Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Revista Colombiana de Ciencias Pecuarias
versão impressa ISSN 0120-0690
Rev Colom Cienc Pecua vol.27 no.4 Medellín out./dez. 2014
ARTICLE
Effect of microencapsulated blends of organic acids and essential oils supplementation on growth performance and nutrient digestibility in finishing pigs¤
Efecto de mezclas microencapsuladas de ácidos orgánicos y suplementos de aceites esenciales sobre el crecimiento y digestibilidad de nutrientes de cerdos en ceba
Efeito de misturas de ácidos orgânicos microencapsulados e suplementação de óleos essenciais sobre o crescimento e a digestibilidade dos nutrientes em suínos em terminação
Jin H Cho1, Animal Scientist, PhD; Min H Song2, Animal Scientist, PhD; In Ho Kim3*, Animal Scientist, PhD.
1 Department of Animal Science, Chungbuk National University, #52 Naesudong–ro, Heungdeok–gu, Cheongju, Chungbuk, 361–763, South Korea.
2 Department of Animal Science and Biotechnology, Chungnam National University, Daejeon 305–764, South Korea.
3 Department of Animal Resource & Science, Dankook University, #29 Anseodong, Cheonan, Choongnam, 330–714, South Korea.
* Corresponding author: In Ho Kim. Dankook University, Department of Animal Resource & Science, Cheonan, Choongnam, South Korea, 330–714. Email: inhokim@dankook.ac.kr
Received: October 21, 2013 Accepted: May 2, 2014
Summary
Background: positive effects of organic acids and essential oils (MOE) on livestock are well documented. Microencapsulation allows the slow release of core materials in a specific moment or environment. Objective: to evaluate the effect of supplementing finishing pigs with microencapsulated blends of organic acids and essential oils (MOE) on growth performance, nutrient digestibility, fecal noxious gas emissions, and meat quality. Methods: 75 crossbred pigs [(Yorkshire × Landrace) × Duroc, 56.15 ± 3.77 kg] were used in this 10–week trial. Pigs were randomly distributed into 1 of 3 dietary treatments on the basis of body weight (BW) and gender. Each treatment had 5 replicate pens with 5 pigs (2 gilts, 3 barrows) per pen. Treatments were as follows: CON (a basal diet); MOE1 (CON + 0.025% MOE); MOE2 (CON + 0.050% MOE). Results: pigs fed the MOE2 diet had higher final BW at 5th and 10th week than those fed the CON diet (p<0.05). During weeks 0 to 5, MOE1 and MOE2 groups had greater average daily gain (ADG) than the CON group (p<0.05). Overall, ADG in MOE2 was greater than that in CON treatment (p<0.05). MOE2 group had higher dry matter (DM) and energy digestibility than the CON group (p<0.05). Conclusion: the present results indicate that dietary supplementation with 0.05% MOE improves growth performance and nutrient digestibility in finishing pigs.
Keywords: average daily gain, dry matter digestibility, meat quality, micro–encapsulation.
Resumen
Antecedentes: los efectos positivos de los ácidos orgánicos y aceites esenciales (MOE) en el ganado han sido documentados. La microencapsulación permite la liberación lenta del material de núcleo en un período o medio ambiente particulares. Objetivo: evaluar el efecto de suplementar cerdos de engorde con mezclas microencapsuladas de ácidos orgánicos y aceites esenciales (MOE) sobre el rendimiento productivo, la digestibilidad de nutrientes, las emisiones de gases fecales, y la calidad de la carne. Métodos: 75 cerdos cruzados [(Yorkshire × Landrace) x Duroc), 56,15 ± 3,77 kg] se utilizaron en las 10 semanas que duró el ensayo. Los cerdos se distribuyeron aleatoriamente en 1 de 3 tratamientos dietarios, de acuerdo con su peso corporal (BW) y género. Cada tratamiento tuvo 5 réplicas (corrales) con 5 cerdos por corral (2 hembras y 3 machos castrados). Los tratamientos fueron: CON (dieta basal); MOE1 (CON + 0,025% MOE); MOE2 (CON + 0,050% MOE). Resultados: los cerdos alimentados con la dieta MOE2 tuvieron mayor BW final en las semanas 5ª y 10ª que los alimentados con la dieta CON (p<0,05). Durante la semana 0 a la 5 los grupos MOE1 y MOE2 tuvieron mayor ganancia media diaria (ADG) que el grupo CON (p<0,05). En general, la ADG de MOE2 fue mayor que en el tratamiento CON (p<0,05). El grupo MOE2 tuvo mayor digestibilidad de la materia seca (DM) y de la energía que el grupo CON (p<0,05). Conclusión: los resultados indican que la suplementación de la dieta con 0,05% MOE mejora el crecimiento y la digestibilidad de nutrientes en cerdos de ceba.
