Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Colombiana de Ciencias Pecuarias
Print version ISSN 0120-0690
Rev Colom Cienc Pecua vol.26 no.4 Medellín Oct./Dec. 2013
ORIGINAL ARTICLES
Effect of water velocity on intermediary metabolism of juvenile matrinxã fish (Brycon amazonicus)¤
Efecto de la velocidad del agua en el metabolismo intermediario de juveniles del pez Yamú (Brycon amazonicus)
Efeito da velocidade da água no metabolismo intermediário de juvenis do peixe Matrinxa (Brycon amazonicus)
Gustavo Alberto Arbeláez-Rojas*, PhD; Gilberto Moraes, PhD.
Departamento de Genética e Evolução, Universidade Federal de São Carlos, São Carlos, SP, Brasil, Rodovia Washington
Luiz Km 235. CEP 13565-905.
(Received: March 11, 2013; accepted: July 30, 2013)
Summary
Background: determination of water velocity for optimum fish growth is fundamental since its duration and intensity can interfere with the metabolic preference for some biochemical paths, resulting in the use of specific substrates for fish growth. Objective: the purpose of this study was to assess the metabolic adjustments of juvenile matrinxã (Brycon amazonicus) reared under various sustained swimming conditions (SS). Methods: fish were subjected to SS for 90 days at five swimming speeds: 0.0 (control), 1.0, 1.5, 2.0, and 2.5 Body Length per second (BL/s). At the end of the experimental period, fish were euthanized; samples of blood, liver, white-muscle, red-muscle and ventral muscle were collected and metabolite concentrations were evaluated. Results: fish reared between 1.0-1.5 BL/s increased the hepatosomatic index (HSI) while those swimming at velocities higher than 1.5 BL/s showed diminished HSI and visceral fat. Fish under moderate swimming utilized visceral fat to supply energy for red muscle contractions while white muscle of fish swimming at higher speeds used carbon backbones from amino acids plus visceral fat. Conclusion: sustained swimming between 1.0-1.5 BL/s enhanced the intermediary metabolism of B. amazonicus, improving fish performance. Future studies linking macronutrient dietary levels and SS should allow adjusting rearing conditions under SS to optimize growth versus metabolic performance.
Key words: energy reserves, fat bulks, metabolic adjustments, performance, sustained swimming.
Resumen
Antecedentes: la determinación de la velocidad del agua para óptimo crecimiento de los peces es importante porque dependiendo de la intensidad y duración, puede interferir sobre la activación de las vías metabólicas, indicando mayor o menor utilización de un determinado substrato energético para el crecimiento. Objetivo: la finalidad de este trabajo fue estudiar los efectos del ejercicio de natación sobre el metabolismo de juveniles de matrinxa (Brycon amazonicus). Métodos: juveniles de B. amazonicus fueron cultivados y condicionados a nadar bajo diferentes velocidades de natación 0,0 (control), 1,0, 1,5, 2,0 y 2,5 longitud total por segundo (LT s-1). Al final del período experimental (90 días), se realizó la eutanasia de los peces y se recolectaron muestras de sangre, tejido hepático, músculo blanco, músculo rojo y músculo ventral para determinar los intermediarios metabólicos. Resultados: peces cultivados en velocidades de natación entre 1,0 y 1,5 LT s-1 presentaron aumento del índice hepasomático (HSI), mientras que los peces que nadaron en velocidades superiores a 1,5 LT s-1 mostraron disminución de los índices HSI y grasa visceral (IGVS). Los peces condicionados a nadar en velocidades moderadas utilizaron lípidos viscerales como fuente de energía para sostener la contracción muscular del músculo rojo. Mientras que los peces que nadaron en las mayores velocidades, el músculo blanco fue más demandado, empleando como combustible, esqueletos de carbono provenientes de los aminoácidos y significativa movilización de grasa visceral. Conclusión: la natación sostenida entre 1,0 – 1,5 LT s-1 mejoró el metabolismo intermediario de B. amazonicus y el desempeño de los peces. Futuros trabajos asociando niveles de macro nutrientes y protocolos de natación moderada pueden permitir ajustes más refinados en la mejoría de las condiciones de cultivo, en la optimización del crecimiento, en la eficiencia alimenticia y en el aumento de la resistencia orgánica a las prácticas de manejo en las pisciculturas.
Palabras clave: ajustes metabólicos, desempeño, grasa visceral, natación sustentada, reservas energéticas.
Resumo
Antecedentes: a determinação da velocidade da água para o ótimo crescimento dos peixes é importante porque dependendo da intensidade e duração, pode interferir sobre a predominância na ativação de uma ou de outra via metabólica, indicando maior ou menor utilização de um determinado substrato energético e agir no crescimento. Objetivo: o propósito deste trabalho foi estudar os efeitos que o exercício de natação tem sobre o metabolismo de juvenis do peixe Matrinxa, Brycon amazonicus. Métodos: juvenis de B. amazonicus foram criados e condicionados a nadar durante 90 dias em cinco velocidades de natação: 0,0 (controle); 1,0; 1,5; 2,0 e 2,5 comprimentos corporais por segundo (CC s-1). Após termino do período experimental, os peixes foram sacrificados, o sangue, fígado, músculos branco, vermelho e ventral foram coletados para avaliar o perfil metabólico. Resultados: velocidades moderadas de natação entre 1,0 a 1,5 CC s-1 aumentaram o índice hepatosomático (HSI), enquanto que, o condicionamento dos peixes a maiores velocidades diminuiu significativamente o índice HSI e a gordura visceral (IGVS) em relação ao controle. Com respeito às respostas metabólicas, o condicionamento dos peixes em velocidades moderadas mostrou que o fornecimento de energia para sustentar a contração muscular dependeu primariamente dos triglicerídeos provenientes principalmente dos lipídios viscerais, ao passo que, em velocidades maiores, a via gliconeogênica foi a responsável pela manutenção na produção de energia para os músculos locomotivos, a qual aumentou progressivamente em função do catabolismo dos aminoácidos dos músculos locomotivos. Conclusão: a natação sustentada entre 1,0 - 1,5 CC s-1 melhorou o metabolismo intermediário do B. amazonicus o que resultou em melhor desempenho dos peixes. Estudos futuros associando níveis dietéticos de nutrientes com a natação moderada, pode permitir aos peixes ajustes mais refinados na melhoria das condições de criação, na otimização do crescimento, na eficiência alimentar e no aumento da resistência orgânica as praticas de manejo nas pisciculturas.
