Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Colombiana de Ciencias Pecuarias
Print version ISSN 0120-0690
Rev Colom Cienc Pecua vol.26 no.3 Medellín July/Sept. 2013
CLINICAL CASES
Detection of macrophages infected with Mycobacterium avium subspecies paratuberculosis in a cow with clinical stage IV of Johne's disease. A case report ¤
Detección de macrófagos infectados con Mycobacterium avium subspecies paratuberculosis en una vaca en fase clínica IV de la enfermedad de Johne. Reporte de un caso
Detecção de macrófagos infectados com Mycobacterium avium paratuberculosis subespécie em uma vaca com uma fase clínica IV da doença de Johne. Um relato de caso
René Ramírez-García, MV, MSc; Juan G Maldonado-Estrada*, MVZ, MS, PhD.
* Corresponding author: Juan G Maldonado-Estrada. Escuela de Medicina Veterinaria, and Sede de Investigación Universitaria (SIU) Calle 62 # 52-59, Lab-233. Universidad de Antioquia. 050011 Medellín, Colombia. E-mail: juanguimal@gmail.com
Grupo de investigación CENTAURO, Escuela de Medicina Veterinaria, Facultad de Ciencias Agrarias. Universidad de Antioquia, Medellín, Colombia.
(Received: October 22, 2012; accepted: January, 29, 2013)
Summary
Anamnesis: a six-year-old milking Holstein cow (Bos taurus taurus) was diagnosed with stage IV Johne's Disease (JD). Clinical and Laboratory findings: the cow suffered intermittent diarrhea during 6 months with no response to antibiotic treatment. Consequently, the cow was subjected to euthanasia. Treatment approach: antemortem milk and peripheral blood samples and postmortem colon, mediastinic, mesenteric lymph nodes, and spleen samples were processed for macrophages isolation. Total DNA was extracted from macrophages and used to diagnose IS900 of Mycobacterium avium paratuberculosis (MAP) through real time PCR. The MAP IS900 segment was successfully amplified from cells of all samples, indicating that these cells were MAP-infected macrophages. Conclusion: macrophages of cows suffering from JD can be used for amplification of the MAP IS900 segment.
Key words: intracellular bacteria, real time PCR, resident macrophages, susceptible host.
Resumen
Anamnesis: una vaca Holstein de 6 años de edad (Bos taurus taurus) presentó sintomatología de la fase IV de la enfermedad de Johne. Hallazgos clínicos y de laboratorio: la vaca presentó diarrea intermitente durante 6 meses sin respuesta al tratamiento con antibióticos. En consecuencia, la vaca fue sometida a eutanasia. Esquema de tratamiento: muestras de leche y de sangre periférica se tomaron ante-mortem; muestras de la mucosa del intestino, bazo y linfonodos mediastínico y mesentérico, se tomaron post-mortem, todas para aislamiento de macrófagos. El ADN total de los macrófagos fue usado para la amplificación del segmento IS900 de Mycobacterium avium paratuberculosis (MAP) por PCR en tiempo real. Conclusión: los macrófagos de vacas con la enfermedad de Johne pueden ser usados para la amplificación del segmento IS900 de MAP.
Palabras clave: bacteria intracelular, hospedero susceptible, macrófagos residentes, PCR en tiempo real.
Resumo
Anamnese: uma vaca Holstein com 6 anos de idade (Bos taurus taurus) apresentou os sintomas da fase IV da doença de Johne. Achados clínicos e laboratoriais: a vaca teve diarreia intermitente por seis meses sem resposta ao tratamento com antibióticos. Por conseguinte, a vaca foi submetida à eutanásia. Abordagem de tratamento: amostras de leite e de sangue periférico foram retiradas ante-mortem, enquanto as amostras da mucosa intestinal, baço e linfonodos mesentéricos e mediastinais foram tomadas todas post-mortem para o isolamento de macrófagos. O ADN total de macrófagos foi utilizado para amplificação do segmento IS900 do Mycobacterium avium paratuberculosis (MAP) por PCR em tempo real. Conclusão: macrófagos isolados a partir de vacas com doença de Johne podem ser utilizados para a amplificação da segmento IS900 do MAP.
Palavras chave: bactérias intracelulares, hospedeiro suscetível, macrófagos residentes, PCR em tempo real.