Palabras clave: calidad de la carne, digestibilidad de la materia seca, ganancia media diaria, microencapsulación
Resumo
Antecedentes: os efeitos positivos de ácidos orgânicos e óleos essenciais (MOE) para o gado estão bem documentados na literatura. A microencapsulação permite a liberação lenta de materiais centrais num momento ou ambiente específico. Objetivos: avaliar os efeitos de suplementação de suínos em terminação com misturas microencapsuladas de ácidos orgânicos e óleos essenciais (MOE) em pontos como desempenho produtivo, digestibilidade dos nutrientes, emissões de gases nocivos fecais e qualidade da carne. Metodologia: 75 suínos mestiços [(Yorkshire × Landrace) x Duroc), 56,15 ± 3,77 kg] foram utilizados neste teste de 10 semanas. Os animais foram selecionados aleatoriamente em 1 de 3 currais de tratamento dietético, com base no peso corporal (BW) e sexo. Cada curral teve 5 réplicas e cada curral, 5 suínos (2 fêmeas, 3 machos castrados). Os tratamentos foram os seguintes: CON (dieta basal); MOE1 (CON + 0,025% MOE); MOE2 (CON + 0,050% MOE). Resultados: os animais que receberam a dieta MOE2 apresentaram significativamente maior PV final em 5 e 10 semanas do que aqueles alimentados com a dieta CON (p<0,05). Durante 0–5 semanas, os grupos MOE1 e MOE2 tiveram maior GMD que o grupo CON (p<0,05). De um modo geral, ADG de MOE2 foi maior do que no grupo CON (p<0,05). O grupo MOE2 teve a matéria seca (DM) e a digestibilidade de energia maiores do que o grupo CON (p<0,05). Conclusão: os resultados indicam que a suplementação dietética com 0.05% de MOE melhora o desempenho do crescimento e digestibilidade de nutrientes em suínos em terminação.
Palavras–chave: Posicionamento de marca. Marcas disfarçadas. Marcas verdadeiras. Experiência exploratória. Efeitos no consumidor.
Introduction
The first ban on farm use of antibiotic growth promoters (APGs) was enacted in 1986 in Sweden due to concerns that antibiotics in livestock feed could increase antibiotic–resistant pathogens and antibiotic residue problems in animal products (Kelley et al., 1998), which may pose a potential health hazard to humans (Dipeolu et al., 2005).
It is well accepted that organic acids (OA) can reduce the gastrointestinal tract pH and change the balance of microorganisms, thus offering an interesting alternative to growth–promoting antibiotics used in pigs (Canibe et al., 2005). Feeding OA to farm animals has been reported to improve growth performance (Partanen and Mroz, 1999) and nutrient digestibility (Wang et al., 2009). Similarly to OA, pure botanicals –also referred to as volatile or ethereal oils from plant materials– can also affect animal growth performance (Simonson, 2004) and nutrient digestibility (Windisch et al., 2008) by enhancing digestive enzyme activity and nutrient absorption (Burt, 2004).