Palavras chave: ajustes metabólicos, desempenho, gordura visceral, natação sustentada, reservas energéticas.
Introduction
Environmental changes usually result in biochemical and physiological adaptations to maintain enantiostasis (Randall and Brauner, 1991). Among the myriad of external changes, variations of water speed throughout environments, or throughout seasons, lead to physiological, biochemical and morphological adaptations (Randall and Brauner, 1991; Wootton, 1999). High swimming speeds may lead to metabolic disturbances affecting homeostasis and growth (Young and Cech, 1994b; Hernández et al., 2002).
Fish reared in running waters are currently reported as physiologically responsive to swimming speeds (Jobling et al., 1993). According to the current knowledge on fish metabolism, swimming speed affects the rearing of several species (Palstra and Planas, 2011). Fish usually move at moderate speeds under aerobic conditions. We also know that excessive swimming is harmful (Brown et al., 2011).
Most glycogen and lipids can be mobilized to supply metabolic demands from excessive swimming (Moyes and West, 1995). Thus, monitoring morphometric indices, such as hepatosomatic index (HSI) and intraperitoneal fat ratio, or fat viscerosomatic index (FVSI) is relevant to evaluate the physiological and health status of the fish (Morgan et al., 2002; Rios et al., 2002). Stored energy is fundamental for juvenile fish survival, indicating their growth potential, which allows inferring anabolism or catabolism status of energy stocks (Sheridan, 1988; Post and Parkinson, 2001; Magnoni et al., 2013).
Matrinxa (Brycon amazonicus; Spix and Agassiz, 1829) is a freshwater fish from the Amazon and Tocantins-Araguaia basins (Howes, 1982). This fish can achieve high growth rates and good feed conversion ratio under farming conditions. It has excellent hydrodynamics to swim in the rapids and also great ability to swim long distances. Matrinxa's muscle plasticity enables it to easily adjust to physiological and metabolic needs in response to environmental demands (Rocha et al., 2004; Brandão et al., 2005).
Recent studies with matrinxa show that a moderate current speed of up to 1.0 body length per second (BL/s) results in growth and metabolism improvements (Arbeláez-Rojas et al., 2002; Hackbarth and Moraes, 2006; Fabrizzi et al., 2013). However, changes in exercise velocity are expected to bring distinct metabolic responses and consequent changes in growth performance. These conditions have not yet been studied in juvenile matrinxa and must be evaluated to take maximum advantage of such processes, avoiding damage and exploring the propitious species limits. Therefore, the aim of this work was to evaluate the effect of water speed on growth and metabolic responses of matrinxa juveniles.
Materials and methods
Ethical considerations
This research was approved by the Animal Experimentation Ethics Committee of the Federal University of São Carlos, Brazil (053 from November 5, 2009).
Type of study
Brycon amazonicus juveniles were purchased at Aguas-Claras Fish Farm in Mococa, São Paulo (Brazil) and transported to the experimental laboratory facilities where they were held in three 2000 L indoor tanks coupled to a closed system with re-circulating, thermostatic, aerated and filtered water. The fish were allowed to acclimatize under a natural photoperiod for three weeks and fed commercial pellets with 36% crude protein (CP). After this period one hundred fish (average length and weight 13.4 ± 0.1 cm and 33.3 ± 0.9 g, respectively) were randomly distributed in five 250 L fiber-glass circular tanks (swimming system), totaling 20 fish per tank.
Sustained swimming system
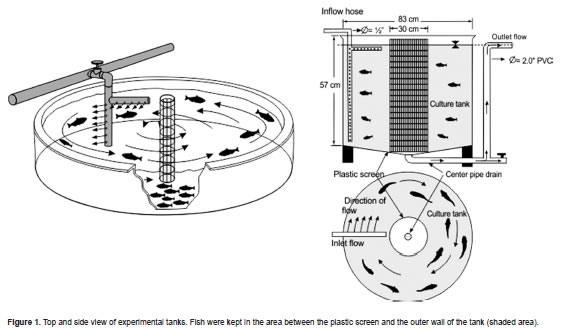
The swimming systems were bottom-funnel shaped tanks with 20° horizontal leaning from the side to a central sewer hole allowing for easy clearance of debris (Figure 1). Water was biologically filtered, thermostatic, and re-fed to the tanks through bored L-shaped pipes. These pipes were fitted to the water column to maintain its speed, obtained by inserting a 3/4 HP pump (Mark Grundfos, model NXDP4, São Paulo, SP, Brazil) into the line, adjusted by a control valve. Water speed was gauged with a mechanical flow meter (General Oceanic Inc., Miami, FL, USA). Since tangential water speed decreases from the edge to the center, fish were prevented from accessing the central water region by a top-to-bottom PVC net column of 1/3 the total tank diameter. The narrower column of water that remained for swimming was maintained at a regular speed. The fish were acclimated for one week before the trials.
