Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Colombiana de Ciencias Pecuarias
Print version ISSN 0120-0690
Rev Colom Cienc Pecua vol.27 no.1 Medellín Jan./Mar. 2014
ORIGINAL ARTICLES
Effectiveness of the aromatase (P450 Arom) inhibitors Letrozole and Exemestane for masculinization of red tilapia (Oreochromis spp.) ¤
Efectividad de los inhibidores de la aromatasa (aromP450) Letrozol y Exemestano para la masculinización de tilapia roja (Oreochromis spp.)
Efetividade dos inibidores da aromatase (aromP450) Letrozol e Exemestano para a masculinização da tilápia vermelha (Oreochromis spp)
John J Betancur López1, Zoot; Juan Carlos Quintero Velez3, MV, MSc; Henry Ostos Alfonso1, MD, MSc; Frank Barreiro-Sanchez1, Est MV; Marta Olivera Angel2, MV, Dr Sci.
1Grupo Laboratorio de Medicina Genómica, Universidad SURCOLOMBIANA.
2Grupo BIOGÉNESIS, Facultad de Ciencias Agrarias, Universidad de Antioquia.
3Grupo de Investigación en Ciencias Veterinarias-Centauro, Escuela de Medicina Veterinaria, Facultad de Ciencias Agrarias, Universidad de Antioquia, Calle 70 No. 52-21, Medellín, Colombia.
* Corresponding author: John J Betancur López. Grupo Laboratorio de Medicina Genómica, Universidad Surcolombiana. Carrera 3 No. 1-31, vía Las Termitas, La plata, Huila. Email: asincronix@gmail.com
(Received: September 13, 2012; accepted: August 8, 2013)
Summary
Background: cytochrome P450 aromatase enzyme (aromP450) is a key enzyme in the conversion of androgens into estrogens, a crucial step in the control of sexual differentiation in fish. Objective: the aim of this study was to evaluate the efficiency of two aromatase inhibitors (AIs) as an alternative method for the masculinization of red tilapia (Oreochromis spp.). Methods: Letrozole (LT) and Exemestane (EM) aromatase inhibitors were used at two experimental doses (25 and 100 mg/Kg). Five days post-hatching larvae (5 dph) were fed the inhibitors for 30 days (35 dph). The control treatments consisted of 17α metil testosterone (MT) at a concentration of 60 mg/ Kg (positive control) and food with 300 ml/Kg ethanol (negative control). On 60 dph, gonadal extraction of fishes was performed for histological processing and staining with hematoxylin-eosin for analysis. Results: there were no significant differences (p>0.05) between any compound and implemented doses with the controls in terms of larval survival. Percentage of male fish increased for LT, EM, and MT (positive control), which showed significant differences (p<0.05) with the negative control. The dose analysis showed significant differences (p<0.05) for 100 mg/Kg dose and positive control with 25 mg/Kg dose and negative control; there were also differences between 25 mg/Kg dose and negative control. Conclusions: our results suggest that oral administration of third generation AIs (Type I or Type II) is effective for increasing the proportion of males without differences between inhibitor types. There is also a direct effect of the dose on male proportion. Suppression of aromatase activity allows guiding sexual differentiation towards final testicular development.
Key words: androgens, competitive inhibition, estrogens, irreversible inhibition, sex differentiation.
