Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Colombiana de Ciencias Pecuarias
Print version ISSN 0120-0690
Rev Colom Cienc Pecua vol.26 no.4 Medellín Oct./Dec. 2013
ORIGINAL ARTICLES
Cellular response of the bovine mammary gland after Weissella confusa infusion to control Streptococcus agalactiae¤
Respuesta celular de la glándula mamaria bovina después de la infusión con Weissella confusa para el control de Streptococcus agalactiae
Resposta celular da glândula mamaria bovina após infusão com Weissella confusa para o controle de Streptococcus agalactiae
Liliana Serna-Cock1*, Bact, PhD; Cruz E Enríquez2, Zoot, Msc; Rómulo Campos Gaona2, MV, PhD; Andrea Vásquez1, Ing Agroind.
* Corresponding author: Liliana Serna. Universidad Nacional de Colombia Sede Palmira, Facultad de Ingeniería y Administración, Valle, Colombia. AA 237, Valle, Colombia. Email: lserna@unal.edu.co
1Universidad Nacional de Colombia Sede Palmira, Facultad de Ingeniería y Administración, AA 237. Valle, Colombia.
2Universidad Nacional de Colombia Sede Palmira, Facultad de Ciencias Agropecuarias, AA 237, Valle, Colombia.
(Received: August 12, 2012; accepted: May 16, 2013)
Summary
Background: the use of lactic acid bacteria (LAB) as a potential therapeutic agent to control bovine mastitis was previously proposed. However, little is known about the cellular response of the bovine mammary gland in cattle infected with Streptococcus agalactiae and treated with LAB. Objective: to assess the cellular response by the mammary gland in lactating cows after infection with Streptococcus agalactiae followed by infusion with Weissella confusa as antibacterial treatment. Methods: healthy udder quarters of lactating cows were infected with S. agalactiae (107 cfu/mL). After 24 h of pathogen infusion, 50% of the quarters were infused with 109 cfu/mL of W. confusa (SW) and the remaining 50% were kept as control units (S). At days 1, 2, 3, 4, 5, 11, and 14 post-infusion of the pathogen, the clinical signs of mastitis and the degree of cellular response by the mammary gland were evaluated using the California mastitis test, somatic cell count, electrical conductivity, and differential leukocyte count in milk. Results: the SW quarters showed clinical inflammation of the mammary gland associated with a significant increase in somatic cell count, California mastitis test, electrical conductivity and high proportion of polymorphonuclear neutrophils. The results suggest that the infusion with W. confusa cells induced a higher cellular immune response in the bovine mammary gland than S. agalactiae alone. Conclusions: results indicate that W. confusa infusions for controlling S. agalactiae should not be adopted. However, the activation mechanism of somatic cells in the mammary gland needs to be elucidated.
Key words: bovine mastitis, lactic acid bacteria, leukocytes.
Resumen
Antecedentes: previamente se propuso el uso de bacterias ácido lácticas (LAB) como agentes terapéuticos potenciales para el control de mastitis bovina. Sin embargo, poco se ha investigado sobre la respuesta celular de la glándula mamaria bovina ante la aplicación de LAB en bovinos infectados con Streptococcus agalactiae. Objetivo: evaluar, en vacas lactantes, la respuesta celular de la glándula mamaria después de la infección con Streptococcus agalactiae y la infusión intrapezón con Weissella confusa como tratamiento. Métodos: cuartos sanos de ganado criollo Hartón del Valle en estado de lactancia, se infectaron con S. agalactiae (107ufc/ mL). Transcurridas 24 horas post-infusión del patógeno, al 50% de los cuartos se les aplicó W. confusa a concentración de 109 ufc/mL (cuartos SW) y el 50% de los cuartos restantes se tomaron como unidades experimentales control (cuartos S). En los días 1, 2, 3, 4, 5, 11 y 14 pos-infusión del patógeno, se evaluaron signos clínicos de mastitis y el grado de respuesta celular de la glándula mamaria, a través de california mastitis test, recuento de células somáticas, conductividad eléctrica y diferencial de leucocitos en leche. Resultados: en los cuartos SW se observaron evidencias clínicas de inflamación de la glándula mamaria asociada con incremento significativo de recuento de células somáticas, California mastitis test, conductividad eléctrica, y altos porcentajes de neutrófilos polimorfonucleares. Los resultados sugieren que la infusión con células de W. confusa genera mayor respuesta celular en la glándula mamaria bovina que S. agalactiae. Conclusiones: los resultados indican que no puede utilizarse la infusión de células vivas de W. confusa como tratamiento para el control de S. agalactiae. Se debe investigar otro mecanismo de uso de la LAB para el tratamiento de mastitis bovina.