Additionally, microencapsulation is a technology recently used to deliver substances into specific sites of the gastrointestinal tract. It allows for the slow release and rumen by–pass of nutrients throughout the gastrointestinal tract (Piva et al, 1997). Piva et al. (2007) reported that microencapsulation can be used for delaying drug absorption and protecting amino acids and proteins from rumen degradation when using corrosive products. Grilli et al. (2010) also reported that 3,000 mg/kg of a microencapsulated blend (organic acids and natural–identical flavors) improved growth performance of weanling pigs. Goksoy et al. (2010) found that dietary supplementation of OA or origanum onites in broiler diets reduced cooking loss, drip loss, and increased pH (24 h), suggesting that supplementation of OA or essential oils could improve meat quality. Another study reported that fecal gas emissions were reduced by including essential oils in weanling pig diets (Cho et al., 2006).
Based on the cited reports, supplementation of microencapsulated blends of organic acids and essential oils (MOE) could improve growth performance, nutrient digestibility and meat quality in pigs, while decreasing noxious gas emission. Therefore, this study was conducted to evaluate the effect of supplementing microencapsulated blends of OA (25% and 16.7% of citric and sorbic acids, respectively) and essential oils (1.7% thymol and 1.0% vanillin) on growth performance, nutrient digestibility, fecal noxious gas emissions, and meat quality in finishing pigs.
Materials and methodst
The experimental protocol used in this study was approved by the Animal Care and Use Committee of Dankook University (ACUCDU 1302406).Animals and diet
A total of 75 crossbred pigs [(Yorkshire × Landrace) × Duroc] with an initial body weight (BW) of 56.15 ± 3.77 kg were randomly assigned to 3 dietary treatments based on gender and BW. Each pen housed 5 pigs (2 gilts and 3 barrows), and 5 pens/treatment were used. All pigs were housed in a temperature and humidity controlled room. The experiment lasted 10 weeks. Each pen was equipped with a one–sided, stainless–steel self–feeder and a nipple drinker that allowed pigs ad libitum access to feed and water. Individual BW and feed intake were recorded at weeks 5 and 10 of the experiment to determine average daily gain (ADG), average daily feed intake (ADFI) and gain/feed (G/F) ratio. Microencapsulated blends of organic acids and essential oils (MOE) from VetAgroSpA (Aviplus–S®, 42100 Reggio Emilia, Italy) contained citric acid (25%), sorbic acid (16.7%), thymol (1.7%), and vanillin (1.0%).
Treatments
Treatments were as follows: basal diet (CON); CON + 0.025% MOE (MOE1); and CON + 0.050% MOE (MOE2). All nutrients were formulated to meet or exceed NRC recommendations (1998) for finishing pigs (Table 1).
Apparent total tract digestibility (ATTD) of dry matter (DM), gross energy (GE) and nitrogen (N) was determined by adding 0.2% chromic oxide (Cr2O3, Duksan Pure Chemical Co., Ansan City, Korea) to the diets as an inert indicator. Pigs were fed diets mixed with chromic oxide from d 63 to 69. Fresh fecal grab samples (10 pigs per treatment) randomly collected from 2 pigs per pen on d 70 were mixed and pooled, and a representative sample was stored in a freezer at –20 °C for chemical analysis.
Before the analysis, fecal samples were thawed and dried at 60 °C for 72 h, after which they were finely ground to a size that could pass through a 1 mm screen. Feed and fecal samples were analyzed for DM, N and GE following procedures outlined by the AOAC (2000). Chromium was analyzed via UV absorption spectrophotometry (Shimadzu UV–1201, Shimadzu, Kyoto, Japan) following the method described by Williams et al. (1962). GE in feed and feces was determined using a calorimeter (Mode 1241, Parr Instrument Co., Moline, IL, USA).