Resumen
Antecedentes: la citocromo P450 aromatasa (aromP450) es una enzima clave en la conversión de andrógenos a estrógenos; un paso crucial en el control de la diferenciación sexual en peces. Objetivo: evaluar la eficiencia de dos inhibidores de la aromatasa (IAs) como método alternativo para la masculinización de tilapia roja (Oreochromis spp.). Métodos: se evaluaron los inhibidores de la aromatasa, letrozol (LT) y exemestano (EM), a dos dosis experimentales de 25 y 100 mg/Kg. Larvas de 5 días post eclosión (dpe) fueron alimentadas durante 30 días (35 dpe) con los tratamientos. Como testigos se implementaron 17α metil testosterona (MT) a una concentración de 60 mg/Kg (control positivo), y alimento con etanol 300 ml/Kg (control negativo). El día 60 dpe se realizó extracción gonadal de los peces para procesamiento histológico y tinción con hematoxilina-eosina para su análisis. Resultados: no hubo diferencias significativas (p>0,05) entre ninguno de los tratamientos y los controles para la variable sobrevivencia larvaria. La proporción de machos aumentó con la implementación de LT, EM y MT (control positivo), los cuales presentaron diferencias significativas (p<0,05) con el control negativo. Hubo diferencias significativas (p<0,05) entre la dosis de 100 mg/Kg y el control positivo con la dosis de 25 mg/Kg y el control negativo; también se presentaron diferencias entre las dosis de 25 mg/kg y el control negativo. Conclusiones: nuestros resultados sugieren que el suministro vía oral de IAs de tercera generación, tipo I o tipo II, es eficiente en aumentar la proporción de machos en los grupos tratados, sin diferencias entre el tipo de inhibidor, pero con un efecto directo de la dosis sobre la proporción de machos. La supresión de la actividad de la aromatasa, permitió orientar la diferenciación sexual hacia el desarrollo testicular.
Palabras claves: andrógenos, diferenciación sexual, estrógenos, inhibición competitiva, inhibición irreversible.
Resumo
Antecedentes: a enzima aromatase citocromo P450 (aromP450) é uma enzima chave na conversão de andrógenos em estrógenos, um passo fundamental no controle da diferenciação sexual em peixes. Objetivo: avaliar a eficiência de dois inibidores da aromatase (AIs), como um método alternativo para a masculinização da tilápia vermelha (Oreochromis spp.). Métodos: avaliaram-se os inibidores da aromatase, letrozol (LT) e exemestano (EM), em duas doses experimentais de 25 e 100 mg/Kg. Larvas 5 dias pós-eclosão (dpe) foram alimentadas durante 30 dias (35 dpe) com os tratamentos. Como testemunho, controle positivo, se implementaram 17α metil testosterona (MT) a uma concentração de 60 mg/Kg e como controle negativo alimento com etanol 300 ml/Kg. No dia 60 dpe extraíram-se as gônadas dos peixes para processamento histológico e coloração com hematoxilina-eosina para sua analise. Resultados: não houve diferenças significativas (p>0,05) entre os tratamentos e os controles para a variável sobrevivência larvária. A percentagem de machos foi aumentada com a utilização de LT, EM e MT (controle positivo), com diferenças significativas (p<0,05) comparados com o controle negativo. A análise da dose mostrou diferenças significativas (p<0,05) entre a dose de 100 mg/Kg e o controle positivo com a dose de 25 mg/Kg e o controle negativo; também apresentaram diferenças entre a dose de 25 mg/kg e o controle negativo. Conclusões: nossos resultados sugerem que o fornecimento oral de IAs de terça geração, tipo I ou II, são eficientes para aumentar a proporção de machos nos grupos tratados, sem diferenças entre o tipo de inibidor, porem com efeito direto da dose sobre a proporção de machos. A supressão da atividade da aromatase permitiu orientar a diferenciação sexual até um desenvolvimento testicular final.
Palavras chave: andrógenos, diferenciação sexual, estrógenos, inibição competitiva, inibição irreversível.
Introduction
Aromatase inhibitors (AIs) are chemicals that inhibit the activity of the aromatase enzyme (aromP450), leading to a reduction of estrogen synthesis. Cytochrome P450 aromatase (cyp19) plays an important role in steroid homeostasis by converting androgens into estrogens. It has been demonstrated that synthesis of 17ß-estradiol from testosterone by the action of aromP450 enzyme is essential for ovarian development (Fenske et al., 2004; González, 2003). Teleost fish differ from the typical vertebrate model in which they have two different and separated loci for genes cyp19, cyp19a1 (predominant in gonads), and cyp19a2 (predominant in brain). It is believed that both loci are involved in the process of sexual differentiation (Tchoudakova et al., 2001; Sawyer et al., 2006).