Palabras clave: bacterias ácido lácticas, leucocitos, mastitis bovina.
Resumo
Antecedentes: previamente foi proposto o uso de bactérias ácido-lácticas (LAB), como agentes terapêuticos potenciais para o controle da mastite bovina. No entanto, pouca pesquisa tem sido feita sobre a resposta celular da glândula mamária de bovinos após aplicação de LAB em bovinos infectados com Streptococcus agalactiae. Objetivo: avaliar, em vacas em período de lactação, a resposta celular da glândula mamária após a infecção com Streptococcus agalactiae e a infusão dentro da teta de Weissella confusa como tratamento. Métodos: tetas saudáveis de gado nativo Harton del Valle em lactação foram infectados com S. agalactiae (107ufc/mL). Ventiquatro horas após a infusão do agente patogénico, a 50% dos quartos foi administrada W. confusa em concentração de 109 ufc/mL (quartos SW) e 50% dos quartos foram usados como unidades experimentais de controle (quartos S). Nos dias 1, 2, 3, 4, 5, 11 e 14 após a infusão do agente patogénico, foram avaliados os sinais clínicos de mastite e o grau de resposta celular da glândula mamária através do Califórnia Mastite Teste, contagem de células somáticas, condutividade elétrica e diferencial de leucócitos do leite. Resultados: nos quartos SW observou-se evidência clínica de inflamação da glândula mamária associado ao aumento significativo na contagem de células somáticas, Califórnia mastite teste, condutividade eléctrica, e altas porcentagens de neutrófilos polimorfonucleares. Os resultados sugerem que a infusão de células de W. confusa gera maior resposta celular na glândula mamária bovina que o S. agalactiae. Conclusões: os resultados indicam que a infusão de células vivas de W. confusa como um tratamento para o controle de S. agalactiae não pode ser usado. Outros métodos de uso da LAB para o tratamento de mastite devem ser pesquisados.
Palavras chave: bactérias do ácido láctico, leucócitos, mastite bovina.
Introduction
Bovine mastitis is an inflammation of the mammary gland usually caused by microbial infections (Bradley, 2002). It is considered one of the most prolific diseases responsible for reducing milk production and quality in infected animals, and requires specific veterinary treatments (Aranguren Parra et al., 2009). During the development of the infection in the mammary gland, a specialized defense system is activated, recruiting polymorphonuclear neutrophils (PMNs), macrophages and lymphocyte cells (Sladek and Rysanek, 2010). The PMNs play a key role in the defense against intramammary infections in cows. They are unique leukocytes in milk capable of producing large quantities of reactive oxygen species that destroy the invading bacteria, and also indirectly interfere in the balance among bactericidal activity, inflammatory response, and tissue damage to the mammary gland (Mehrzad et al., 2010).