Water quality
Water quality parameters were measured daily. The average values during the trials were temperature 27.5 ± 0.9 °C; dissolved oxygen 5.3 ± 0.07 mg/L; pH 7.2 ± 0.4; ammonia 0.03 ± 0.04 mg/L, and conductivity 71.6 ± 4.8 μS/cm.
Experimental design
The experimental design involved five speed treatments randomly assigned to tanks, with experimental units represented by 20 tagged fish per treatment, i.e., 20 replicates per treatment. Fish ordinarily swam counter water flow. Water speed was monitored at distinct positions and adjusted every two weeks according to the biometric data. Water speed was adjusted to keep fish swimming at 1.0, 1.5, 2.0, and 2.5 body lengths per second (BL/s). One of the tanks was used as a control treatment, with fish left in a motionless water system. Fish were fed to satiety three times a day at 09:00, 13:00 and 17:00 hours, with extruded commercial pellets (32% CP) for 90 days. Fish were kept under a natural photoperiod. Before handling for biometry gauging, animals were anesthetized with eugenol (40 mg/L; Inoue et al., 2003). Feeding was discontinued for 24 hours at the end of the experimental period. Ten fish were randomly sampled for blood drawn from the caudal vein with a heparinized syringe. Afterwards, fish were euthanized through cervical separation. Liver, white muscle, ventral muscle, and red muscle were quickly excised, dissected over a Petri dish into cold saline solution and immediately frozen in liquid nitrogen for posterior analysis. Liver and mesenteric fat were weighed and stored in a freezer at -20 ºC for later determination of biometric indices.
Metabolic intermediates
Plasma samples were deproteinized using 20% trichloroacetic acid (TCA) at 1:10 plasma:TCA ratio. Liver and muscle samples were subsampled, keeping the same ratio (1:10 tissue:TCA), homogenized under an ice bath with a Teflon pestle motor-driven cell disruptor by two 30-second strokes at 1000 rpm. Cell extracts were centrifuged at 3000 x g and supernatants were used to determine metabolite concentration. The following metabolites were determined by colorimetry: ammonia (Gentzkow and Masen, 1942), protein (Lowry et al., 1951), free amino acids (Copley, 1941), glucose (Dubois et al., 1956), lactate (Harrower and Brown, 1972), pyruvate (Lu, 1939), free fatty acids (FFA; Norvák, 1965), glycogen (Bidinotto et al., 1997), triglycerides (TG; Chernecky et al., 1993), and total lipids (Folch et al., 1957). Triglycerides, fatty acids, and lipids were determined from ventral muscle sub-samples.
Morphometric indices
At the ninetieth day of sustained swimming (SS) feeding was discontinued for 24 hours and 10 fish per treatment were sampled, anesthetized, and euthanized. Liver and visceral fat were excised to determine hepatosomatic index (HSI) and fat viscerosomatic index (FVSI). Both indices were calculated as follows:
Index = (tissue weight/body weight) × 100
Hepatosomatic index (HIS) = ((LW (g)/(BW(g)) x 100
Fat viscerosomatic index (FVSI) = ((FVW (g)/ BW(g)) x 100
Where LW and BW are liver weight and body weight, respectively. FVW is fat visceral weight. FW and IW are final and initial weight, respectively.
Statistical analysis
The variables (morphometric indices and metabolic intermediates) for each swimming speed were compared through a one-way ANOVA followed by a Tukey's multiple range test. Differences were considered significant at p<0.05. The experimental unit was each fish (n=20). Data are expressed as mean ± SD. The Statistical Analyses System (SAS) version 8.0 was used for data analysis (SAS, 1990).
Results
Morphometric indices
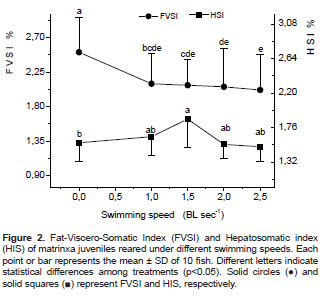
The highest HSI corresponded to matrinxa exercised for 90 d at 1.5 BL/s (p<0.05), and no changes were observed among the other groups. The FVSI (2.72% ± 0.44) of fish under motionless water conditions was significantly greater (p<0.05) than FVSI (2.24 ± 0.45) of fish farmed at faster swimming. There was a tendency for decreased VSI with increased water speed (Figure 2).
Metabolic responses
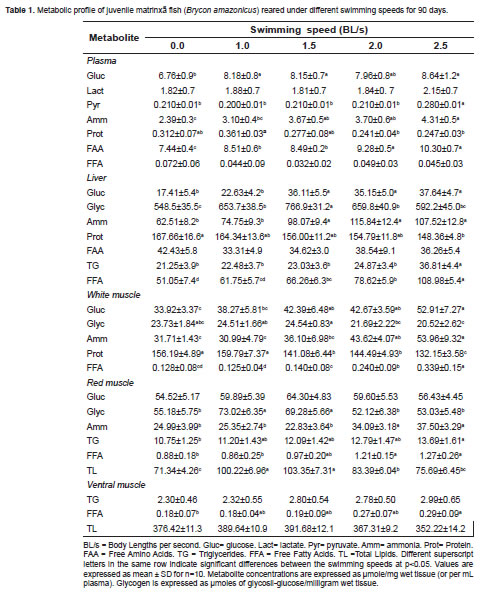
Metabolite variations in response to SS are shown in table 1. Plasma glucose, FAA, and N-NH4 +concentrations increased with swimming speed while protein decreased from 1.5 BL/s. FFA levels decreased for all swimming conditions. The TG and lactate concentrations did not change slightly to 4.62 ± 0.3, and 1.90 ± 1.14 μmole/mg wet tissue).