The mRNA of aromP450 has been detected in female gonads — but not in those of the males — during sexual differentiation (Sawyer et al., 2006; Gardner et al., 2005). The activity of aromP450 is higher during ovarian differentiation in comparison with testis differentiation in reptiles and amphibians (Hecker et al., 2005). The expression of cyp19a isoform in Atlantic halibut (Hippoglossus hippoglossus) is high in ovaries and testes, while cyp19b is mostly expressed in the nervous system (Matsuoka et al., 2006). Similar results were reported for Killfish (Fundulus heteroclitus) in ovaries (cyp19a>cyp19b) and nervous system (cyp19a<cyp19b) (Greytak et al., 2005). Immunoexpression of steroidogenic enzymes, including aromatase (aromP450), is correlated with ovarian differentiation in tilapia (Oreochromis niloticus) (Nakamura et al., 2003).
Aromatase inhibitors can be classified into first generation (aminoglute thimide), second generation (Formestane, Fadrozole), and third generation (Anastrozole, Letrozole, and Exemestane), which in turn can be divided into Type I and Type II inhibitors. Type I inhibitors have steroidal structure, similar to androgens, and inactivate the enzyme by irreversibly blocking the substrate-binding site. Type II inhibitors are non-steroidal and are reversible (Mokbel, 2002).
Several studies conducted in all-female (XX) Nile tilapia (Oreochromis niloticus) populations have demonstrated that implementing AIs, such as Fadrozole, ATD (1,4,6- androstatriene-3–17- dione), and Exemestane increases male proportions in groups exposed to oral treatments (Kwon et al., 2000; Ruksana et al., 2010; Afonso et al., 2001; Guiguen et al., 1999).
The objective of this study was to evaluate two AIs: Type I (Exemestane) and Type II (Letrozole) at two concentrations in the feed as an alternative method for sexual reversion in Red tilapia.
Materials and methods
Ethical considerations
This study was approved by the Ethics Committee for Animal Research of the Universidad de Antioquia (Medellín, Colombia), Act No. 36 of May 15, 2007.
Location
Red tilapia (Oreochromis spp.) fingerlings were produced at Pérez Chiquito farm (Agroindustrial y Comercial 3C Ltd. Company, Aipe municipality, Huila, Colombia) located 650 meters above the sea level, with 29 °C average temperature, 66% relative humidity, and 25 °C average water temperature. Animal dissection, gonadal extraction, histology, and microscopic evaluation of the plates were conducted at the Medicina Genómica laboratory (Health Department, Universidad Surcolombiana, Neiva, Huila-Colombia).
Preparation of experimental feed
Aromatase inhibitors (AIs), Letrozole LT (Femara®, 2.5 mg tablets, Novartis AG, Basel, Switzerland) and Exemestane EM (Aromasin®, 25 mg tablets, Pfizer Japan Inc., Tokyo, Japan) were prepared at 25 mg and 100 mg/Kg feed. The control treatments consisted of 17α metil testosterone (MT) added to the feed at 60 mg/Kg (positive control) and feed treated with 300 ml ethanol/Kg (negative control). Same amounts of ethanol were used to dilute AIs and MT. Treated feed was dried in a dark room at a 26 °C until ethanol completely evaporated. Feed was then stored in amber glass vials throughout the experimental period to avoid oxidative photodegradation of the active ingredients.
Sex reversal by feed
Eggs were collected from the oral cavity of females. Collected material was evaluated by stereomicroscope (Leica© M60, Leica Microsystems, Wetzlar, Germany) to ensure homogeneity of larvae. The stage of embryonic development was defined with the scale proposed by Fujimura et al. (2007). Pharyngula stage embryos (66 to 70 hpf – 2.5 to 3.5 dpf) were selected to complete incubation. The terms post-fertilization hours (hpf), post-fertilization days (dpf), and post-hatching days (dph) refer to specific stages.