Fecal NH3, H2S and total mercaptan emissions were measured in week 10. A total of 300 g excreta were collected in a plastic box (polyvinyl, W25 × L35 cm) from each pen. Gas was determined after 1, 3, 5 and 7 d fermentation using Gastec gas sampling pumps (Gastec, GV–100S, Japan) during 1 min.
At the end of the experiment, all of the pigs were slaughtered at a local commercial slaughterhouse. After chilling at 2 °C for at least 24 h, one 2.54 cm thick longissimus muscle (LM) sample was removed at the level of the 10th rib and allowed to bloom for 30 min. The subjective color, marbling and firmness scores of the LM cut surface were then evaluated following procedures established by the NPPC (1991). Color, marbling and firmness were scored by a sensory panel using a 5–point scale (1 = pale, devoid of marbling, very soft; 5 = dark, moderately abundant marbling or greater, very firm). The sensory panel was comprised of 11 panelists, all of whom were trained to evaluate the sensory attributes of color, marbling and firmness (NPPC, 1991). Training involved presenting the panelists with samples of known color, marbling and firmness. Water–holding capacity (WHC) was estimated by determining expressible juice using a modification of the filter paper press method described by Wierbicki and Deatherage (1958), as follows: a meat sample (0.2 g) was placed on a filter paper (11 cm diameter) between plexiglass plates and pressed at 3,000 psi for 2 min. The outline area of the expressible juice and the meat film were traced. Both areas were determined using a compensating polar planimeter. Expressible juice was calculated as follows:
Sample color was determined using a Chromameter (Model CR-410, Minolta Co., Japan) and are reported in CIE system values: lightness (L*), redness (a*), and yellowness (b*). Color was measured in duplicate with one reading of the anterior side and one of the posterior side from each sample. All color readings were taken on the skin side surface in an area free of obvious color defects (over scalded, bruises, and blood accumulation).
Meat pH was measured at 20 min postmortem using a pH meter with a pH electrode (NWK biner pH, K-21, Landsberg, Germany) inserted for 10 s approximately 2.5 cm below the surface of the anterior portion of the sample. The electrode was calibrated at 20 °C in buffers with pH 4.00 and 7.00.
For the drip loss test, 4.5 g meat samples (1.5 cm diameter core; 4 cm length) were taken from the loin, placed perpendicular to the length of the muscle and suspended into a plastic bag for 7 days. The sample weight was measured at 1, 3, 5, and 7 days.
To determine cooking loss, two 25 mm slices of LM samples were weighed and placed into individual polyethylene bags. The samples were then cooked for 60 min in a water bath at 70 °C. After cooking, the fluid was poured from the bags and the samples were refrigerated (0~1 °C) overnight. The samples were patted dry with paper towels the following morning and then reweighed to determine cooking loss, which was expressed as a percentage of the uncooked sample weight.
Statistical analysis
Data were analyzed using a randomized complete block design following the GLM procedure of SAS (1996, SAS Institute Inc., Cary, North Carolina, USA), with each pen being used as the experimental unit. Polynomial contrasts (linear and quadratic) were used to test the effects of MOE supplementation. Data variability was expressed as SEM and the level of significance was set at 0.05.
Results
The BW increased linearly (p<0.05) 1.22% from CON to MOE1 and 1.59% from CON to MOE2 at the end of the week 5, and it also increased (p<0.05) 1.99% from CON to MOE1 and 2.81% from CON to MOE2 at the end of week 10 (Table 2). During weeks 0 to 5, ADG was linearly (p<0.05) increased 4.27% from CON to MOE1 and 6.19% from CON to MOE2. Overall, ADG was also linearly (p<0.05) increased 4.28% from CON to MOE1 and 6.36% from CON to MOE2. No significant differences (p>0.05) were observed for G/F among treatments.
The digestibility of DM was linearly increased (p<0.05) 3.90% from CON to MOE1 and 8.19% from CON to MOE2. Energy digestibility was also linearly increased (p<0.05) 5.79% from CON to MOE1 and 11.39% from CON to MOE2 (Table 3). There was no effect (p>0.05) on N digestibility by the inclusion of MOE.
