Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Colombiana de Ciencias Pecuarias
Print version ISSN 0120-0690
Rev Colom Cienc Pecua vol.28 no.1 Medellín Jan./Mar. 2015
ARTICLE
Effect of fixed feeding time on growth, body composition, and hepatic histology of hybrid catfish (Pseudoplatystoma reticulatum x Leiarius marmoratus) fed with carbohydrates and lipids ratios¤
Efecto de la alimentación a tiempo fijo sobre el crecimiento, composición corporal e histología hepática del bagre híbrido (Pseudoplatystoma reticulatum x Leiarius marmoratus) alimentado con proporciones de carbohidratos y lípidos
Efeito do tempo de alimentação fixa no crescimento, composição corporal e histologia hepática do bagre híbrido (Pseudoplatystoma reticulatum x Leiarius marmoratus) alimentados com relações de carboidrato e lipídios
Martha J Prieto-Guevara1, MSc; Rodrigo F Silva2*, PhD; Leandro S Costa3, MSc; Raquel T Pereira3, MSc; Priscila V Rosa3, PhD.
1Departamento de Ciencias Acuícolas, Universidad de Córdoba, 230002 Montería, Colombia.
2Centro de Ciências Agrárias, Ambientais e Ciências Biológicas da Faculdade de Engenharia de Pesca da Universidade Federal do Recôncavo da Bahia, Cruz das Almas 44380-000, Brasil.
3Departamento de Zootecnia da Universidade Federal de Lavras, Lavras 37200-000, Brasil.
*Corresponding author: Rodrigo Fortes da Silva, Center of Agricultural Science, Environmental and Biological, Faculty of Fishing Engineering, University of Bahia Recôncavo, 44380-000, Cruz das Almas, Brazil, Tel.: +55 75 3621-1558, Fax: +55 75 3621-6389, Email: fortes@ufrb.edu.br
Received: October 12, 2013; accepted: May 23, 2014
Summary
Background: feed and light are the most important factors affecting the biological rhythms of fish. This work studies fish adaptation to those factors. Objetive: to determine the influence of feeding time and dietary starch and lipid levels on growth, body composition, and liver histology of hybridized Brazilian catfish. Methods: two isoenergetic diets were formulated to contain two levels of crude starch (CHO, %) and lipids (L, %): 5/11 CHO/L and 25/2.2 CHO/L. Sixty animals (260 ± 10 g) were randomly distributed into twelve tanks (100 L). Using self-feeders, two fish groups were fed a diet containing either 5% or 25% starch during the light period (ML), while other two groups were fed the same diets during the dark period (MD). The following parameters were measured: final weight, weight gain, specific growth rate, food intake, hepatosomatic index, and viscerasomatic index. The experiment was carried out in triplicate for 60 days. Results: growth parameters such as specific growth rate (SGR), final weight, and weight gain showed statistical differences between groups, with the best results for the group fed the 25% starch diet during ML. Significant differences between groups on body lipid content, energy, and dry weight were also recorded for those feed 25% starch in the MD. A significant effect was also observed on liver lipid and glycogen content, with values generally higher for ML with 5% starch. Fish fed 25% starch showed significantly lowest lipid and glycogen content during ML. Surprisingly, the opposite occurred regarding liver composition for fish fed in MD. Conclusions: we suggest diurnal feeding should be practiced for optimal performance of juvenile fish, however dark or light phases could be used, taking into consideration its relationship with carbohydrate levels.
Key words: automatic feeder, feeding schedule, food utilization, starch.