Larvae (5 dph) with complete yolk sac resorption and initiating exogenous feeding were fed for 30 days (35 dph). Three replications were used per experimental group (200 larvae/replication). Larvae were located in 40 L aquariums. Water turnover was 10% of total daily volume. Feed (45% protein) was offered at 10% of total biomass in 8 servings per day. Replications were transferred into 1 m2 earthen ponds after the experimental phase. After 65 dph, individuals were anesthetized with MS-222 (Tricaine methane sulphonate; 100 mg/L), killed by thermal shock, and morphometric data (weight, standard and total length) were recorded. Finally, all the individuals were preserved for subsequent gonad extraction and assessment.
Sample fixation and histological analysis
Larvae were fixed in formalin (4% formaldehyde in Phosphate buffer saline), and then dissected and gonads extracted. Subsequently, blocks of tissue were sectioned using a microtome (Microm Gmbh- HM 325, Thermo Scientific, Walldorf, Germany). The obtained cuts (2 to 3 μm thick) were stained with hematoxylin–eosin. Fenotipic gonadal sex was determined by observing the plates under the light microscope (40X). Histological diagnosis of gonadal sex was defined by the presence of ovarian-type structures (oogonia) or testis (espermatogonies).
Statistic analysis
A completely randomized design in a 4 x 2 extended factorial transformation to data BOX – COX was used to validate the ANOVA assumptions. The estimated optimal lambda was 1, obtained by the maximum-likelihood method. Mean differences were determined by the Tukey test (5% significance). Descriptive analysis (mean ± standard deviation) is presented for grouped data by compound, dose, and compound-dose interaction. Finally, the Spearman correlation coefficient was calculated for the morphometric data. Data were analyzed using the statistic Software SAS (Version 8.1, SAS Institute, Inc., Cary, NC, USA).
Results
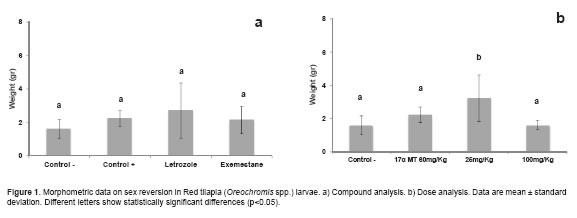
Morphometric data
Correlations between total length and weight (r = 0.97094; p<0.0001), total length and standard length (r = 0.96429; p<0.0001) and between standard length and weight (r = 0.95166; p<0.0001) show a high degree of relationship between the variables analyzed. Weight variation was not significant (p>0.05) between AIs and controls (Figure 1a). The 25 mg/kg doses were different (p<0.05) from 100 mg/Kg doses and controls (Figure 1b).
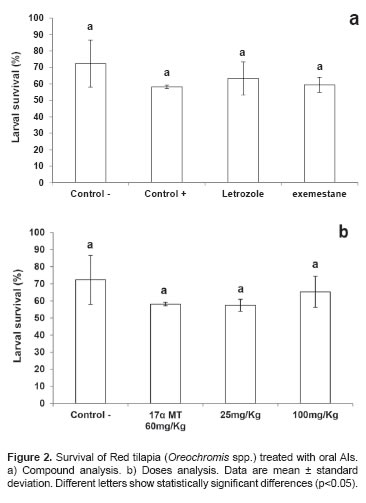
Larval survival (%)
Survival differences were not significant (p>0.05) between controls and other doses of aromatase inhibitor (Figure 2a, b). The highest survival rate was obtained in the negative control (72.3 ± 14.2%) while the lowest was recorded in the positive control (58.1 ± 1.04%).