Introduction
Paratuberculosis or Johne's disease (JD) is caused by the intracellular bacterium Mycobacterium avium subspecies paratuberculosis (MAP). MAP causes a devastating chronic disease in the affected animal. The prevalence and incidence of JD ranges from 5% to 100% (McNab et al., 1991). Our research group documented the presence of JD in the northern dairy region of Antioquia province (Colombia) based on clinical, pathological (Ramirez et al., 2011), and molecular results (Zapata-Restrepo et al., 2010; Fernandez-Silva et al., 2011b). Macrophages infected with MAP can circulate and re-circulate from Peyer's patches of infected intestinal mucosa to other tissues via blood and lymph compartments (Mutharia et al., 2010; Bimczok and Rothkötter, 2006). Consequently, we wondered if it would be possible to take advantage of this recirculation process for early detection and diagnosis in cows. The main objective of this work was to evaluate the usefulness of circulating and resident macrophages for amplification of the IS900 sequence of MAP.
Patient examination
Anamnesis
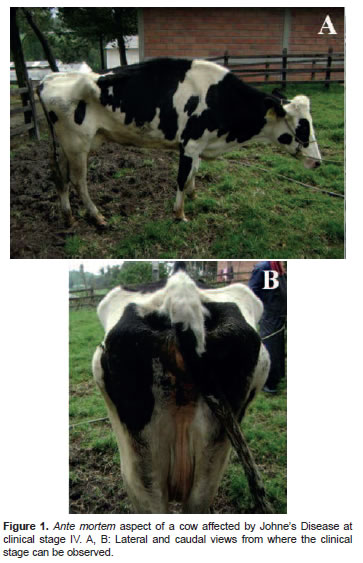
A six-year-old lactating Holstein cow (Bos taurus taurus) from the University of Antioquia's dairy herd was diagnosed with stage IV JD. The farm is located at 2200 m above sea level in a high-humidity region at San Pedro de los Milagros (Antioquia, Colombia). The farm is enzootic for JD as reported by Ramírez et al. (2011). The cow suffered from recurrent episodes of profuse diarrhea with progressive weight loss for 6 months.
Clinical findings
The cow's body condition score (BCS) was 1.5 (scale 1 to 5) and showed severe depression. Cardiac frequency was 80 beats/min. The animal was tachypneic and evidenced diarrhea. It had not eaten for several days (Figure 1). Rectal temperature was 39.5 °C. Mucosae were pale and dry. Lack of intestinal motility was also evidenced at auscultation of abdominal quadrants. The last milking record of the cow—taken 1 month before the clinical exam—indicated a yield of 20 kg milk per day. Based on the enzootic status of the farm, anamnesis, and clinical exam, the cow was diagnosed as clinically compatible with stage IV JD. In addition, the cow had tested positive when evaluated for antibodies against MAP by ELISA test. This test was performed as reported by Fernández-Silva et al. (2011a) and the specific result of this cow was taken from the official record reported to the staff of the farm. The farm and veterinarian staff decided to euthanize the cow. Blood samples were taken ante-mortem in sterile vacutainer tubes containing citrate as anticoagulant. A milk sample was obtained by manual milking in sterile 50 ml Coning centrifuge tubes (Corning, NY, USA). Samples were transported under refrigeration to the laboratory and processed to isolate mononuclear cells as previously reported (Zapata- Restrepo et al., 2010).
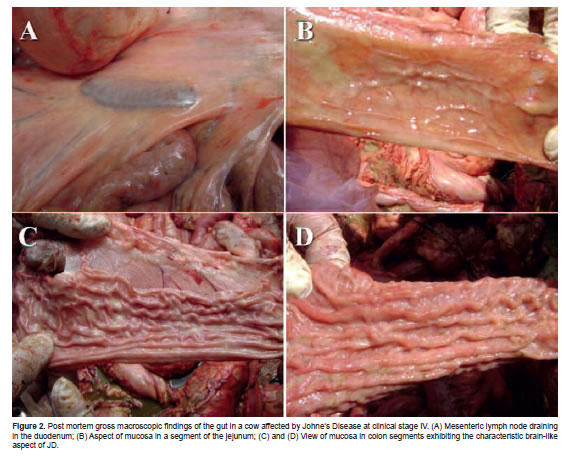
The cow was then euthanized by intrathecal injection of 25 ml lydocaine through the foramen magnum. Significant carcass alterations were not observed at the macroscopic examination during necropsy but a generalized pale condition suggested anemia. Abundant brown fat was observed around the pericardium near the insertion of the aorta adventitia. Adherences were present at the left costal side of the pericardium. Lung atelectasis was present. The heart was cardiomegalic and marked atrophy of the spleen was observed. The liver borders were rounded, bile ducts were thick, and several adult forms of Fasciola hepatica were observed in the bile ducts. Mediastinal and mesenteric lymph nodes were hypertrophic (Figure 2A). Jejunum and ileum were normal (Figure 2B), but the terminal ileum and the colon were thickened and the macroscopic aspect of intestinal mucosa was typical of JD (Figures 2C and 2D).