Supplementation with MOE had no effect (p>0.05) on fecal NH3, H2S, or total mercaptan emissions (Table 4).
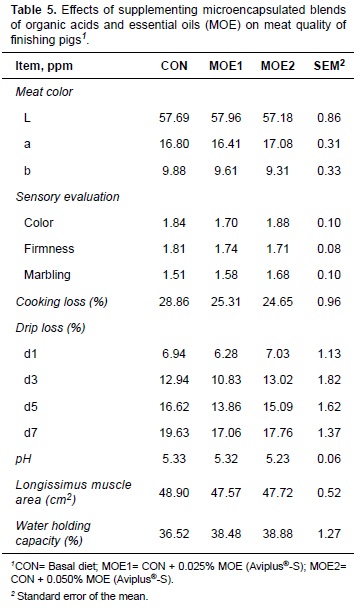
Dietary supplementation with MOE had no effect (p>0.05) on meat color, sensory evaluation, cooking loss, drip loss, pH, longissimus muscle area, or WHC (Table 5).
Discussion
In the present study, the addition of microencapsulated feed additives containing OA and essential oils (EO) improved growth performance and nutrient digestibility. The ability of these compounds to kill bacteria strongly depends on their chemical structure (Si et al., 2006). Thymol, a phenolic molecule from thyme, has high in vitro activity against S. typhimurium (Si et al., 2006), presumably by damaging the cytoplasmatic membrane integrity of the pathogen. Previous work has demonstrated that thymol can inhibit the production of odor compounds and also eliminate coliforms and generic E. coli populations (Varel and Miller, 2004). However, the results of in vivo experiments are contradictory, using various extracts from thyme and lower doses than that used in vitro. This may be the reason for the improved growth performance and nutrient digestibility resulting from MOE inclusion in this study.
Flavors, as well as OA, have recently gained attention in animal nutrition because of their natural antimicrobial properties and growth promoting effects. The factor limiting the use and efficacy of these compounds is the need to reach the intestine to exert their antibacterial activity, without being absorbed too rapidly upon leaving the stomach. Tsiloyiannis et al. (2001) demonstrated that dietary supplementation with OA reduces the incidence and severity of diarrhea post–weaning. No significant differences were seen in behavior or skin lesions between pH groups, indicating that overall welfare (behavior and aggression) was not influenced by the doses of OA used. The impact of OA on coliform diversity was, however, not investigated. It should be noted that a possible disadvantage of strong acidification might be a significant reduction in water intake, which could compromise animal welfare. An improvement of nutrient digestibility was shown in our study. Possible mechanisms for these effects include a reduction in the stomach pH and a direct effect on the microbiota of the gastro–intestinal tract (GIT). A reduction of the stomach pH increases pepsin activity and reduces the passage rate through this organ, which may lead to an increase in protein digestibility (Busser et al., 2011). Dietary supplementation of OA, especially blends of formic acid and essential oils, improved nutrient digestibility. Inclusion of formic acid and essential oils improved digestibility of crude fiber, total carbohydrates and NSP (Gerritsen et al., 2010). Positive effects of OA on nutrient digestibility have been reported in other studies (Biggs and Parsons, 2008; Ghazalah et al., 2011). Tung and Pettigrew (2006) also found increased dry matter and protein digestibility. Nevertheless, N digestibility was not affected by MOE in the present study.
Dietary supplementation of thymol increased the number of parietal cells for 100 μm depth of oxyntic gland (Trevisi et al., 2007). Thymol supplementation reduced feed intake rather than performance. Some gut barrier factors were also positively influenced. Feeding a microencapsulated blend resulted in changes in gut luminal metabolites, such as volatile fatty acids (VFA) and polyamines (Grilli et al., 2010). This indicates that microencapsulation would increase the effects of OA and essential oils. VFA from bacterial fermentation are the major anions in colonic contents and are rapidly absorbed by the colonic epithelial cells of pigs, being metabolized to supply energy (Bergman, 1990), which could explain the increased growth and nutrient digestibility observed in the present study. Previous studies have also shown that specific encapsulated blends can be slowly released along the intestine and interact with the resident microflora by reducing bacterial pressure in the upper intestine, while enhancing microbial metabolism in the lower part (Piva et al., 2007). The improvement of the intestinal environment favors nutrient digestibility.
