Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Colombiana de Ciencias Pecuarias
Print version ISSN 0120-0690
Rev Colom Cienc Pecua vol.27 no.1 Medellín Jan./Mar. 2014
ORIGINAL ARTICLES
Presence of Salmonella spp. in reused broiler litter¤
Presencia de Salmonella spp. en cama reutilizada de pollos de engorde
Presença de Salmonella spp. em cama reutilizada de frangos de corte
Eduardo Muniz1, MV, MSc; Dany Mesa1*, MV; Rocío Cuaspa2, Biol; Alexandre M Souza1, MV; Elizabeth Santin1, MV, MSc, PhD.
1Laboratório de Microbiologia e Ornitopatologia, Departamento de Medicina Veterinária, Universidade Federal do Paraná, Brasil.
2Departamento de Bioquímica e Biologia Molecular, Universidade Federal do Paraná, Brasil.
* Corresponding author: Dany Mesa. Laboratório de Microbiologia e Ornitopatologia, Departamento de Medicina Veterinária, Universidade Federal do Paraná, Rua dos Funcionários 1540, Curitiba, Parana, Brazil. Email: dmesaf@unal.edu.co
(Received: December 14, 2012; accepted: April 11, 2013)
Summary
Background: reutilization of poultry litter for multiple broiler flocks is a common practice in modern production systems due to the increasing scarcity and cost of bedding materials, and the necessity to reduce environmental impact. However, this practice has been associated with sanitary risks, such as the presence of Salmonella spp. in broiler meat. Objective: a study was conducted to detect the presence of Salmonella spp. in reused litters. Methods: 1,280 litter samples from Midwestern Brazilian poultry farms were analyzed during seven consecutive flocks. Samples were collected from flocks aged 28 to 32 days. Disposable shoe covers were used for sample collections. Presence of Salmonella spp. was determined by microbiological isolation. During the interval period between flocks the litter was fermented prior to its reuse by covering it with a black plastic canvas for 7 days. Results: positive samples for Salmonella spp. decreased when the number of litter reuses increased compared with the first reuse of the litter. An anaerobic digestion process with biological and physicochemical changes in the litter material and microbial communities may explain the low survival of pathogenic bacteria such as Salmonella spp. Conclusions: our study demonstrates that litter reused after the fermentation process is a safe and recommended practice to reduce the presence of Salmonella spp.
Key words: ammonia, anaerobic digestion, composting, fermentation, microbiota.
Resumen
Antecedentes: la reutilización de la cama de pollos de engorde es una práctica común en el sistema moderno de producción avícola, sustentada por la reducción en el impacto ambiental, escasez de este material y disminución de costos de producción. Sin embargo, esta reutilización se ha asociado con riesgos sanitarios, tales como presencia de Salmonella spp. en los lotes de pollo. Objetivo: se realizó un estudio con el fin de detectar la presencia de Salmonella spp. en camas reutilizadas y fermentadas de pollos de engorde pertenecientes a granjas comerciales. Métodos: se analizaron 1280 muestras de cama de diversas granjas avícolas ubicadas en el centro oeste de Brasil durante siete lotes consecutivos de pollos. Las muestras de cama fueron tomadas de galpones con aves entre los 28 y 32 días de edad, utilizando polainas. La presencia de Salmonella spp. se determinó mediante aislamiento microbiológico. Durante el intervalo entre lotes, la cama fue fermentada antes de cada reutilización cubriendo la superficie entera de la cama con una lona de plástico negra por siete días. Resultados: fue observada una disminución en las muestras positivas para Salmonella con la reutilización y fermentación de las camas entre lotes, significativa con respecto al primer reuso. Esto indica que puede estar ocurriendo un proceso de digestión anaeróbica que conduce a que los procesos biológicos y físico-químicos entre el material de la cama y la comunidad microbiana allí presentes, estén afectando la supervivencia de bacterias patógenas como Salmonella. Conclusiones: nuestro estudio demuestra que la reutilización de la cama es una práctica segura y recomendable cuando se realiza después del proceso de fermentación, debido a que reduce la presencia de Salmonella spp.
Palabras clave: amonio, compostaje, digestión anaeróbica, fermentación, microbiota.