Despite advances in veterinary science, nutrition, and molecular biology, mastitis is still a problem in dairy farms. Prevention strategies such as antibiotic therapy have been proposed (Pellegrino et al., 2008). However, these therapies are costly and occasionally cause the elimination of innocuous or beneficial organisms due to the low specificity of antibiotics (Synnott et al., 2009). Although the use of antibiotics has been effective against mastitis, it has resulted in higher pathogen resistance (Ochoa- Zarzosa et al., 2008). Consequently, there is a growing need for alternative methods to control mastitis in lactating cows. In recent years, the use of lactic acid bacteria (LAB) strains for the control of bovine mastitis has interested the research community. Klostermann et al. (2008) reported that intramammary applications of Lactococcus lactis were as effective as conventional antibiotic treatments against mastitis. Crispie et al. (2008) reported that intramammary administration of L. lactis to uninfected cows prompted an immune modulation effect with significant recruitment of PMNs and lymphocytes in the udder quarter where LAB was applied. Furthermore, Beecher et al. (2009), using L. lactis to modulate the immune response in the mammary gland, suggested the potential of LAB to control mastitis without the use of antibiotics.
Serna-Cock et al. (2010) found that W. confusa, a LAB isolated from the ruminal liquid of bovine females, exhibited in vitro antimicrobial activity against S. agalactiae and Staphylococcus aureus (main pathogens responsible for bovine mastitis). The objective of this study was to evaluate the cellular response by the mammary gland in lactating cows after induction of infection with S. agalactiae and intrammary infusion with W. confusa as the antibacterial treatment.
Materials and methods
Ethical considerations
The experiment was conducted at the Mario Gonzales Aranda Farm at the National University of Colombia in Palmira, Valle del Cauca, Colombia. This research was approved by the National University of Colombia, Palmira's Animal Experimentation Ethics Committee (record number 5, dated May 23, 2012). The animals were under the supervision of a veterinarian throughout the entire experiment. The use of antibiotics was not required at the end of the experiment. On day 14, cows showed normal clinical signs in the mammary glands (negative CMT test, somatic cell count < 199,526 cells/mL).
Type of study
Healthy udder quarters of Hartón-del-Valle lactating cows (native Colombian breed) were the experimental units. Three conditions were used to diagnose healthy udders: physical appearance, a negative California Mastitis Test (CMT), and fewer than 200,000 somatic cells/mL count (SCC) in milk. The selected quarters were washed with potable water, dried with a towel, and disinfected with 70% (v/v) ethanol in water.
Intramammary infusion of S. agalactiae
A commercial strain of S. agalactiae (ATCC® 13813TM) was used to induce mastitis in selected quarters. The commercial pathogen strain was reconstituted in nutrient broth (OXOID, England) and incubated at 37 °C for 24 h. Each selected quarter received 1 mL aqueous solution of S. agalactiae (107 colony-forming units: cfu/mL) via a 5 cm intramammary infusion through the nipple.
Intramammary infusion of W. confusa
A cryo-preserved W. confusa strain effective against S. aureus and S. agalactiae was used (Serna- Cock et al., 2010). Bacterial growth was conducted in MRS broth (De Man et al., 1960) using 1000 mL Erlenmeyer flasks (700 mL effective volume) without aeration, under continuous agitation in an orbital shaker (model 5000I, VWR, Radnor, PA, USA) set at 33 °C and 100 rpm for 4 h. In previous experiments under these conditions, the highest antimicrobial activity of W. confusa against S. aureus was observed (Serna-Cock et al., 2010). The initial inoculum of W. confusa was 10% of the substrate volume after pH was adjusted to 6.0 using NaOH 4M. After incubation, the substrate was aseptically poured into 45 mL centrifuge sterile tubes and centrifuged for 31 min at 2860 x G (model 5804R CITI, Eppendorf, Germany). Then, the supernatant was removed and the precipitate from each tube was re-suspended in 1 mL physiological water (0.9% w/v NaCl), gently agitated, and centrifuged for 5 min at 2860 x g. The supernatant was again discarded and cells of W. confusa with a concentration of 109 cfu/mL were obtained. These concentrated cells were aseptically stored in 15 mL sterile tubes (5 mL effective volume) and lyophilized at -20 °C (0.120 mB absolute pressure, and -50 °C condenser temperature; LabConco, England) for later use.