Metabolic responses of the liver
Glucose, N-NH4 +, TG, and FFA levels increased with swimming speed. Glycogen stores were higher in fish under exercise and the highest value was observed at 1.5 BL/s. The highest FFA and TG values were observed at 2.5 BL/s. Protein and FAA showed a slight tendency to decrease with increased swimming speed (Table 1). Lactate and pyruvate concentrations approximated 5.51 ± 0.58 and 1.02 ± 0.05 μmole/mg wet tissue, respectively. The average TL level was 59.75 ± 1.25μmole/mg wet tissue.
White muscle metabolic responses
Glucose, N-NH4 + and FFA levels increased with swimming speed. Protein levels decreased from 1.5 BL/s. Glycogen bulk decreased from 1.5 BL/s, and the other metabolites remained invariable: Lactate 55.35 ± 2.4, pyruvate 1.82 ± 0.1, TG 1.49 ± 0.1, and TL 12.95 ± 2.9 μmole/mg wet tissue, respectively (Table 1).
Red muscle metabolic responses
No significant decrease was observed in this tissue as a result of swimming speed, except for glycogen and TL, from 1.5 BL/s. The N-NH4 +, TG, and FFA levels increased. Glycogen stores were higher at 1.0-1.5 BL/s. A small tendency for decrease, from 0.0 to 2.5 BL/s swimming speed, was observed in the protein levels, dropping from 169.99 ± 2.99 to 162.04 ± 4.21 μmole/mg wet tissue, while FAA showed a contrary profile increasing from 14.59 ± 1.40 to 16.51 ± 0.84 μmole/mg wet tissue. Lactate and pyruvate concentration remained constant and near to 31.27 ± 1.95 and 0.71 ± 0.1 μmole/mg wet tissue, respectively (Table 1).
Ventral muscle metabolic responses
This tissue responded to swimming exercise with a slight tendency to increase for TG (from 2.30 ± 0.46 to 2.99 ± 0.65 μmole/mg wet tissue) and FFA (from 0.18 ± 0.07 to 0.29 ± 0.09 μmole/ mg wet tissue). The maximum TL content (390.66 ± 1.5μmole/mg wet tissue) was observed between 1.0 and 1.5 BL/s. The other values were significantly lower and were grouped near 365.32 ± 12.2 μmole/mg of wet tissue (Table 1).
Discussion
Morphometric indices
In spite of statistical similarity for hepatosomatic index (HSI) from fish kept under SS this index in matrinxa exercised at 1.5 body lengths per second (BL/s) was higher than that of sedentary fish. The HSI can be defined as the ratio of liver weight to body weight. It indicates the status of energy reserves in an animal. HIS is regarded as an indirect way of measuring growth rate and energy balance of hepatic metabolism (Jobling, 2001). The high HSI induced by SS at 1.5 BL/s—which was coincident with the highest hepatic glycogen level—indicates a feed efficiency increase and a nutritional status improvement due to moderate physical exercise (Jobling et al., 1993). Increases in HSI have also been reported in trout (Oncorhynchustshawytscha) swimming at 1.5 BL/s (Kiessling et al., 1994) and Atlantic cod (Gadusmorhua) exercising at a speed of 1.0 BL/s (Bjornevik et al., 2003). Higher speeds cause reduction of such index to levels equal to those observed in the reference groups. Hackbarth and Moraes (2006) further supported these results when they submitted matrinxa juveniles to 2.3 BL/s swimming speed for 72 days. They found no significant changes in HSI between fish reared either in motionless water or under that condition. Therefore, HSI and liver glycogen profile suggest that an increase in swimming speed above 1.5 BL/s is detrimental to matrinxa.
Another index used to evaluate energy metabolism in fish is the fat viscerosomatic index (FVSI). Variations in FVSI depend primarily on nutritional composition, especially dietary energy: protein ratio (Guillaume et al., 2002), and the intensity and duration of exercise (Jobling, 2001). Significant reduction in fat bulk was observed in matrinxa reared under SS. There is abundant information concerning the influence of exercise on body composition, but little in regard to mesenteric fat tissue. Some studies have shown that juvenile channel catfish (Ictaluruspunctatus) living under 4 cm/s swimming results in a visceral fat decrease (Jarboe and Grant, 1996). This data, in spite of being incipient, suggests that fat stores are consumed first when conditions such as exercise increase the energy demand.
Lipid metabolism
Lipids are the main source of metabolic energy ready available to meet metabolic demands of tissues with high physiological activity (Sheridan, 1988). Some factors can modulate this availability in tissues such as the muscles involved in the swimming processes. Among those factors are feed composition, physiological status, seasonality, and other species-specific traits (Shearer, 1994). Physical activity strongly affects biochemical composition, especially of lipids, in the swimming muscles of fish (Jobling, 2001). In matrinxa, both red and white muscles were responsive to swimming speed in regard to the lipid profile, and this responsiveness was slightly different between them. Both muscles mobilized lipids. However, while white muscle seemed to break down TG, increasing FFA content, red muscle promoted lipid anabolism, increasing FFA and lipid stores. The levels of FFA in red muscle increased with swimming speed as well as TG synthesis. This metabolic response calls attention to the possible harmful effects of high speeds during SS. Since TG levels were stable in white muscle, the remarkable FFA increase was possibly due to its uptake from plasma, wherein levels were low in all swimming conditions. Similar responses have been previously reported in matrinxa (Hackbarth and Moraes, 2006). The main source of lipids to supply white muscle seems to be the liver. In this physiological compartment, both FFA and TG levels increased with swimming speed, a biochemical fact that arose from such conditions, which can be adverse. Red muscle is more taxed than white muscle during SS. Its dense capillary bed and high activity of mitochondrial enzymes make it highly adapted to metabolize amino and fatty acids (Moyes and West, 1995; Anttila et al., 2010). However, under continuous exercise these limits must be established. Lipid accumulation in red-muscle can be a genetic trait acquired over the species' history, since its rheophilic characteristic demand large amounts of fat stores used in growth and maturation of gonads, muscle contraction for reproduction activities, and migration (Zaniboni-Filho et al., 2006; Bombardier et al., 2009).