Resumo
Antecedentes: a reutilização de cama aviária por vários lotes é uma prática moderna do sistema de produção de aves, baseada na redução do impacto ambiental, escassez de este material e diminuição nos custos de produção. Porém, dita prática é associada com riscos sanitários como a presença de patógenos como Salmonella spp. nos lotes de frango. Objetivo: uma pesquisa foi realizada para detectar a presença de Salmonella spp. na cama reutilizada e fermentada de produtores de frango. Métodos: foram analisadas 1280 amostras de cama de diferentes produtores do Centro-oeste do Brasil durante sete lotes consecutivos. As amostras de cama foram coletadas com aves na idade entre 28 e 32 dias usando pró-pés descartáveis e a presença de Salmonella spp. foi determinada por isolamento bacteriológico. Durante o intervalo dos lotes a cama foi tratada antes da reutilização por meio da cobertura através de uma lona plástica preta em toda a superfície interna do aviário por sete dias. Resultados: foi observada uma diminuição no número de amostras positivas de Salmonella spp. com a reutilização e fermentação das camas entre os lotes, significativa em relação ao primeiro reuso. Isto indica que o processo de reutilização, seguido de fermentação anaeróbia do material da cama pela comunidade de microrganismos afetou a sobrevivência de bactérias patogênicas como Salmonella spp. Conclusões: este estudo evidencia que o reuso da cama é seguro e recomendado quando realizado após o processo de fermentação no intervalo do lote, devido a que diminui a presença de Salmonella spp.
Palavras chave: amônia, compostagem, digestão anaeróbica, fermentação, microbiota.
Introduction
Multiple broiler flocks are commonly reared on a single batch of litter in intensive poultry production systems. In these systems, birds are placed into grow-out houses one day after hatching, directly on litter bedding (Volkova et al., 2009). Management and processing is required to decrease the microbial load before reusing the litter (Vizzier Thaxton et al., 2003). With this purpose, fermentation has been proposed as an optimal alternative to ensure the microbiological quality of the litter (Macklin et al., 2006). However, some doubts regarding potential sanitary risks associated to this practice have been also posed.
Many microorganisms in poultry litter originate in bird excrement, including Enterobacteriaceae and other bacteria with zoonotic capacity (Cook et al., 2012; Fries et al., 2005). Continuous exposure to undesirable bacteria from litter can increase contamination of the birds' digestive tract. Even though enterobacteria do not cause health problems in chickens, it may become a human sanitary problem during slaughtering due to contamination when the carcass accidentally come in contact with contents from infected crop or intestine, compromising food safety and public health (Haapapuro et al.,1997).
Transference of pathogens into the food chain may also occur when litter is applied to soil as an organic fertilizer, resulting in the contamination of fresh produce (Lovanh et al., 2007; Volkova et al., 2009). Contaminated litter can promote pathogen perpetuation from one flock to another when it is reused more than once. For this reason, reuse of litter is not recommended when sanitary episodes have occurred.
Independent of litter destination (reuse for subsequent flocks or used as fertilizer), treatment to reduce or inactivate bacteria is vital for decreasing animal and human health risks. Thus, litter treatment is considered a needful condition in good poultry production practices (Larrison et al., 2010; Pope and Cherry, 2000). Total replacement of the litter after every flock results in considerable environmental impact due to the high amounts of substrate required (e.g. wood shavings, straw or sawdust) and the destination of this residue in the environment (Pandey and Soupir, 2011; Pote et al., 2011, Watts et al., 2011). Furthermore, changing the litter after every flock represents a significant cost in poultry production.
The purpose of this study was to evaluate the presence of Salmonella after fermenting the litter by covering it with a canvas prior to each reuse.
Materials and methods
Experiments
A total of 1,280 litter samples from several poultry farms in Midwestern Brazil were analyzed during seven consecutive flocks. The population corresponds to 196 producers.
Litter management
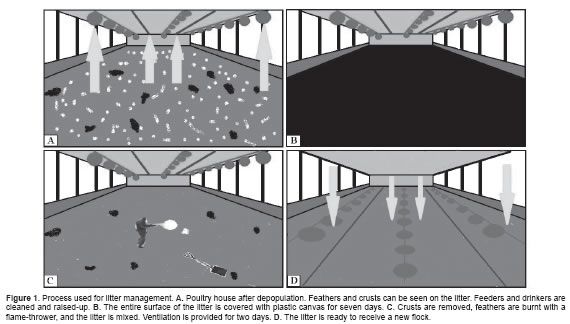
The procedure performed between flocks (Figure 1) is briefly described as follows:
1. Cleaning and rising of the equipment (feeders, drinkers, etc.) with soap and water immediately following depopulation (Figure 1A).