To treat the udder quarters infected with S. agalactiae, lyophilized cells of W. confusa were first reconstituted. At the time of the application into the udder quarter, each tube of lyophilized cells was reconstituted in 5 mL of sterile distilled water and poured in sterile syringes. The intramammary LAB infusion was performed 24 h after the S. agalactiae infusion. Half of the infected quarters were randomly infused with 5 mL of the 109 cfu/mL LAB aqueous solution and half of the remaining quarters were not infused and represented the experimental control units.
Milk sample collection and test determinations
Milk samples were taken during morning hours in sterile bottles at 1, 2, 3, 4, 5, 11, and 14 d postinfusion of S. agalactiae. The degree of cellular response of the mammary gland was assessed by analyzing the milk samples for CMT, SCC, electrical conductivity (EC), and differential leukocyte count (polymorphonuclear neutrophils, lymphocytes, and macrophage cells).
The CMT test results were grouped according to the following classification (Contreras et al., 1996): (0) absence of any variation in viscosity, no thickening of the mixture; (1) distinct thickening, no gel formation; (2) gel formation, immediate thickening; and (3) gel formed and stacked to the paddle. The SCC was measured using an automatic cell counter (DeLaval Cell counter DCC 2828, DeLaval, Sweden). The EC was conducted using a Mastitis Detector (Milk Checker N-4 L, Oriental Instruments Co. Ltd., Japan).
The differential leukocyte count test was conducted using light microscopy observations of milk smears, stained with Wright dye and Giordano buffer (MOL LABS, Colombia) as described by Lindmark-Manson (2006), with modifications. The modified differential leukocyte technique is described as follows: 20 mL of milk was mixed with 20 mL of buffer solution (1L of di-ionized water, 7.65 g NaCl, 1.994 g Na2HPO4, and 0.21 g KH2PO4) at pH 7.45. The milk-buffer mixture was centrifuged for 10 min at 2860 x G and 4 °C. The supernatant was discarded and the pellet was re-suspended in 1 mL buffer solution and centrifuged for 10 min at 2800 x G and 4 °C. Once again, the supernatant was discarded and the pellet re-suspended in 0.6 mL soy broth. A 0.01 mL portion of the obtained suspension was spread into a plate slide forming a thin film. The film was dried at room air conditions, methanol added (MERCK, Germany) for 5 min and then removed, dried again, and stained with Wright dye and Giordano buffer for 8 min. Distilled water was used to wash the film and 70% (v/v) ethanol in water was used to remove any excess of water. Once the film dried, each cell was differentiated by light microscopy at 100x (Gargouri et al., 2008).
Experimental Design
A completely randomized 2 x 7 factorial design with three replicates was used. Factors were: infusion at two levels S. agalactiae (S) and S. agalactiae + W. confusa (SW) and evaluation time at seven levels 1, 2, 3, 4, 5, 11 and 14 d postinfusion of S. agalactiae.
The response variables were degree of cellular response of the mammary gland through California Mastitis Test (CMT), somatic cell count (SCC), electrical conductivity (EC), and differentiation of leukocytes in milk (polymorphonuclear neutrophils, lymphocytes and macrophages cells). For the SCC, a logarithmic transformation (Log10) was performed before conducting the analysis of variance.
Analysis of variance tested the treatment effects. Significant differences among means were determined using the least significant difference (LSD) test. SAS software (Version 9.2; SAS Institute Inc., Cary, NC, USA) was used for statistical analysis.