Resumen
Antecedentes: la alimentación y la luz son los factores más importantes que afectan los ritmos biológicos en peces. En este artículo se presenta un poco de conocimiento acerca de la plasticidad de los peces para la utilización del alimento. Objetivo: este estudio se realizó para determinar la influencia del horario de alimentación y los niveles de almidón y lípidos de la dieta en el crecimiento, la composición corporal y la histología hepática en el bagre híbrido. Métodos: fueron formuladas dos dietas isoenergéticas para contener dos niveles de almidón (CHO, %) y lípidos (L, %): 5/11 CHO/L y 25/2.2 CHO/L. Sesenta animales (260 ± 10 g) fueron distribuidos aleatoriamente en 12 tanques (100 L). Un grupo de peces fue alimentado con 5% de almidón en el periodo diurno (ML), y otro grupo fue alimentado en el periodo nocturno (MD). Se realizaron los mismos procedimientos para el grupo alimentado con 25% de almidón. Los siguientes parámetros fueron medidos: peso final, ganancia de peso, tasa de crecimiento especifico, ingestión alimentaria, índice hepatosomático y índice vicerosomático. El experimento se realizó por triplicado durante 60 días. Resultados: los parámetros de crecimiento como la tasa de crecimiento específico (SGR), peso final y ganancia de peso mostraron diferencias estadísticas entre los grupos, con mejores resultados en los grupos alimentados con 25% de almidón en ML. Se observaron diferencias significativas entre los grupos para el contenido de lípidos en el cuerpo, energía y materia seca, para los animales alimentados con 25% de almidón en MD. Hubo un efecto significativo sobre los lípidos hepático y glucógeno, con los valores generalmente más altos en ML para los peces alimentados con 5% de almidón. Sin embargo, los peces alimentados con 25% de almidón presentaron significativamente menor contenido de lípidos y glucógeno en la condición de ML. Sin embargo, ocurrió lo contrario con los peces alimentados en MD, para la composición del hígado. Conclusiones: la alimentación diurna se sugirió para un mejor rendimiento de los juveniles, sin embargo, la fase nocturna o de luz se podrían utilizar, teniendo en cuenta su relación con los niveles de carbohidratos.
Palabras clave: alimentador automático, almidón, horario de alimentación, utilización del alimento.
Resumo
Antecedentes: alimentos e luz são os fatores mais importantes que arrastam os ritmos biológicos em peixes. Neste trabalho, trazemos um pouco do conhecimento sobre a plasticidade dos peixes para a utilização dos alimentos. Objetivo: este estudo foi realizado para determinar a influencia do horário de alimentação e níveis de amido e lipídio dietético no crescimento, composição corporal e histologia hepática em um bagre hibrido. Métodos: duas dietas isoenergéticas foram formuladas para conter dois níveis de amido (CHO, %) e lipídeo (L, %): 5/11 CHO/L e 25/2.2 CHO/L. Sessenta animais (260 ± 10 g) foram distribuídos aleatoriamente em 12 tanques (100 L). Um grupo de peixes foi alimentado com 5% de amido no período diurno (ML), e outro grupo foi alimentado no período noturno (MD). Os mesmos procedimentos foram realizados para o grupo que de alimentava com 25% de amido. Os seguintes parâmetros foram medidos: peso final, ganho de peso, taxa de crescimento especifico, consumo, índice hepatossomático y índice vicerossomático. O experimento foi conduzido em triplicado por 60 dias. Resultados: os parâmetros de crescimento como SGR, peso final e ganho de peso mostraram diferença estatística entre os grupos, com melhores resultados nos grupos alimentados com 25% de amido em ML. Diferença significativa entre grupos para conteúdo de lipídio na carcaça, energia e material seca foram observados para os animais alimentados com 25% de amido em MD. Foi observado efeito significativo no lipídio hepático e glicogênio com valores em geral mais altos em ML para os peixes alimentados com 5% de amido. Entretanto, os peixes alimentados com 25% de amido mostraram significativamente baixo conteúdo de lipídio e glicogênio sobre a condição de MD. Surpreendentemente, o oposto correu com os peixes alimentados em MD, para a composição do fígado. Conclusões: a alimentação diurna foi sugerida para um melhor desempenho dos juvenis, no entanto, a fase noturna ou de luz poderiam ser usados , levando em consideração sua relação com os níveis de carboidratos.
Palavra chave: alimentador automático, amido, horário de alimentação, utilização do alimento.
Introduction
Feeding fish to appetite may lead to improved growth, feed efficiency and fish welfare, given that fish display daily variations in appetite (Anthouard et al., 1993; Juell et al., 1993). It should not be surprising that periodic access to food has a profound influence on animal behavior and physiology (Sánchez- Vázquez et al., 1995). Thereby, the light–dark and feeding cycles are the most important factors that entrain biological rhythms in animals (Montoya et al., 2010). The synergistic effect of photoperiod and feeding on growth performance seems to be critical for fish (Villamizar et al., 2011). Fish raised under different feeding schedules show physiological variations in glucose regulation capacity (Hemre et al., 2002), amylase and protease activity (Montoya et al., 2010), hepatic nucleic acids, stress response (Sánchez et al., 2009), food ingestion (Bucking and Wood, 2009), as well as levels of T3, T4, and growth hormone (Gélineau et al., 1996). These changes highlight the importance of the feeding schedule (dark or light period) on fish metabolism and growth.