2. Watering the litter (20 liters/m2).
3. All columns inside the shed were covered with plastic canvas (approximately one square meter) to protect them from the fermentation process.
4. Displacing the litter from the shed sides to create a space between the walls and the litter.
5. Covering the litter with plastic canvas avoiding air entrance (Figure 1B).
6. Removing the canvas after seven days of fermentation, discarding the crusts and mixing the litter.
7. Applying the flame-thrower uniformly to the entire surface to burn residue such as feathers (Figure 1C).
8. Ventilating the house for two days before placing the new flock of birds (Figure 1D).
After this process was completed, one-day-old chicks were housed directly on the reused litter.
Sample collection
Litter samples were collected when birds were between 28 and 32 days of age. Collectors cleaned their hands carefully before. Plastic boots with shoe cover swabs were used for collection. Collectors walked on the litter for about 10 min, focusing placement of steps between feeders and drinkers, given that these sites maintain a high concentration of animals and therefore a greater amount of feces. The sample was collected on the shoe cover surface in contact with the litter. The shoe cover swabs were placed inside sterile bags containing 1% buffered peptone water solution and stored in cool boxes or coolers with ice while microorganism samples solubilized. Sample bags were sent to laboratory after collection.
Laboratory processing
Shoe cover swabs were discarded. Two aliquots of the solution were transferred to selective enrichment broths: 0.5 ± 0.05 mL into 10 mL Tetrathionate Broth and 0.1 ± 0.02 mL into 10 mL Rappaport-Vassiliadis. Both were incubated separately at 35 ± 2 °C and 42 ± 2 °C for 18 h and 24 h, respectively, and carefully mixed by vortexing. Then, each culture was streaked both in Brilliant Green agar and MacConkey agar plates using 10 μL inoculum for each. Agar plates were incubated overnight at 35 ± 2 °C and examined for the presence of Salmonella spp. colonies (BRASIL, 1995).
Statistical analysis
The chi-squared test was used to verify whether the frequency of positive samples for Salmonella spp. was related to the number of litter reuses. The criterion for statistical significance was p<0.05.
Results
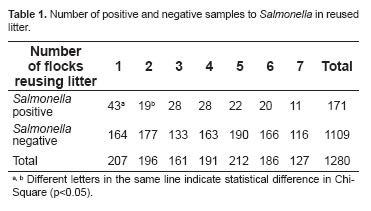
Detection of Salmonella spp. in litter reused up to seven times is shown in table 1. A decrease in positive samples was observed when comparing the new litter with all subsequent reused litters after the covering process, significant between the first flock with new litter and the second flock with the first reuse.
Besides the observed Salmonella reduction and beyond the aim of this report, a noteworthy decrease of darkling beetle (Alphitobius diaperinus) infestation was also observed after the covering process. Although it was not measured, we consider this an interesting observation for further studies since the covering and fermentation processes could also eliminate most insects and larvae without requiring the use of chemicals.
Discussion
The farms included in this study used the littercovering method between flocks as a strategy for poultry waste management. Ammonia concentration increased during the anaerobic digestion and fermentation process that probably occurred. We believe this leads to a microbicidal action in different populations, including Salmonella spp. These results are in agreement with Roll et al. (2011) who analyzed the presence of Salmonella in reused litters of 14 consecutive flocks and observed a reduction in the presence of Salmonella after treatment with lime.
The fermentation process consists of the hydrolysis of complex components, including fats, proteins and polysaccharides, which are broken down by microorganisms to their component subunits with the posterior production of collectable biogases (mainly methane and CO2) (Chen et al., 2008; Kelleher et al., 2002). Poultry waste is composed of litter, bird manure, and other residues. Due to high protein and amino acid metabolism, poultry manure is rich in organic nitrogen in the form of urea, which is mostly converted into ammonia by microbial activity, consequently undergoing a nitrification process (i.e. conversion to nitrate) (Kelleher et al., 2002). In this pathway ammonia exists as either a gas (NH3) or as ammonium (NH4 +), which is a hydrophobic, watersoluble and highly permeable molecule to biological membranes.
Ammonia is proposed as an anaerobic digestion inhibitor by the leak of proton-motive forces or interference with the tricarboxilic acid cycle. The first requires both a pH and electron gradient. The second involves the amination of α-ketoglutarate, an intermediate needed for the metabolism of organic compounds (Chen et al., 2008; Krylova et al., 1997).