The level of lipids stores in fish can be related not only to exercise but also to nutrient levels. Some species, even when fed low levels of dietary fat, present high levels of lipids (Yogata and Oku, 2000). It has been suggested that these responses are adaptive; however, special care must be taken regarding composition of the fish's diet during exercise. High levels of dietary fat may result in high lipid deposition in muscle, reducing the fish's swimming performance (Anttila et al., 2010). The Japanese flounder, exposed to various swimming speeds, increases lipid content in dorsal and anal fin muscles in response to moderate speeds (Ogata and Oku, 2000). Generally, fish exposed to moderate swimming speeds present increased lipid deposition (Totland et al., 1987; Young and Cech, 1994b; Ozório, 2008). In brief, lipids are accumulated in tissues and organs involved in activities that require more energy.
When swimming is extended for long periods, rainbow trout's demand for carbohydrates decreased by activation of LCFA (long chain fatty acids) oxidation. In fact, the greatest capacity to oxidize LCFA by red muscle has been reported (Richards et al., 2004). This tissue uptakes palmitate twice as fast as white muscle. This ability to oxidize LCFA is probably due to the greater number of protein transporters in the cell membrane of red muscle tissue. Brown trout accumulates nine times more lipids than white muscle (Anttila et al., 2010). Utilization of stored lipids as an aerobic energy source is increased by the addition of carnitine to fish diet (Ozorio et al., 2005); otherwise the burning of protein for energy takes significance in the energetic input to increase muscular activity. In this scenario, the cumulative increase of ammonia observed in fish fed low-fat diets is due to increased muscle protein catabolism, exacerbated by carnitine deficiency.
Another muscle whose primary function is to accumulate lipids, working as a fatty acids reservoir, is the ventral muscle or the belly flap (Jobling, 2001). Matrinxa increased fatty acids mobilization in this compartment according to the swimming speed increase. Nevertheless, lipid mobilization was not enough to alter muscle TL levels. This was likely due to ventral muscle be the largest deposit of fat in this body structure; one of the most important lipid reservoirs in addition to visceral mesenteric tissue, which stores significant amounts of fat as triglycerides (Navarro and Gutierrez, 1995). Sustained swimming of matrinxa at increasing speeds (1.0 to the faster 2.5 BL/s) stimulated lipid metabolism in all tissues. The reduction of fatty acids in the bloodstream is likely linked to insulin levels and hormone-sensitive lipase. Considering that a substantial reduction of lipid stores was only observed in visceral fat, inferred from FVSI, it is possible to attribute this compartment a primary role in lipid mobilization. As previously proposed (Van den Thillart and Van Raaij, 1995; Navarro and Gutierrez, 1995), Viscera covering lipids are the first to mobilize, also observed in matrinxa. In fact, this is the main fat store in matrinxa, encasing the highest caloric density per gram of tissue, followed by red muscle, liver, and white muscle. The present data allows concluding that matrinxa is rather dependent on lipids as primary substrate and red muscle is its main consumer due to its aerobic preference and high capacity for oxidizing longchain fatty acids such as palmitate (Richards et al., 2002; Magnoniet al., 2013).
Protein metabolism
Ammonia is the main waste product of protein catabolism in muscle and the liver. This wastage increases with infectious processes, sub-nutrition and physical activity (Moyes and West, 1995; Wright, 1995) as observed in the present work. High ammonia levels (>1.5 BL/s) are explained by an increase in protein catabolism following exercise. Such catabolism may be harmful when occurring in plasma, as suggested by the decrease observed in plasma protein content. However, this inconvenience seems to occur at 1.5 BL/s as well. In fact, protein catabolism inferred from the plasma profile reflects protein metabolism in peripheral tissues. The increase of plasma FAA should corroborate such an assumption, since its source cannot be plasma proteins only. The other body compartments suggest that protein catabolism in the liver can contribute to plasma FAA increase, particularly considering the liver FAA decrease. Interestingly, hepatic protein catabolism was relevant only at the highest swimming speed. Protein breakdown did not occur only in the liver; white muscle consumed nearly 15% of its content in fish kept under SS at 2.5 BL/s. Indeed, such catabolism started at 1.5 BL/s. The red muscle of matrinxã started protein amino acids catabolism only from 2.0 BL/s. However, this tissue seems to be fed by FAA sources other than its own. Considering that muscle represents 50 to 60% of the total body weight, any changes occurring in them will impact growth (Jobling, 2001). This data elucidates the decrease of WG from 1.5 BL/s and allows concluding that protein synthesis is impaired when fish swim at higher speeds. In some salmonids, increased protein catabolism in response to swimming speed is also evident (Davison and Golspink, 1977; Davison, 1997). When swimming is moderated, less ammonia is excreted since less protein is burned as fuel, showing a fine control in allocating protein for somatic growth (Ozório, 2008; Felipet al., 2013). There is a net increase of muscle protein deposition at the expense of lipid storages as fish swim continuously at moderate speeds (Lauff and Wood, 1997; Richards et al., 2002; Mckenzie et al., 2007). However, matrinxa seem to be very sensitive to SS and speeds higher than 1.5 BL/s can be harmful.