Busser et al. (2011) conducted a study to investigate the effects of water pH on performance and health of weaned pigs. They evaluated whether very acidic water (pH 4) was advantageous compared to higher pH levels (pH 5 or 6) and neutral water. In this study, MOE supplementation also decreased GIT pH to a certain extent and improved the health status of finishing pigs. Short chain fatty acids (e.g., acetic, propionic, and n–butyric acid) have been shown to stimulate epithelial cell proliferation (Gálfi and Bokori, 1990; Lupton and Kurtz, 1993; Sakata et al., 1995), resulting in increased villous height and absorptive capacity. Furthermore, the use of OA may reduce the coliform load along the gastrointestinal tract (Scipioni et al., 1978; Thomlinson and Lawrence, 1981; Bolduan et al., 1988; Mathew et al., 1991; Tsiloyiannis et al., 2001).
The previous study also showed that organic acid supplementation did not affect carcass quality of entire male pigs, except for an increased dressing percentage in animals fed benzoic acid. This increase could be explained by the antimicrobial effect and subsequent gut–wall thinning effect and/or lower GIT weight caused by antimicrobial growth promoters, as reported by Visek (1978). However, OA did not affect meat quality in our study, which could be explained by the previous study results.
In conclusion, a microencapsulated feed additive containing organic acids and EO added at 0.05% to a finisher pigs diet could increase growth performance and digestibility of dry matter and energy.
Acknowledgements
This work was supported by the Cooperative Research Program for Agriculture Science & Technology Development (Project No. 00849401) Rural Development Administration, Republic of Korea.
Conflicts of interes
The authors declare they have no conflicts of interest with regard to the work presented in this report.
Notes
¤ To cite this article: Cho JH, Song MH, Kim IH. Effect of microencapsulated blends of organic acids and essential oils supplementation on growth performance and nutrient digestibility in finishing pigs. Rev Colomb Cienc Pecu 2014; 27:264–272.
References
AOAC Association of Official Analytical Chemists. Official Methods of Analysis. 17th ed. Gaithersburg, MD; 2000. [ Links ]
Bergman EN. Energy contribution of volatile fatty acids from the gastrointestinal tract in various species. Physiol Rev 1990; 70:567–590. [ Links ]
Biggs P, Parsons CM. The effects of several organic acids on growth performance, nutrient digestibilities, and cecal microbial populations in young chicks. Poult Sci 2008; 87:2581–2589. [ Links ]
Bolduan G, Jung H, Schneider R, Block J, Klenke B. Influence of fumaric acid and propanediol–formate on piglets. J Anim Physiol Anim Nutr 1998; 59:143–149. [ Links ]
Burt S. Essential oils: Their antibacterial properties and potential applications in food–A review. Int J Food Microbiol 2004; 94:223-253. [ Links ]
Busser EVD, Dewulf J, Zutter LD, Haesebrouck F, Callens J, Meyns T, Maes W, Maes D. Effect of administration of organic acids in drinking water on fecal shedding of E.coli, performance parameters and health in nursery pigs. Vet J 2011; 188:184–188. [ Links ]
Canibe N, Højberg O, Højsgaard S, Jensen BB. Feed physical form and formic acid addition to the feed affect the gastrointestinal ecology and growth performance of growing pigs. J Anim Sci 2005; 83:1287–1302. [ Links ]