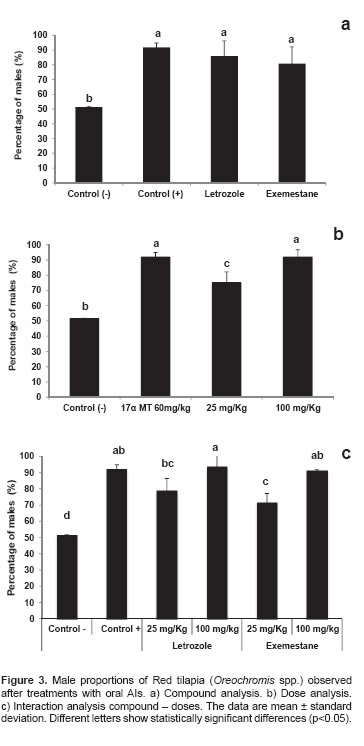
Percentage of males (%)
Positive control, LT, and EM had the highest male percentage, and were different (p<0.05) from the negative control (Figure 3a). Experimental doses differed (p<0.05) between 25 mg/Kg and 100 mg/Kg for percentage of males. The 25 mg/Kg dose also differed (p<0.05) from the experimental controls. The 100 mg/Kg dose did not differ from the positive control (17αMT 60 mg/Kg) (Figure 3b).
A compound x dose interaction was observed (p<0.05), with the negative control differing from all treatments and positive control. Interactions between dose and treatment evidenced that EM and LT at 100 mg/kg were different (p<0.05) from interactions between EM and LT at 25 mg/ Kg, which demonstrates an effect of dose on masculinization percentage (Figure 3c).
High masculinization values were recorded for the positive control (91.6 ± 3.09) and treatments 100 mg/ Kg, LT – 100 mg/ Kg (92.6 ± 5.81) and EM – 100 mg/Kg, which showed no significant differences. The negative control showed a 50:50 sex ratio, which approaches the theoretical data (Figure 3c).
Discussion
Aromatase inhibitors have been widely used to control sexual differentiation in fish. Most reports have primarily evaluated Fadrozole, a steroidal AI widely used in a wide variety of fish at a wide range of effective doses. In all-female (XX) Nile tilapia (Oreochromis niloticus) populations, Fadrozole (200 and 500 mg/Kg) offered for 30 days from day 7 post hatching (7 dph) resulted in 92.5 and 96 masculinization percentages, respectively (Kwon et al., 2000). Mixed populations of the same species treated with Fadrozole (75 and 100 mg/Kg for 30 days) resulted in 100% all-male populations (Afonso et al., 2001). Japanese flounder (Paralichthys olivaceus) treated with Fadrozole (100 mg/Kg during 70 days, starting on the day 30 dph) resulted in allmale populations (Kitano et al., 2000). Komatsu et al. (2006) administered Fadrozole (500 mg/Kg) to golden rabbitfish larvae (Siganus guttatus) for 30 and 90 days, obtaining 87 and 100% masculinization responses, respectively. Masculinization values were between 38 and 60% using lower doses (10 and 100 mg/Kg). Other researchers used 150 mg/Kg ATD (1,4,6- androstatriene-3–17-dione), which is another type I AI, for 30 days in Oreochromis niloticus, obtaining 75.3% males (Guiguen et al., 1999). Ruksana et al. (2010) treated Oreochromis niloticus with exemestane from day 9 to 35 (dph), achieving all-male populations with doses of 1000 and 2000 mg/Kg. They reported that those individuals who exhibited testicular differentiation showed no expression of P450scc, 3 β-HSD, and aromP450 enzymes at 35 dph, upon immunohistochemistry evaluation. At 120 dph, individuals showed immunopositive reaction to P450scc and 3 β-HSD, but there was no evidence of aromP450 expression. This same pattern of steroidogenic enzymes was observed in ovarian to testicular forwarding on tilapia larvae treated with 17α methyl testosterone (Bhandari et al., 2006). In our study, exemestane treatment (100 mg/Kg) resulted in 90.6 ± 1.21% masculinization. Even when values were not 100% males, we observed a direct relationship between doses and masculinization percentage. Our results differ from those by Ruksana et al. (2010) who obtained 8.3 and 83.3% males using 100 and 500 mg/ Kg, respectively. Although both species belong to the same Oreochromis gender, handling conditions, initiation of treatment, time and supply frequency, may represent substantial changes that can be reflected in the masculinization percentage. Although its effectiveness for sex control has been proven, species–specific standardizations according to the handling conditions of each farm must be performed to obtain conclusive results on the most appropriate doses. Unfortunately, fadrozole, the most studied AI, is not commercially available at the present time.