Carbohydrate metabolism
In fish, carbohydrates contribution as an energy source to supply muscular energy demand is dependent on exercise intensity and duration (Richards et al., 2002; Ozório, 2008; Felip et al., 2013). Muscular glycogen is not enough to support energy requirements for long swimming periods (Moyes and West, 1995). The liver and muscular glycogen in fish may vary with species, physiological state, seasonality, nutritional composition, and food intake (Navarro and Gutierrez, 1995; Van den Thillart and Van Raaij, 1995). In addition, exercise intensity and span can affect glycogen levels. Sustained swimming at 1.5 BL/s increased the bulk of liver glycogen in matrinxa; higher speeds dropped these values to the basal contents. A very similar pattern was observed in both white and red muscles. This metabolic frame can improve swimming performance and resistance to fatigue (Kieffer, 2000; Brown et al., 2011) around that swimming speed range. Under continuous swimming for 90 days at speeds higher than 1.5 BL/s, matrinxa spent their glycogen stores, missing the benefits of exercise. The increase of glycogen bulk observed, swimming at speeds around 1.0-1.5 BL/s, was possibly due to gluconeogenesis from nitrogenous substrates or even from glycerol. The intense protein and lipid metabolism observed in matrinxa under SS corroborates such an assumption. Higher speeds usually lead to glycogen depletion and lactate accumulation. In such circumstances, fish turn to anaerobic metabolism and basically depend on carbohydrates, as observed in brown-trout (Magnoni et al., 2013). However, matrinxa, even at the highest swimming speed, did not show any anaerobic preference. At the highest swimming speed plasma lactate reached the highest value; however, it was only followed by one pyruvate peak. This fact made the ratio L/P (lactate/pyruvate) equal 7.7, very close to the L/P average for all conditions (8.6).
Glucose is widely used as an energy substrate in the animal kingdom. In spite of the metabolic lipid preference by matrinxa under SS, liver and white muscle glucose levels increased. However, a glucose increase in white muscle was observed from 1.5 BL/s. This increase would supply the metabolic energy demanded for swimming; however, the liver's glucose increase should deliver it to the white muscle. The straight correlation between liver versus white muscle glucose sustains such assumption. The need for glucose by white muscle cannot be ignored; from the swimming speed of 1.5 BL/s, the liver's glycogen stores start to be consumed, likely in order to supply the white muscle's requirements. Even white muscle demand for glucose seems to have increased, since its glycogen stores were decreased from that point. In spite of the anaerobic characteristics of white muscle and the glucose needs of matrinxa under SS, the L/P ratio indicated anaerobic preference in experimental conditions. This strategy may be grounded in adjustments of the blood capillary bed, increasing blood perfusion (Davie et al., 1986; Young and Cech, 1994a; Sänger, 2000). However, such morphological adaptation must be further investigated (Milligan and Wood, 1987; Xavier et al., 2002).
In conclusion, sustained swimming adaptively rearranges the metabolic profile of matrinxa. Exercise enhances lipid mobilization in matrinxa, presenting a lipotropic effect. It improves the mobilization of visceral fat, reducing FVSI. Catabolism of glucose is observed in white muscle. However, exercise brings benefits that help avoid lactate fermentation. It is clear that a precise adjustment of swimming speed must be made to prevent protein consumption and metabolic overload. The best swimming speed for juvenile matrinxa under continuous exercise is around 1.0 to 1.5 BL/s. Under such conditions, the largest reserves of glycogen can probably provide a larger energy contribution to meet glycolysis demands of anaerobic white muscles, since these muscles are required in explosive swimming situations (capturing food, escaping predators or avoiding natural obstacles during reproductive migration and feeding). Future studies should be aimed at linking nutrient levels and SS in order to optimize the rearing conditions of intensive B. amazonicus farming.
¤ To cite this article: Arbeláez-Rojas GA, Moraes G. Effect of water velocity on intermediary metabolism of juvenile matrinxã fish (Brycon amazonicus). Rev Colomb Cienc Pecu 2013; 26:288-299.
Acknowledgements
This work was sponsored by the National Council for Scientific and Technological Development CNPq (Brazil). Authors thank colleagues at the Adaptive Biochemistry Laboratory for their logistic support.
References
Anttila K, Jäntti M, Mänttäri S. Effects of training in swimming of sea trout (Salmotrutta). J Comp Physiol B 2010; 180:707- 714. [ Links ]
Arbeláez-Rojas GA, Fracalossi DM, Fim JDI. Composição corporal de tambaqui, Colossomamacropomum e matrinxa, Bryconcephalus, em sistemas de criação intensiva, em igarapé, e semi-intensiva, em viveiros. Brazilian J Animal Sci 2002; 31:1059-1069. [ Links ]
Bidinotto PM, Souza RHS, Moraes G. Hepatic glycogen in eight tropical freshwater teleost fish: A procedure for field determinants of microsamples. Bol Tec CEPTA – Pirassununga 1997; 10:53-60. [ Links ]