Additional abiotic factors should be considered; the temperature reached during fermentation may also play a microbicidal role. The maximum temperature in our study was 60 °C (data not shown). Kim et al. (2012) observed that reduction of Salmonella can be achieved by exposing fresh chicken litter to 70 °C for 80.5 to 100.8 min. Wilkinson et al. (2011) reported that Salmonella typhimurium in fresh chicken litter was completely eliminated in 1 h at 55 to 65 °C under laboratory conditions. Other abiotic parameters that regulate microbiological conditions are pH (Payne et al., 2007) and moisture (Eriksson de Rezende et al., 2001). Different litter management and treatment methods can modify those factors (Miles et al., 2011; Torok et al., 2009).
Microorganisms and their complex microbial communities are responsible for most biochemical transformations. Using a combination of culture and molecular detection in intestinal chicken, Lu et al. (2003a) reported that Gram-positive bacteria and proteobacteria were the predominant populations (including Lactobacillus, Clostridium, Staphylococcus and Streptococcus) involved in the decomposition of organic material, including wood. This may explain the absence of important animal and human pathogens from the environment. Our study was conducted in Midwestern Brazil. Composition of litter microbiota depends on the geographical region (Cressman et al., 2010) and environmental conditions (Dumas et al., 2011). It should be noted that most of the studies have been conducted in North America.
It has been demonstrated that diversity of intestinal bacterial populations increases as birds age (Lu et al., 2003b). Deposition of excreta onto the litter rapidly alters biotic and abiotic environments simultaneously as litter conditions affect the intestinal microbiota and immune responses (Chapman and Rayavarapu, 2007; Lee et al., 2011). This supports our hypothesis that reusing the litter under proper management conditions may improve intestinal microbiota composition, important for the growth and health of the bird.
In conclusion, by reusing litter we observed a reduction in the presence of Salmonella spp., which could contribute to reducing costs and usage of raw litter material. Further studies are needed to evaluate the impact of reusing the litter on the presence of other relevant poultry pathogens such as Clostridium and Eimeria, as well as its effect on other physical traits, production cost, and its impact on natural resources.
¤ To cite this article: Sánchez-Jiménez MM, Ortiz-Román LF, Castrillón-Salazar LL, Giraldo-Echeverri CA, Olivera-Angel M. Application of a polymerase chain reaction test for the detection of Brucella canis from clinical samples of canines and humans. Rev Colomb Cienc Pecu 2014; 27:3-11.
References
BRASIL. Método Analítico de Carcaças de Aves e Pesquisa de Salmonella. Portaria n°8 de 23 de janeiro de 1995. Brasília. Programa Nacional de Sanidade Avícola (PNSA) 1995. [ Links ]
Cook A, Odumeru J, Lee S, Pollari F. Campylobacter, Salmonella, Listeria monocytogenes, verotoxigenic Escherichia coli, and Escherichia coli prevalence, enumeration, and subtypes on retail chicken breasts with and without skin. J Food Prot 2012; 75:34-40. [ Links ]
Cressman MD, Yu Z, Nelson MC, Moeller SJ, Lilburn MS, Zerby HN. Interrelations between the microbiotas in the litter and in the intestines of commercial broiler chickens. Appl Environ Microbiol 2010; 76:6572-6582. [ Links ]
Chapman HD, Rayavarapu S. Acquisition of immunity to Eimeria maxima in newly hatched chickens reared on new or reused litter. Avian Pathol 2007; 36:319-323. [ Links ]
Chen Y, Cheng J, Creamer K. Inhibition of anaerobic digestion process: A review. Bioresour Technol 2008; 99:4044-4064. [ Links ]
Dumas MD, Polson SW, Ritter D, Ravel J, Gelb J, Jr., Morgan R, Wommack KE. Impacts of poultry house environment on poultry litter bacterial community composition. PLoS One 2011; 6:1-12. [ Links ]