Results
Assessment of CMT, SCC, and EC
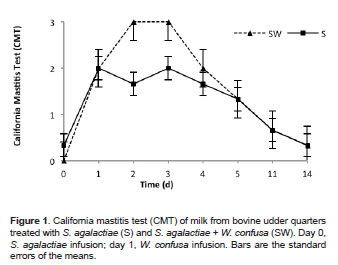
Figures 1, 2 and 3 show CMT, SCC, and EC test results of milk from quarters inoculated with S. agalactiae and then with W. confusa, respectively. Significant statistical differences were found (p<0.05) due to evaluation time and treatments (S vs. SW). The CMT in milk samples from the control quarters (S) showed subclinical mastitis grade 2 at 24 h post-infusion of the pathogen (Figure 1). After day 2 post-infusion, the presence of subclinical mastitis in the S quarters started decreasing until average values were 0.33 at day 14. Milk samples from the SW-treated quarters, showed mastitis grade 3 at 24 h and 48 h post-infusion of W. confusa (i.e., at days 2 and 3 post-infusion with S. agalactiae), with apparent clinical signs of inflammation. Severe induration was observed in addition to temperature changes, suggesting that W. confusa intensified the inflammatory response of the mammary gland. At days 4, 5, 11, and 14, this response gradually decreased (Figure 1).
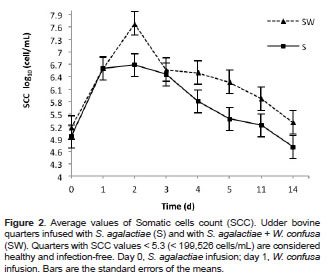
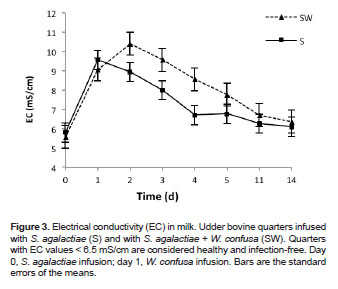
After 24 h of the pathogen infusion, SCC and EC evidenced high values (p<0.05), indicating that the presence of S. agalactiae increased cell counts and altered the normal ion composition of milk (Figures 2 and 3). EC values in milk from the S treated quarters started decreasing at day 2 post-infusion, while SCC values remained high until day 3 postinfusion. A significant reduction in cell counts was observed at day 4 (p<0.05), reaching normal Log10 SCC values of 4.7 (i.e., 5.0x104 cells/mL) at day 14 (Figure 2).
Significant differences (p<0.05) were found in SCC between S and SW treatments at 24 h after infusion of W. confusa (day 2). The highest Log10 SCC value of 7.68 (i.e., 4.7x107 cells/mL) corresponded to milk taken from the SW treated quarters. At day 3, no significant differences between S and SW treated quarters were found (p = 0.1216). However, at days 4, 5, and 11 SCC were significantly high in those quarters treated with SW (p = 0.0185), (p = 0.0054), and (p = 0.0309), respectively (Figure 2). At day 14, Log10 SCC values of 5.3 (i.e., 1.9x105 cells/mL) were obtained in the SW treated quarters.
Significant differences (p<0.05) were found in EC between the S and SW treatments at 2, 3, 4, 5, and 11 days, with higher EC values in quarters treated with W. confusa, which suggests that milk from these quarters had a greater alteration of sodium and chlorine ions due to the cellular response in the mammary gland.
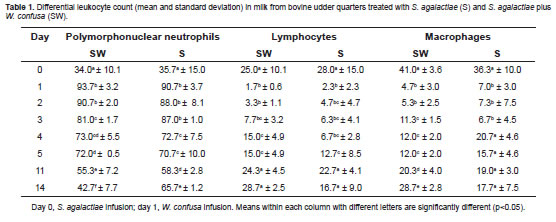
Differential Leukocyte Count Determination
Table 1 shows the differential leukocyte count for the S and SW treatments. After 24 h postinfusion of S. agalactiae, more than 90% PMNs were found on the udder nipples. No significant differences were found in the proportions of PNMs (p = 0.3067), lymphocytes (p = 0.1002), and macrophage cells (p = 0.3422) between the SW and S treated quarters.