This variability may be due to the specific fish strain used and environmental factors, including temperature, light regimen and season (Hemre et al., 2002). Several authors found that light/dark cycles alter appetite and feed intake (Petit et al., 2003; Biswas et al., 2005; Siikavuopio et al., 2008), but the question remains whether it also influences the utilization of specific nutrients (Hemre et al., 2002). Variations of food ingestion during daytime and nighttime and its consequences have been widely reported in rats (Larue-Achagiotis and Le Magnen, 1983; Ruis et al., 1989; Tallett et al., 2009).
Omnivorous (Leiarius marmoratus), known as ''jandia'', and carnivorous (Pseudoplatystoma reticulatum), known as ''cachara'' or ''barred sorubim'', are among the largest fish in the main South-American basins. Hybridization between both species shows interesting characteristics favoring commercial application. In addition, these hybrids would be superior to carnivorous catfish for digesting vegetal diets.
The aim of the present study was to investigate which carbohydrate and lipid levels are more efficiently used for growing hybrid cachandiá (Pseudoplatystoma reticulatum x Leiarius marmoratus) under a fixed feeding time schedule. An additional aim was to evaluate the influence of dietary carbohydrate:lipid ratio and fixed feeding time on body composition and hepatic histology of these fish.
Materials and methods
Ethical considerations
This research is in agreement with the ethics principles in animal experimentation, adopted by the Animal Experimentation Ethics Committee of Universidade Federal de Lavras, Brasil (Protocol 028/12 of September 6, 2012).
Experimental diets
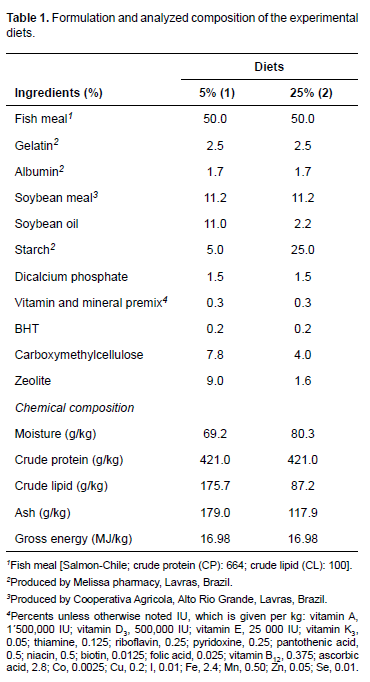
Two 42% crude protein diets were formulated (Table 1). Diet 1 (low ration) was formulated with a ratio of carbohydrate (CHO) to lipid (L) of 5.0/11.0 (CHO/L, %). The CHO/L ratio for Diet 2 was 25.0/2.2 (high ration).
The dry ingredients were mixed and distilled water was added until adequate consistency was obtained. Then, the mixture was cold pelleted to appropriate size. Prepared pellets (approximately 2 mm diameter) were oven dried at 58 °C for 48 h. All diets were then stored at -5 °C.
Fish and feeding trial
Hybrid catfish juveniles used in the experiment were obtained from Grupo Águas Claras-Colpani®, Mococa, Brazil. Upon arrival in the Fish Laboratory of Lavras University, Lavras, Brazil, 60 fish (260 ± 10g) were held in 12 indoor tanks (100 L) for a three-week acclimatization period. The stocking density was five fish per tank. Fish were familiar with self-feeders prior to the experiment. During acclimatization, the fish were exposed to a 12L: 12D light/dark cycle and were fed commercial food using automatic self-feeders (Eheim, model 3581, Germany). The experimental design was a 2x2 factorial (two rations of CHO and L and two feeding times). The self-feeders were scheduled to provide feed both in the daytime and nighttime period. The room had artificial illumination and light intensity measured at the water surface of the tank was 0.6 lux when the lights were on and 0.01 lux when lights were off. Fish were randomly allotted to one of two dietary treatments containing 5 or 25% starch, and each group was fed in light cycle and dark cycle. Each tank was equipped with mechanical and biological filters. Dissolved oxygen (DO), pH and NH3 content were monitored every day in each aquarium (DO: 6.5 ± 0.3 mg/L, pH: 7.5 ± 0.2, NH3: 0.2 ± 0.03 mg/L). During the experiment, temperature was maintained at 28 ± 2 °C.