Several mediastinic and mesenteric lymph nodes, a spleen sample (5 x 5 cm), and transversal colon segments were taken during post-mortem examination. Samples were imbibed in Phosphate Buffer Saline (PBS, 1mM, pH = 7.2; Sigma- Aldrich-Aldrich, St. Louis, MO, USA) containing 1% penicillin/streptomycin and amphotericin B. Samples were kept on ice and transported to the laboratory in 75 cm2 Falcon culture flasks (Falcon, San Jose, CA, USA). After necropsy the carcass was deposited into a septic fossa according to the farm's sanitary protocol, which includes deep underground disposition (1.5 m) followed by abundant lime covering and complete carcass burial.
Tissue samples and secondary lymphoid tissues were cut and processed by mincing with dissecting scissors sterilized in PBS with added antibiotics as previously indicated. Samples were processed for macrophage isolation according to the protocol by Ramirez-Garcia et al. (personal communication, 2012). Tissues were minced with surgical scissors in petri dishes with added PBS and RPMI-1640 complete medium. The supernatant was recovered and centrifuged in a 1.077 density Ficoll gradient (Sigma, St. Louis, MO, USA) at 800 x g/40 min. Blood and milk samples were processed directly for isolation of mononuclear cells by centrifugation in 1.077 Ficoll gradient at 800 x g/40 min. After gradient centrifugation, the buffy-coats were recovered with a sterile plastic Pasteur pipette and washed three times in PBS at 400 x g/10 min. Pellets were recovered from mesenteric lymph nodes, spleen, mediastinal lymph nodes, blood, and milk. Cell pellets were resuspended in RPMI-1640 complete media (Mediatech Inc., Herndon, VA, USA) and cultured at 37 °C/2 hours in sterile polypropylene petri dishes. The nonadherent mononuclear cells were removed after this incubation period while the adherent cells were washed twice with RPMI-1640 complete media and cultured under standard conditions at 37 °C/5% CO2 in a humidified atmosphere.
The cells obtained by this protocol were bovine macrophages according to CD14 expression (PEconjugated mouse anti-human CD14 moAb [clone 61D3]) (Biosurce, San Diego, CA, USA) and phagocytosis of polypropylene spheres coupled to Phycoerythrin (CaliBRITE PE-beads; Becton- Dickinson, CA, USA). Plastic-adherent cells were cultured for at least 48 hours, after which time cells showed the characteristic pattern of macrophages. After 48 hours of macrophages culture cells were processed for total DNA isolation using the DNA Qiagen-mini kit (Qiagen, TX, USA) according to the manufacturer's instructions. The DNA purity and concentration was evaluated in a Nanodrop® ND1000 spectrophotometer (Thermo Fisher Scientific, Wilmington, DE, USA); samples were stored at -80 °C until processing.
Amplification of IS900 MAP fragment by real time PCR was performed using DNA samples obtained from all sources of macrophages as previously indicated, according to the protocol by O'Mahony and Hill (2002), with modifications in the thermal profile as follows: hot start at 15 min/95°C (instead of 10 min); 40 cycles (instead of 50) of amplification phase at 30 sec/95°C (instead of 15 sec), 10 sec/55°C (instead of 30 sec/60°C) and 30 sec/72° (instead of 12 sec). Primers used for IS900 amplification were: (FW) 5'CGA CGT GTC CTT ACA CAG C 3'; and reverse (RW) 5G'GT ATG GTT TCA TGT GGT T 3'. Real time PCR were processed in a Rotor-GeneTM 6000 real time rotary analyzer (Corbett Research, Australia). The PCR curves and florescence normalization were established by the Threshold value (Ct). LinREGPCRTM software was used for the efficiency calculation. All samples were processed in triplicate and data were analyzed by the –(ΔΔct) method (Livak and Schmittgen, 2001). The GAPDH gene was used as control for calculation of ΔCt. The results were analyzed using the Rotor- Gene 6000 series 1.7 software (Corbett Research, Sydney, Australia). The IS900 segment of MAP was amplified from all samples of macrophages evaluated (Figure 3).
The strongest real time-PCR signal of IS900 MAP fragment was observed in macrophages isolated from mesenteric lymph nodes (Figure 3B) and colon mucosa (Figure 3D), whereas the lowest signal was obtained from mediastinal lymph nodes (Figure 3A), milk (Figure 3C) or peripheral blood macrophages (data not shown). The Ct value for the IS900 sequence was 0.003 whereas it was 0.001 for the GAPDH gene. Ct was 9 and 16 for 1/10 and 1/100 positive control samples, respectively, whereas Ct of samples from macrophages ranged between 16 and 24.
Treatment approach
The cow was subjected to euthanasia due to the advanced clinical stage of the disease. For this reason the treatment schedule is not reported here.