Discussion
Previous in vitro studies demonstrated the effectiveness of LAB against S. aureus and S. agalactiae. Based on CMT, SCC, and EC results, it is suggested that W. confusa generates a high and rapid cellular response in the mammary gland. The quarters infused with W. confusa showed greater changes when compared to those infected with S. agalactiae. The results also showed evidence of clinical mastitis (severe induration, local fever, and clot formations in milk).
Greene et al. (1991) compared the effects of treating subclinical mastitis with intramammary infusions of Lactobacillus or an antibiotic preparation on cure rates and milk SCC. They warned that the administration of Lactobacillus or antibiotic treatment to all quarters based on elevated composite SCC should not be adopted. Lactobacillus increased SCC with no effect on the infection rate. Similar results (i.e., increase in SCC) were found in this study with LAB W. confusa.
According to our PMNs, lymphocytes, and macrophages results, the cellular response was the same in both S and SW quarters. More studies are needed to better understand cellular response in the mammary gland after infusing W. confusa. It is important to identify and differentiate how cell activation occurs, or to identify the type of proin flammatory cytokines that are activated in the mammary gland in the presence of W. confusa.
Studies with LAB Lactococcus lactis DPC 3147 on immune response after mammary gland infusion with a probiotic culture showed that the greatest increase in immune gene expression was observed in interleukin (IL)-1β and IL-8, which corresponded with peaks in somatic cell counts (Beecher et al., 2009). In this study, proportions of neutrophils, lymphocytes and macrophages were assessed in milk; therefore, more studies are needed to identify and differentiate the specific type of lymphocytes present in both S and SW quarters.
The mammary gland response is specific for each bacterial pathogen. Soltys and Quinn (1999) reported that there was a difference in the type of cells present in milk depending on the specific mastitis-causing pathogen. These authors found that S. aureus prompted a strong T CD4+ lymphocyte response, whereas Streptococcus spp. prompted a CD4+ and CD8+ lymphocyte response.
According to these results, further research should be conducted to elucidate (a) the ability of W. confusa to adhere to the epithelial cells of the teat canal, (b) the period of time the strain remains in the teat canal, (c) canal and cistern histology post inoculation, and (d) the activation mechanism of somatic cells in the mammary gland post inoculation. These further studies will determine whether W. confusa should be included or excluded in non-antibiotic formulations for the prevention and treatment of bovine mastitis.
Infusion of LAB W. confusa in bovine mammary gland previously infected with S. agalactiae produced further inflammation, high CMT, EC, and high SCC as a result of a high proportion of PMNs when compared to mammary glands where only the pathogen was present. Results indicate that W. confusa infusion, as a treatment for controlling S. agalactiae, should not be adopted. However, the activation mechanism of somatic cells in the mammary gland needs to be elucidated.
¤ To cite this article: Serna-Cock L, Enríquez CE, Campos R, Vásquez A. Cellular response of the bovine mammary gland after Weissella confusa infusion to control Streptococcus agalactiae. Rev Colomb Cienc Pecu 2013; 26:280-287.
Acknowledgments
The authors would like to express their appreciation to Banco Santander and the Programa Virginia Gutierrez de Pineda para jóvenes Investigadores e innovadores (COLCIENCIAS) for funding this project.