Reports on the use of non-steroidal (Type II) aromatase inhibitors are limited. Gao et al., (2010) used letrozole at increasing doses (50, 150, 250 and 500 mg/Kg) in bluegill sunfish (Lepomis macrochirus). During the critical 60 day period (from day 30 to 90 dph) for sexual differentiation and where this species has increased sensitivity to exogenous treatments, they obtained between 59 and 70% masculinization percentages. Male proportions increased as dose increased. Uchida et al. (2004) fed Letrozole to young zebrafish (Danio rerio) genetic females (XX) from 14 to 40 dph. They reported 62.5, 100, and 100% masculinization results at doses of 10, 100, 1000 mg/Kg, respectively. They suggested that letrozole or exposure to high temperature (35 to 37 °C) during the sexual differentiation period induces suppression of aromP450 gene and apoptosis of oocytes favoring spermatogonies differentiation.
Our results show a direct dose-effect of Letrozole on masculinization percentage when Exemestane was used. Other researchers using both AIs have observed the same effect. Masculinization percentages obtained in the present study using 25 and 100 mg/Kg letrozole were 78 and 92.6%, respectively.
In conclusion, our results demonstrate that the oral use of third generation AIs (Type I or Type II) is effective in increasing the male proportions in Red tilapia. Finding the minimum effective dose requires further research because the existing reports for this new generation of inhibitors is limited and inconclusive. It is necessary to conduct studies aimed to elucidate the molecular and cellular aspects of the sexual differentiation phase of individuals treated with AIs in greater detail.
¤ To cite this article: Betancur JJ, Quintero JC, Ostos H, Barreiro-Sanchez F, Olivera-Angel M. Effectiveness of the aromatase (P450 Arom) inhibitors Letrozole and Exemestane for masculinization of red tilapia (Oreochromis spp.). Rev Colomb Cienc Pecu 2014; 27:47-53.
Acknowledgements
The authors wish to thank CODECTY-Huila for it's funding support, and Compañía Agroindustrial y Comercial 3C for providing facilities and personnel required for this study.
References
Afonso L, Wassermann G, De Oliveira R. Sex reversal in Nile tilapia (Oreochromis niloticus) using a nonsteroidal aromatase inhibitor. J Exp Zool 2001; 290:177-181. [ Links ]
Bhandari R, Nakamura M, Kobayashi T, Nagahama Y. Suppression of steroidogenic enzyme expression during androgen-induced sex reversal in Nile tilapia (Oreochromis niloticus). Gen Comp endocrinol 2006; 145:20-24. [ Links ]
Fenske M, Segner H. Aromatase modulation alters gonadal differentation in developing zebrafish (Danio renio). Aquat Toxicol 2004; 67:105 -126. [ Links ]
Fujimura K, Okada N. Development of the embryo, larva and early juvenile of Nile tilapia Oreochromis niloticus (Pisces: Cichlidae). Developmental staging system. Develop Growth Differ 2007; 49:301-324. [ Links ]
Gao Z, Wang H, Wallat G, Yao H, Rapp D, O'Bryant P. Effects of a nonsteroidal aromatase inhibitor on gonadal differentiation of bluegill sunfish (Lepomis macrochirus). Aquac Res 2010; 41:1282-1289. [ Links ]
Gardner L, Anderwson T, Place A, Dixon B, Elizur A. Sex change strategy and the aromatase genes. J Biochem Mol Biol 2005; 94:395- 404. [ Links ]