Cho JH, Chen YJ, Min BJ, Kim HJ, Kwon OS, Shon KS, Kim IH, Kim SJ, Asamer A. Effects of Essential oils supplementation on growth performance, IgG concentration and fecal noxious gas concentration of weaned pigs. Asian–Aust J Anim Sci 2006; 19:80–85. [ Links ]
Cogliani C, Goossens H, Greko C. Restricting antimicrobial use in food animals: lessons from Europe. Banning nonessential antibiotic uses in food animals is intended to reduce pools of resistance genes. Microbe 2011; 6:274–279. [ Links ]
Dibner JJ, Buttin P. Use of organic acids as a model to study the impact of gut microflora on nutrition and metabolism. J Appl Poultry Res 2002; 11:453–463. [ Links ]
Dipeolu MA, Eruvbetine D, Oguntona EB, Bankole OO,Sowunmi KS. Comparison of effects of antibiotics and enzyme inclusion in diets of laying birds. Archivos De Zootecnia 2005; 54:311. [ Links ]
Environmental Protection Agency. Thymol and Eucalyptus Oil; Exemptions from the Requirement of a Tolerance. Fed Regist 2003; 68:33876–33882. [ Links ]
Gálfi P, Bokori J. Feeding trial in pigs with a diet containing sodium n–butyrate. Acta Vet Hung 1990; 38:3–17. [ Links ]
Gerritsen R, van Dijk AJ, Rethy K, Bikker P. The effect of blends of organic acids on apparent faecal digestibility in piglets. Livest Sci 2010; 134:246–248. [ Links ]
Ghazalah AA, Atta AM, Elkloub K, Moustafa MEL, Shata RFH. Effect of dietary supplementation of organic acids on performance, nutrients digestibility and health of broiler chicks. Int J Poultry Sci 2011; 10:176–184. [ Links ]
Goksoy EO, Aksit M, Kirkan S. The effects of organic acid and origanum onites supplementations on some physical and microbial characteristics of broiler meat obtained from broilers kept under seasonal heat stress. KafkasUniv Vet FakDerg 2010; 16 Suppl–A:S41–S46. [ Links ]
Grilli E, Messina MR, Tedeschi M, Piva A. Feeding a microencapsulated blend of organic acids and nature identical compounds to weaning pigs improved growth performance and intestinal metabolism. Livest Sci 2010; 133:173–175. [ Links ]
Hoffman–Pennesi D, Wu C. The effect of thymol and thyme oil feed supplementation on growth performance, serum antioxidant levels, and cecal Salmonella population in broilers. J Appl Poult Res 2010; 19:432–443. [ Links ]
Kelley TR, Pancorbo OC, Merka WC, Barnhart HM. Antibiotic resistance of litter isolates. Poult Sci 1998; 77:243–247. [ Links ]
Lupton JR, Kurtz PP. Relationship of colonic luminal short–chain fatty acids and pH to in vivo cell proliferation in rats. J Nutr 1993; 123:1522–1530. [ Links ]
Mathew AG, Sutton AL, Scheidt AB, Forsyth DM, Patterson JA, Kelly DT. Effects of a propionic acid containing feed additive on performance and intestinal microbial fermentation of the weanling pig. In: 5th International Symposium on Digestive Physiology in Pigs; Wageningen, Pudoc. EAAP Publication No. 54, The Netherlands, 1991; 464–469 p. [ Links ]
NPPC, National Pork Producers Council. Procedures to Evaluate Market Hogs. 3rd ed. Washington, D.C. 1991. [ Links ]
NRC, National Reaserch Council. Nutrient requerements of swine. 9th rev. ed. Washington, DC: National Academy Press; 1998. [ Links ]
Øverland M, Kjos NP, Borg M, Skjerve E, Sørum H. Organic acids in diets for entire male pigs: Effect on skatole level, microbiota in digesta, and growth performance. Livest Sci 2008; 115:169–178. [ Links ]