References
Aranguren Parra AJ, López Ortega AA, Mendoza CA, Ortega Rivas E. Effect of clinic and subclinic mastitis on the plasmatic concentration of metabolites, total protein, and albumen in bovine females. Zootecnia Trop 2009; 27:57-63. [ Links ]
Beecher C, Daly M, Berry DP, Klostermann K, Flynn J, Meaney W, Hill C, Mccarthy TV, Ross RP, Giblin L. Administration of a live culture of Lactococcus lactis DPC 3147 into the bovine mammary gland stimulates the local host immune response, particularly IL-1 beta and IL-8 gene expression. J Dairy Res 2009; 76:340-348. [ Links ]
Bradley AJ. Bovine mastitis: an evolving disease. Vet J 2002; 164:116-128. [ Links ]
Contreras A, Sierra D, Corrales JC, Sanchez A, Marco J. Physiological threshold of somatic-cell count and California mastitis test for diagnosis of caprine subclinical mastitis. Small Rumin Res 1996; 21:259-264. [ Links ]
Crispie F, Alonso-Gomez M, O'loughlin C, Klostermann K, Flynn J, Arkins S, Meaney W, Ross RP, Hill C. Intramammary infusion of a live culture for treatment of bovine mastitis: effect of live lactococci on the mammary immune response. J Dairy Res 2008; 75:374-384. [ Links ]
De Man JC, Rogosa M, Sharpe ME. A medium for the cultivation of lactobacilli. J Applied Bacteriol 1960; 23:130- 135. [ Links ]
Gargouri A, Hamed H, Elfeki A. Total and differential bulk cow milk somatic cell counts and their relation with lipolysis. Livest Sci 2008; 113:274-279. [ Links ]
Greene WA, Gano AM, Smith KL, Hogan JS, Todhunter DA. Comparison of probiotic and antibiotic intramammary therapy of cattle with elevated somatic cell counts. J Dairy Sci 1991; 74:2976–2981. [ Links ]
Klostermann K, Crispie F, Flynn J, Ross RP, Hill C, Meaney W. Intramammary infusion of a live culture of Lactococcus lactis for treatment of bovine mastitis: comparison with antibiotic treatment in field trials. J Dairy Res 2008; 75:365-373. [ Links ]
Lindmark-Mansson H, Branning C, Alden G, Paulsson M. Relationship between somatic cell count, individual leukocyte populations and milk components in bovine udder quarter milk. Int Dairy J 2006; 16:717-727. [ Links ]
Mehrzad J, Paape M, Burvenich C. Role of neutrophils in protection of udder from infection in high yielding dairy cows. Iran J Vet Res 2010; 11:102-118. [ Links ]
Ochoa-Zarzosa A, Loeza-Lara PD, Torres-Rodriguez F, Loeza- Angeles H, Mascot-Chiquito N, Sanchez-Baca S, Lopez- Meza JE. Antimicrobial susceptibility and invasive ability of Staphylococcus aureus isolates from mastitis from dairy backyard systems. Antonie Van Leeuwenhoek 2008; 94:199-206. [ Links ]
Pellegrino M, Giraudo J, Raspanti C, Nagel R, Odierno L, Primo V, Bogni C. Experimental trial in heifers vaccinated with Staphylococcus aureus avirulent mutant against bovine mastitis. Vet Microbiol 2008; 127:186-190. [ Links ]
Serna-Cock L, Valencia-Hernandez LJ, Campos-Gaona R. Cinética de fermentación y acción antimicrobiana de Weissella confusa contra Staphylococcus aureus y Streptococcus agalactiae. Rev Fac Ing Univ Antioquia 2010; 55:55-65. [ Links ]
Sladek Z, Rysanek D. Apoptosis of resident and inflammatory macrophages before and during the inflammatory response of the virgin bovine mammary gland. Acta Vet Scan 2010; 52:12. [ Links ]
Soltys J, Quinn MT. Selective recruitment of T-cell subsets to the udder during staphylococcal and streptococcal mastitis: Analysis of lymphocyte subsets and adhesion molecule expression. Infect Immun 1999; 67:6293-6302. [ Links ]
Synnott AJ, KuangY, Kurimoto M, Yamamichi K, Iwano H, Tanji Y. Isolation from sewage influent and characterization of novel Staphylococcus aureus bacteriophages with wide host ranges and potent lytic capabilities. Appl Environ Microb 2009; 75:4483-4490. [ Links ]