González A. Actividad citocromo P450 aromatasa en la lubina (Dicentrarchus labrax L). [Tesis Doctoral]. Barcelona, España: Universitat de Barcelona; 2003. [ Links ]
Greytak S, Champlin D, Callard G. Isolation and charaterization of two cytocrhome P450 aromatase forms in Killifish (Fundulus hetroclitus): Differential expression in fish from polluted and unpolluted environments. Aquat Toxicol 2005; 71:371-389. [ Links ]
Guiguen Y, Baroiler J, Ricordel M, Iseki K, Mcmeel O, Martin S. Involvement of estrogens in the process of sex differentiation in two fish species: The Rainbow trout (Oncoyhynchus mykiss) and a tilapia (Oreochromis niloticus). Mol Reprod De 1999; 54:154-162. [ Links ]
Hecker M, Park W, Murphy M, Jones P, Solomon K, Van Der Kraak G. Effects of atrazine on CYP19 Gene Expression and aromatase activity in testes and on plasma sex steroid concentrations of male African clawed frogs (Xenopus laevis). Toxicol Sci 2005; 86:273-280. [ Links ]
Kitano T, Takamune K, Nagahama Y, Abe S. Aromatase inhibitor and 17Α-methyltestosterone cause sex-reversal from genetical females to phenotypic males and suppression of P450 aromatase gene expression in Japanese flounder (Paralichthys olivaceus). Mol Reprod De 2000; 56:1-5. [ Links ]
Komatsu T, Nakamura S, Nakamura M. Masculinization of female golden rabbitfish Siganus guttatus using an aromatase inhibitor treatment during sex differentiation. Comp Biochem Physiol Part C 2006; 143:402-409. [ Links ]
Kwon J, Haghpanah V, Kogson-Hurtado L, McAndrew B, Penman D. Masculinization of genetic female Nile tilapia (Oreochromis niloticus) by dietary administration of an aromatase inhibitor during sexual differentiation. J Exp Zool 2000; 287:46-53. [ Links ]
Matsuoka M, Van Nes S, Andersen Ø, Benfey T, Reith M. Realtime PCR analysis of ovary- and brain-type aromatase gene expression during Atlantic halibut (Hippoglossus hippoglossus) development. Comp Biochem Physiol Part B 2006; 144:128- 135. [ Links ]
Mokbel K. The evolving role of aromatase inhibitors in breast cáncer. Int J Clin Oncol 2002; 7:279-283. [ Links ]
Nakamura M, Bhandari R, Higa M. The role estrogens play in sex diferentiation and sex changes of fish. Fish Physiol Biochem 2003; 28:113-117. [ Links ]
Ruksana S, Pandit NP, Nakamura M. Efficacy of exemestane, a new generation of aromatase inhibitor, on sex differentiation in a gonochoristic fish. Comp Biochem Physiol C Toxicol Pharmacol 2010; 152:69-74. [ Links ]
SAS Institute Inc: SAS for Windows, Version 8. Cary, NC: SAS Institute Inc; 2000. [ Links ]
Sawyer S, Gerstner K, Callard G. Real-time PCR analysis of cytochrome P450 aromatase in Zebrafish: gene specific tissue distribution, sex differences, development programming, and estrogen regulation. Gen Comp endocrinol 2006; 147:108-117. [ Links ]
Tchoudakova A, Kishida M, Wood E, Callard G. Promoter Characteristics of two cyp19 genes differentially expressed in the brain and ovary of teleost fish. J Steroid Biochem 2001; 78:427-439. [ Links ]
Uchida D, Yamashita M, Kitano T, Iguchi T. An aromatase inhibitor or high water temperature induce oocyte apoptosis and depletion of P450 aromatase activity in the gonads of genetic female zebrafish during sex-reversal. Comp Biochem Physiol Part A 2004; 137:11-20. [ Links ]