Partanen KH, Mroz Z. Organic acids for performance enhancement in pig diets. Nutr Res Rev 1999; 12:117–145. [ Links ]
Piva A, Anfossi P, Meola E, Pietri A, Panciroli A, Bertuzzi T, Formigoni A. Effect of microencapsulation on absorption processes in swine. Livestock Production Science 1997; 51:53–61. [ Links ]
Piva A, Pizzamiglio V, Morlacchini M, Tedeschi M, Piva G. Lipid microencapsulation allows slow release of organic acids and natural identical flavors along the swine intestine. J Anim Sci 2007; 85:486–493. [ Links ]
Sakata T, Adachi M, Hashida M, Sato N, Kojima T. Effect of n–butyric acid on epithelial cell proliferation of pig colonic mucosa in shortterm culture. Dtsch Tierarztl Wochenschr 1995; 102:163–164. [ Links ]
SAS, Statistical Analysis Systems. Language guide for Personal Computers. Institute Inc. Release 6.03 ed. Cary, NC: SAS institute Inc. 1996 [ Links ]
Scipioni R, Zaghini G, Biavati B. The use of acidified diets for early weaning of piglets. Zootec Nutr Anim 1978; 4:201–218. [ Links ]
Si W, Gong J, Chanas C, Cui S, Yu H, Caballero C, Friendship RM. In vitro assessment of antimicrobial activity of carvacrol, thymol and cinnamaldehyde towards Salmonella serotype Typhimurium DT104: effects of pig diets and emulsification in hydrocolloids. J Appl Microbiol 2006; 101:1282–91. [ Links ]
Simonson RR. Antimicrobial properties of herbs and spices and their potential use in diets for pigs. Newport Laboratories, Inc. submitted to CRIS 2004. [ Links ]
Thomlinson JR, Lawrence TLJ. Dietary manipulation of gastric pH in the prophylaxis of enteric disease in weaned pigs: some field observations. Vet Rec 1981; 109:120–122. [ Links ]
Trevisi P, Merialdi G, Mazzoni M, Casini L, Tittarelli C,De Filippi S, Minieri L, Lalatta–Costerbosa G, Bosi P. Effect of dietary addition of thymol on growth, salivary and gastric function, immune response, and excretion of Salmonella enteric serovar Typhimurium, in weaning pigs challenged with this microbe strain. Ital J Anim Sci 2007; 6:374–376. [ Links ]
Tsiloyiannis VK, Kyriakis SC, Vlemmas J, Sarris K. The effect of organic acids on the control of porcine post–weaning diarrhoea. Res Vet Sci 2001; 3:287–293. [ Links ]
Tung CM, Pettigrew JE. Critical Review of Acidifiers. Report NPB 05–169, Department of Animal Sciences, University of Illinois 2006. [ Links ]
Varel VH, Miller DN. Eugenol stimulates lactate accumulation yet inhibits volatile fatty acid production and eliminates coliform bacteria in cattle and swine waste. J Appl Microbiol 2004; 97:1001–1005. [ Links ]
Visek WJ. The mode of growth promotion by antibiotics. J Anim Sci 1978; 46:1447–1469. [ Links ]
Wang JP, Yoo JS, Lee JH, Jang HD, Kim HJ, Shin SO, Seong SI, Kim IH. Effect of phenyllactic acid on growth performance, nutrient digestibility, microbial shedding, and blood profile in pigs. J Anim Sci 2009; 87:3235–3243. [ Links ]
Wierbicki E, Deatherage FE. Water Content of Meats, Determination of Water–Holding Capacity of Fresh Meats. J Agric Food Chem 1958; 6:387–392. [ Links ]
Williams CH, DavidDJ, Iismaa O. The determination of chromic oxide in faeces samples by atomic absorption spectrophotometry. J Agric Sci 1962; 59:381–385. [ Links ]
Windisch W, Schedle K, Plitzner C, Kroismayr A. Use of phytogenic products as feed additives for swine and poultry. J Anim Sci 2008; 86:140–148. [ Links ]