Discussion
Typical findings of stage IV JD were observed at the clinical exam and gross findings at necropsy, confirmed by amplification of the IS900 fragment in DNA samples isolated of macrophages from all tissues, peripheral blood, and milk. The cow was bred in a dairy farm that was enzootic for JD as demonstrated by clinical, histopathological (Ramirez et al., 2011), serological (Fernandez et al., 2011b), and molecular (Zapata et al., 2010; Fernandez et al., 2011a) evidence. During 6 months the cow had presented intermittent episodes of stage III and IV JD that were treated according to standard procedures with apparent transient recovery for several weeks, followed by episodes of diarrhea. The cow had also a Fasciola hepatica infection that could be related to an immunosuppressive condition (considering its chronicity, evidenced by the gross findings in the biliary ducts and liver).
The IS900 MAP fragment was successfully amplified in DNA obtained from circulating macrophages (peripheral blood and milk) and resident macrophages (lymph nodes, colon, and spleen), suggesting that MAP uses the key processes in the physiology of leukocyte trafficking (Bimczok and Rothkötter, 2006) for its dissemination through all tissues in the affected animal (Cheville et al., 2001). Colon mucosa and mesenteric lymph nodes displayed the strongest signal of MAP genome amplification (Figure 3). This finding probably represents the normal consequence of a high amplification rate of MAP in macrophages present in the lamina propria of the bowel and suggests that circulation and recirculation of macrophages is a mechanism by which MAP disseminates throughout the organism.
Conclusions
Bovine macrophages isolated from lymphoid tissues, peripheral blood, and milk samples obtained from cows suffering stage IV JD can be used for the amplification of the MAP IS900 segment.
Notas
¤To cite this article: Ramírez-García R, Maldonado-Estrada JG. Detection of macrophages infected with Mycobacterium avium subspecies paratuberculosis in a cow with clinical stage IV of the disease. A case report. Rev Colomb Cienc Pecu 2013; 26:219-225.
Acknowledgements
The authors wish to thank Dr. Carlos Muskus (PECET Group, University of Antioquia), The Colombian Institute for Tropical Medicine (CES University), Giovanny Torres, and Ronald Pelaez (Medellín, Colombia). Gratitude is also extended to the University of Antioquia (CODI, Convocatoria Mediana Cuantía 2007) for the financial support of this work.
References
Bimczok D, Rothkötter HJ. Lymphocyte migration studies. Vet Res 2006; 37:325-338. [ Links ]
Cheville NF, Hostetter J, Thomsen BV, Simutis F, Vanloubbeeck Y, Steadham E. Intracellular trafficking of Mycobacterium avium ss. paratuberculosis in macrophages. Dtsch Tierarztl Wochenschr 2001; 108:236-243. [ Links ]
Fernández-Silva JA, Abdulmawjood A, Akineden O, Bülte M. Serological and molecular detection of Mycobacterium avium subsp. paratuberculosis in cattle of dairy herds in Colombia. Trop Anim Health Prod 2011a; 43:1501-1507. [ Links ]
Fernández-Silva JA, Abdulmawjood A, Bülte M. Diagnosis and molecular characterization of Mycobacterium avium subsp. paratuberculosis from dairy cows in Colombia. Vet Med Int 2011b; 2011:352-561. [ Links ]
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001; 25:402-408. [ Links ]
McNab WB, Meek AH, Martin SW, Duncan JR. Associations between dairy production indices and lipoarabinomannan enzyme-immunoassay results for paratuberculosis. Can J Vet Res 1991; 55:356-361. [ Links ]
Mutharia LM, Klassen MD, Fairles J, Barbut S, Gill CO. Mycobacterium avium subsp. paratuberculosis in muscle, lymphatic and organ tissues from cows with advanced Johne's disease. Int J Food Microbiol 2010; 136:340-344. [ Links ]
O'Mahony J, Hill C. A real time PCR assay for the detection and quantitation of Mycobacterium avium subsp paratuberculosis using SYBR Green and the Light Cycler. J Microbiol Methods 2002; 51:283-293. [ Links ]
Ramírez NF, Rodríguez B, Fernandez JA. Diagnóstico clínico e histopatológico de paratuberculosis bovina en un hato lechero en Colombia. Rev MVZ Córdoba 2011; 16:2742-2753. [ Links ]
Zapata-Restrepo M, Arroyave-Henao O, Ramírez-García R, Piedrahita-Ochoa C, Rodas-González JD, Maldonado- Estrada JG. Identification of Mycobacterium avium subspecies paratuberculosis by PCR techniques and establishment of control programs for bovine paratuberculosis in a dairy herds. Rev Colomb Cienc Pecu 2010; 23:17-27. [ Links ]