Bjørnevik M, Karlsen Ø, Johnston IA, Kiessling A. Effect of sustained exercise on white muscle structure and flesh quality in farmed cod (Gadusmorhua L.). Aqua Res 2003; 34:55-64. [ Links ]
Bombardier E, Booth K, Green J, McKinley RS. Metabolic adaptations of oxidative muscle during spawning migration in the Atlantic salmon Salmosalar L. Fish Physiolog Biochem 2009; 36:355-365. [ Links ]
Brandão FR, Gomes LC, Chagas EC, Araújo DL, Ferreira AL. Densidade de estocagem de matrinxa, Bryconamazonicus na recria em tanque-rede. Pesq Agrop Brasil 2005; 40:299-303. [ Links ]
Brown EJ, Bruce M, Pether S, Herbert NA.Do swimming fish always grow fast? Investigating the magnitude and physiological basis of exercise-induced growth in juvenile New Zealand yellowtail kingfish, Seriolalalandi. Fish Physiol Biochem 2011; 37:327-336. [ Links ]
Chernecky CC, Krech RL, Berger BJ. Laboratory tests and diagnostic procedures. Philadelphia: Saunders; 1993. [ Links ]
Copley NG. Alloxan and ninhydrin test. Analyst 1941; 66:492- 493. [ Links ]
Davie PS, Wells RM, Tetens V. Effects of sustained swimming on rainbow trout muscle structure, blood oxygen transport, and lactate dehydrogenase isozymes: evidence for increased aerobic capacity of white muscle. J Exp Zool 1986; 237:159-171. [ Links ]
Davison W, Goldspink G. The effect of prolonged exercise on the lateral musculature of the Brown trout (Salmotrutta). J Exp Biol 1977; 70:1-12. [ Links ]
Davison W. The effects of exercise training on Teleost fish, a review of recent literature. Comp Biochem Physiol 1997; 117A:67-75. [ Links ]
Dubois MG, Gilles KA, Hamilton JK, Rebers PA, Smith F. Colorimetric method for determination of sugars and related substances. Analyt Chem 1956; 28:350-358. [ Links ]
Fabrizzi F, Moraes G, Hackbarth A, Almeida LC, Arbeláez- Rojas G and Nunes CS. Intermittent sustained swimming in 'matrinxa' Bryconamazonicus(Bryconidae: Bryconinae): hematological and metabolic responses. Neotropical Ichthyology 2013; 11 2:425-432. [ Links ]
Felip O, Blasco J, Ibarz A, Martin-Perez M. Fernández-Borrás J. Beneficial effects of sustained activity on the use of dietary protein and carbohydrate traced with stable isotopes 15N and 13C in gilthead sea bream (Sparusaurata). J Comp Physiol B 2013; 183:223-234. [ Links ]
Folch GD, Less M, Stome-Stanley GH. A simple method for the isolation and purification of total lipids from animal tissue. J Biol Chem 1957; 226C:497-509. [ Links ]
Gentzkow CJ, Masen JM. An accurate method for the determination of blood urea nitrogen by direct nesslerization. J Biol Chemist 1942; 143:531-544. [ Links ]
Guillaume J, Kaushik S, Bergot P, MetaillerR. Nutrition and Feeding of Fish and Crustaceans. Chichester (UK): Praxis Publishing; 2001. [ Links ]
Hackbarth A, Moraes G. Biochemical responses of matrinxa Bryconcephalus (Günther, 1869) after sustained swimming. Aqua Res 2006; 37:1070-1078. [ Links ]
Harrower JR, Brown CH. Blood lactic acid. A micromethod adapted to field collection of microliter samples. J Appli Physiol 1972; 32:224-228. [ Links ]
Hernándes MD, Mendiola P, Costa J, Zamora S. Effects of intensive exercise on rainbow trout growth, body composition and metabolic responses. J Physiol Biochem 2002; 58:1-8. [ Links ]
Howes GJ. Review of the genus Brycon (Teleostei: Characoidei). Bulletin Br Museum (Nat His) Zool 1982; 43:1- 47. [ Links ]
Inoue LAKA, Santos-Neto C, Moraes G. Clove oil as anesthetic for juveniles of matrinxa, Bryconcephalus (Gunther, 1869). Ciên Rur 2003; 33:943-947. [ Links ]
Jarboe H, Grant W. The effects of water velocity on the growth, dressout, and body composition of channel catfish, Ictaluruspunctatus, raised in circular tanks. J Appl Aqui 1996; 6:13-21. [ Links ]
Jobling M, Baarvik BM, Christiansen JS, Jorgensen EH. The effects of prolonged exercise training on growth performance and production parameters in fish. Aqua Inter 1993; 1:95-111. [ Links ]
Jobling M. Nutrient partitioning and the influence of feed composition on body composition. In: Houlihan D, Boujard T, Jobling M, editors. Food intake in Fish. Oxford: Blackwell Science Ltd.; 2001. p.354-75. [ Links ]
Kieffer JD. Limits to exhaustive exercise in fish. Comp Biochem Physiol Part A 2000; 126A:161-179. [ Links ]
Kiessling A, Higgs DA, Dosanjh BS, Eales JG. Influence of sustained exercise at two ration levels on growth and thyroid function of all-female Chinook salmon (OncorynchustshawyschaWalbaum) in seawater. Can J Fish Aqua Sci 1994; 51:1975-1984. [ Links ]
Lauff RF, Wood CM. Effects of training on respiratory gas exchange, nitrogenous waste excretion, and fuel usage during aerobic swimming in juvenile rainbow trout (Oncorhynchusmykiss). Can J Fish Aquatic Sci 1997; 54:566- 571. [ Links ]
Lowry DH, Rosenbrough NJ, Far AL, Randal RJ. Protein measurement with folin phenol reagent. J Biol Chem 1951; 193:265-275. [ Links ]
Lu GD. The metabolism of pyruvic acid in normal and vitamin B-deficient states. I. A rapid specific and sensitive method for the estimation of blood pyruvate. Biochem J 1939; 33:249-254. [ Links ]
Mackenzie DJ, Pedersen PB, Jokumsen A. Aspects of respiratory physiology and energetics in rainbow trout (Oncorhynchusmykiss) families with different size-at-age and condition factor. Aquaculture 2007; 263:280-294. [ Links ]