Eriksson de Rezende CL, Mallinson ET, Gupte A, Joseph SW. Salmonella spp. are affected by different levels of water activity in closed microcosms. J Ind Microbiol Biot 2001; 26:222-225. [ Links ]
Fries R, Akcan M, Bandick N, Kobe A. Microflora of two different types of poultry litter. Br Poult Sci 2005; 46:668-672. [ Links ]
Haapapuro ER, Barnard ND, Simon M. Review--animal waste used as livestock feed: dangers to human health. Prev Med 1997; 26:599-602. [ Links ]
Kelleher BP, Leahy JJ, Henihan AM, O'Dwyer TF, Sutton D, Leahy MJ. Advances in poultry litter disposal technology-a review. Bioresour Technol 2002; 83:27-36. [ Links ]
Kim J, Diao J, Shepherd MW, Jr., Singh R, Heringa SD, Gong C, Jiang X. Validating thermal inactivation of Salmonella spp. in fresh and aged chicken litter. Appl Environ Microbiol 2012; 78:1302-1307. [ Links ]
Krylova NI, Khabiboulline RE, Naumova RP, Nagel MA. The influence of ammonium and methods for removal during the anaerobic treatment of poultry manure. J Chem Technol Biot 1997; 70:99-105. [ Links ]
Larrison EL, Byrd JA, Davis MA. Effects of litter amendments on broiler growth characteristics and Salmonella colonization in the crop and cecum. J Appl Poult Res 2010; 19:132-136. [ Links ]
Lee KW, Lillehoj HS, Lee SH, Jang SI, Ritter GD, Bautista DA, Lillehoj EP. Impact of fresh or used litter on the posthatch immune system of commercial broilers. Avian Dis 2011; 55:539-544. [ Links ]
Lovanh N, Cook KL, Rothrock MJ, Miles DM, Sistani K. Spatial shifts in microbial population structure within poultry litter associated with physicochemical properties. Poult Sci 2007; 86:1840-1849. [ Links ]
Lu J, Sanchez S, Hofacre C, Maurer JJ, Harmon BG, Lee MD. Evaluation of broiler litter with reference to the microbial composition as assessed by using 16S rRNA and functional gene markers. Appl Environ Microbiol 2003a; 69:901-908. [ Links ]
Lu J, Idris U, Harmon B, Hofacre C, Maurer JJ, Lee MD. Diversity and succession of the intestinal bacterial community of the maturing broiler chicken. Appl Environ Microbiol 2003b; 69:6816-6824. [ Links ]
Macklin KS, Hess JB, Bilgili SF, Norton RA. Effects of inhouse composting of litter on bacterial levels. J Appl Poult Res 2006; 15:531-537. [ Links ]
Miles DM, Rowe DE, Cathcart TC. High litter moisture content suppresses litter ammonia volatilization. Poult Sci 2011; 90:1397-1405. [ Links ]
Pandey PK, Soupir ML. Escherichia coli inactivation kinetics in anaerobic digestion of dairy manure under moderate, mesophilic and thermophilic temperatures. AMB Express 2011; 1:18. [ Links ]
Payne JB, Osborne JA, Jenkins PK, Sheldon BW. Modeling the growth and death kinetics of Salmonella in poultry litter as a function of ph and water activity. Poult Sci 2007; 86:191-201. [ Links ]
Pope MJ, Cherry TE. An evaluation of the presence of pathogens on broilers raised on poultry litter treatment-treated litter. Poult Sci 2000; 79:1351-1355. [ Links ]
Pote DH, Way TR, Kleinman PJ, Moore PA, Jr., Meisinger JJ, Sistani KR, Saporito LS, Allen AL, Feyereisen GW. Subsurface application of poultry litter in pasture and no-till soils. J Environ Qual 2011; 40:402-411. [ Links ]
Roll VF, Dai Pra MA, Roll AP. Research on Salmonella in broiler litter reused for up to 14 consecutive flocks. Poult Sci 2011; 90:2257-2262. [ Links ]
Torok VA, Hughes RJ, Ophel-Keller K, Ali M, Macalpine R. Influence of different litter materials on cecal microbiota colonization in broiler chickens. Poult Sci 2009; 88:2474-2481. [ Links ] Vizzier Thaxton Y, Balzli CL, Tankson JD. Relationship of broiler flock numbers to litter microflora. J Appl Poult Res 2003; 12:81-84. [ Links ]
Volkova VV, Bailey RH, Wills RW. Salmonella in broiler litter and properties of soil at farm location. PLoS One 2009; 4:e6403. [ Links ]
Watts DB, Way TR, Torbert HA. Subsurface application of poultry litter and its influence on nutrient losses in runoff water from permanent pastures. J Environ Qual 2011; 40:421-430. [ Links ]
Wilkinson KG, Tee E, Tomkins RB, Hepworth G, Premier R. Effect of heating and aging of poultry litter on the persistence of enteric bacteria. Poult Sci 2011; 90:10-18. [ Links ]