironiusflavolineatus and Chironiusquadricarinatus (Serpentes: Colubridae). Amphibia- Reptilia 2008; 29:149-160. [ Links ]
Rivas JA, Burghardt GM. Understanding sexual size dimorphism in snakes: wearing the snake's shoes. Anim Behav 2001; 62:F1-F6. [ Links ]
Rohlf FJ, Slice D. Extensions of the Procrustes method for the optimal superimposition of landmarks. Syst Zool 1990; 39:40- 59. [ Links ]
Rohlf FJ. TpsDig, Version 1.31. Department of Ecology and Evolution. State University of New York at Stony Brook, Stony Brook, New York, USA. 2001. [ Links ]
Rohlf FJ. TpsRelw, Version 1.29. Department of Ecology and Evolution, State University of New York at Stony Brook, New York, USA. 2003. [ Links ]
Rohlf FJ. TpsSplin, Version 1.20. Department of Ecology and Evolution. State University of New York at Stony Brook, Stony Brook, New York, USA. 2004. [ Links ]
Salazar AM, Rodriguez-Acosta A, Giron ME, Aguilar I, Guerrero B. A comparative analysis of the clotting and fibrinolytic activities of the snake venom (Bothrops atrox) from different geographical areas in Venezuela. Thromb Res 2007; 120:95-104. [ Links ]
Saldarriaga MM, Otero R, Nunez V, Toro MF, Diaz A, Gutierrez JM. Ontogenetic variability of Bothrops atrox and Bothrops asper snake venoms from Colombia. Toxicon 2003; 42:405- 411. [ Links ]
Saldarriaga MM, Sasa M, Pardo R, Méndez MA. Phenotypic differences in a cryptic predator: Factors influencing morphological variation in the terciopelo Bothrops asper (Garman, 1884; Serpentes: Viperidae). Toxicon 2009; 54:923- 937. [ Links ]
Sasa M, Wasko DK, Lamar WW. Natural history of the terciopelo Bothrops asper (Serpentes: Viperidae) in Costa Rica. Toxicon 2009; 54:904-22. [ Links ]
Sasa M. Morphological variation in the lancehead pitviper Bothrops asper (Garman) (Serpentes: Viperidae) from middle America. Rev Biol Trop 2002; 50:259-271. [ Links ]
Shine R, Crews D. Why male gartner snakes have small heads: The evolution and endocrine control of sexual dimorphism. Evolution 1988; 42:1105-1110. [ Links ]
Shine R. Sexual size dimorphism in snakes revisited. Copeia 1994; 2:326-346. [ Links ]
Shine R. Vertebral numbers in male and female snakes: the roles of natural, sexual and fecundity selection. J Evol Biol 2000; 13:455-465. [ Links ]
Smith MT, Collyer ML. Regional variation and sexual dimorphism in head form of the prairie rattlesnake (Crotalusviridisviridis): comparisons using new analytical and collection methods. In: Beaman KR, Caldwell MD, Bush SP, editors. The biology of rattlesnakes: Loma Linda university Press, California; 2008. [ Links ]
Solorzano A, Cerdas L. Reproductive Biology and Distribution of the Terciopelo, Bothrops asper Garman (Serpentes: Viperidae), in Costa Rica. Herpetologica 1989; 45:444-450. [ Links ]
Stahl SJ, editor. Reptile production medicine. In: Seminars in Avian and ExoticPet Medicine. Ciudad Saunders; 2001:140-150. [ Links ]
Taylor EN, Denardo DF. Sexual size dimorphism and growth plasticity in snakes: an experiment on the Western Diamondbacked Rattlesnake (Crotalusatrox). J Exp Zool A Comp Exp Biol 2005; 303:598-607. [ Links ]
Tomovic LM, Crnobrnja-Isailovic JM, Ajtic RD, Aleksic ID, Djordjevic SZ. When do meadow vipers (Viperaursinii) become sexually dimorphic? ontogenetic patterns of sexual size dimorphisms. J Zool Syst Evol Res 2010; 48:279-282. [ Links ]
Valenzuela N, Adams DC, Bowden RM, Gauger AC. Geometric morphometric sex estimation for hatchling turtles: A powerful alternative for detecting subtle sexual shape dimorphism. Copeia 2004; 4:735-742. [ Links ]
Vincent SE, Herrel A, Irschick DJ. Ontogeny of intersexual head shape and prey selection in the pitviper Agkistrodonpiscivorus. Biol J Linn Soc 2004; 81:151-159. [ Links ]
Vincent SE, Herrel A, Irschick DJ. Sexual dimorphism in head shape and diet in the cottonmouth snake (Agkistrodonpiscivorus). J Zool 2004; 264:53-59. [ Links ]
Zelanis A, Tashima AK, Rocha MMT, Furtado MF, Camargo ACM, Ho PL, Serrano SMT. Analysis of the ontogenetic variationin the venom proteome/peptidomeof Bothrops jararacareveals different strategies to deal with prey. J Proteome Res 2010; 9:2278-2291. [ Links ]
Zuffi, M. A. L., S. Fornasiero, R. Picchiotti, P. Poli, and M. Mele. Adaptive significance of food income in European snakes: body size is related to prey energetics. Biol J Linn Soc 2010; 100:307-317. [ Links ]