Magnoni LJ, felip O, Blasco J, Planas JV. Metabolic Fuel utilization During swimming: optimizing Nutricionalrequeriments For enhanced Performance. In: Palstra AP and Planas JV, editors. Swimming Physiology of Fish: Towards using exercise for farming a fit fish in sustainable aquaculture. Berlin Heidelberg: Springer-Verlag; 2013. p.203- 35. [ Links ]
Milligan CL, Wood CM. Effects of strenuous activity on intracellular and extracellular acid-base status and H+ exchange with the environmental in the inactive, benthic starry flounder Platychthysstellatus. Physiol Zool 1987; 60:37-53. [ Links ]
Morgan IJ, McCarthy ID, Metcalfe NB. The influence of lifehistory on lipid metabolism in over wintering juvenile Atlantic salmon. J Fish Biol 2002; 20:674-686. [ Links ]
Moyes CD, West TG. Exercise metabolism of fish. In: Hochachka PW, Mommsen TP, editors. Metabolic Biochemistry. Biochemistry and Molecular Biology of Fish. Vol.4. Amsterdam: Elsevier Science; 1995. p.367-92. [ Links ]
Navarro I, Gutierrez J. Fasting and starvation. In: Hochachka PW, Mommsen TP, editors. Metabolic Biochemistry. Biochemistry and Molecular Biology of Fish.Vol.4. Amsterdam: Elsevier Science; 1995. p.393-432. [ Links ]
Norvák M. Colorimetric ultramicro method for the determination of free fatty acids. J of Lipid Research 1965; 6:431-433. [ Links ]
Ogata HY, Oku H. Effects of water velocity on growth performance of juvenile Japanese flounder, Paralichthysolivaceus. J World Aqua Society 2000; 31:225-230. [ Links ]
Ozório ROA. Swimming Activity and Non-Protein Energy (NPE) Metabolism in Fish. Current Nutrition & Food Science 2008; 4:282-289. [ Links ]
Ozório ROA, Van Ginneken V, Van den Thillart G, Verstegen M, Verreth J. Dietary carnitine maintains energy reserves and delays fatigue of exercised African catfish (Clariasgariepinus) fed high fat diets. Sci Agric 2005; 62:208-213. [ Links ]
Palstra AP, Planas JV. Fish under exercise. Fish Physiol Biochem 2011; 37:259-272. [ Links ]
Post J, Parkinson EA. Energy allocation strategy in young fish: allometry and survival. Ecology 2001; 82:1040-1051. [ Links ]
Randall D, Brauner C. Effects of environmental factors on exercise in fish. J Exp Biol 1991; 160:113-126 [ Links ]
Richards JG, Bonen JF, Heigenhauser GJF, Wood CM. Palmitate movement across red and white muscle membranes of rainbow trout. Am J Physiol Regul Integr Comp Physiol 2004; 286:R46- 53. [ Links ]
Richards JG, Mercado AJ, Calyton CA, Heigenhauser GJ, Wood CM. Substrate utilization during graded aerobic exercise in rainbow trout. Exp Biol 2002; 205:2067-2077. [ Links ]
Rios FS, Kalinin AL, Rantin FT. The effects of long-term food deprivation on respiration and haematology of the neotropical fish Hopliasmalabaricus. J Fish Biol 2002; 61:85-95. [ Links ]
Rocha RM, Carvalho EG, Urbinati EC. Physiological responses associated with capture and crowding stress in matrinxa Bryconcephalus (Gunther, 1869), Teleostei: Characidae. Aqua Res 2004; 35-3:245-249. [ Links ]
Sänger AM, Pötscher U. Endurance exercise training affects fast white axial muscle in the cyprinid ChalcalburnuschalcoidesMento (Agassiz, 1832), Cyprinidae, Teleostei. Basic Appl Myol 2000; 6:297-300. [ Links ]
SAS. 1990. SAS/STAT. User's Guide. SAS Inst., Inc., Cary, NC, USA. [ Links ]
Shearer KD. Factors affecting the proximal composition of cultured fishes with emphasis on salmonids. Aquaculture 1994; 119:63-88. [ Links ]
Sheridan MA. Lipid dynamics in fish: aspects of absorption, transportation, deposition and mobilization. Comp Biochem Physiol 1988; 90B:679-690. [ Links ]
Totland GK, Kryvi H, Jodestol KA, Christiansen EN, Tangeras A, Slinde E. Growth and composition of the swimming muscle of adult atlantic salmon, Salmosalar during long-term sustained swimming. Aquaculture 1987; 66:299-313. [ Links ]
Van den Thillart G, Van Raaij M. Circulatory substrate fluxes and their regulation. In: Hochachka PW, Mommsen TP, editors. Metabolic Biochemistry. Biochemistry and Molecular Biology of Fish. Vol.4. Amsterdam: Elsevier Science; 1995. p.14-63. [ Links ]
Wootton RJ. Ecology of teleost fishes.Netherlands Kluwer: Academic Publishers; 1999. [ Links ]
Wright P. Nitrogen excretion: three end products, many physiological roles. J Exp Biol 1995; 198:273-281. [ Links ]
Young PS, Cech JR. Optimum exercise conditioning velocity for growth, muscular development, and swimming performance in young-of-the-year striped bass, (Moronesaxatilis). Can J Fish Aqua Sci 1994a; 51:1519-1527. [ Links ]
Young, PS, Cech JR. Effects of different exercise conditioning velocities on the energy reserves and swimming stress responses in young-of-the-year striped bass, (Moronesaxatilis). Can J Fish Aqua Sci 1994b; 51:1528-1534. [ Links ]
Yogata H, Oku H. The effects of swimming exercise on growth and whole-body protein and fat contents of fed and unfed fingerling yellowtail. Fish Sci 2000; 66:1100-1105. [ Links ]
Zaniboni Filho E, Reynalte-Tataje D, Weingartner M. Potencial del género Brycon en la piscicultura brasileña. Rev Colomb Cien Pecu 2006; 19:233-240. [ Links ]
Xavier AR, Roselino JE, Resano NM, Garófalo MA, Migliorini RH, Kettellhut IC. Glyconeogenic pathway in isolated skeletal muscles of rats. Can J Physiol Pharm 2002; 80:164-169. [ Links ]