Fish were fed the experimental diets from selffeeders during the middle of the light cycle (ML) at 12:00 (i.e., 6 h after lights were on and 6 h before lights were off [López-Olmeda et al., 2009]) and during the dark cycle (MD) at 00:00. Daily food allowance was set at 5% of the aquarium biomass, and was adjusted every four weeks after weighing the fish. Uneaten pellets were collected from the bottom of the aquariums 1 h after food was offered.
The following parameters were measured: initial weight, weight gain, specific growth rate, food intake, hepatosomatic index, and viscera somatic index. At the end of the experiment (60 days) fish were weighed to determine growth performance. Before measuring body weight, the fish from each group (ML and MD) were starved for 24 hours and anaesthetized with benzocain (250 mg/L). All fish were sampled for viscera somatic index (VSI), hepatosomatic index (HIS), and carcass composition. Viscera and liver were removed from the fish and weighed. Fish carcasses were stored in a freezer at -5 °C for subsequent analysis.
Analytical methods
Moisture, crude protein, lipid, and ash content of raw ingredients, experimental diets, and fish were determined by standard methods of association of official analytical chemists (AOAC 2003). Gross energy was measured in an adiabatic calorimeter (NRC, 1993).
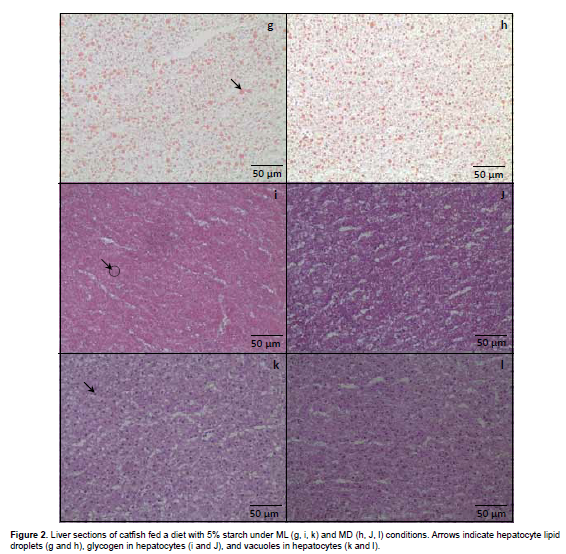
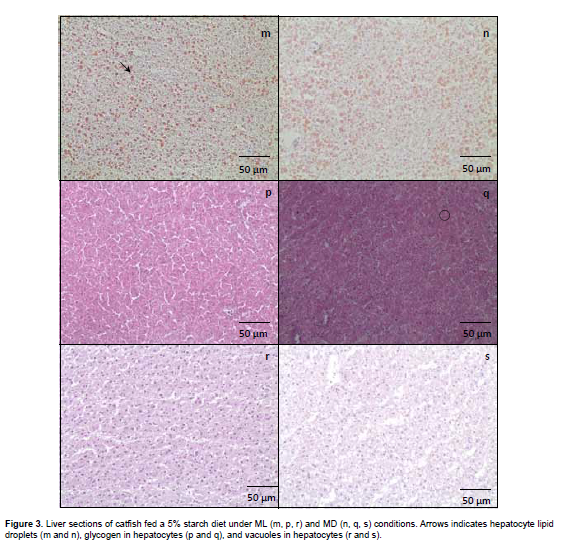
Histological preparation and image analysis of liver samples
All fish were sampled for analysis. A liver sample was collected, frozen in liquid nitrogen and stored at -80 °C. Histological frozen sections (10 µm thick) were cut in cryostate (Leica CM 1850, Microsystems GmbH, Wetzlar, Germany) and stained with hematoxylin and eosin (HE), periodic acid schiff (PAS), and Sudan III. Tissue changes induced by treatment were photographed and analyzed. Sections were observed and photographed (40x) under a light biological microscope (Olympus CX31, Miami, USA) with a digital color camera (Olympus SC30, Miami, USA) with AnalySISgetIT software (Olympus image) and analyzed with a public domain Java image processing program (ImageJ 1.45 m; Rasband W., National Institute of Health, USA).
Data analysis
Data on approximate analysis and body indexes were analyzed using SPSS 11.5. Data were expressed as mean ± SEM. Two-way ANOVA was used, followed by a Tukey's Test for post hoc analysis. P-value was fixed at 0.05.
Results
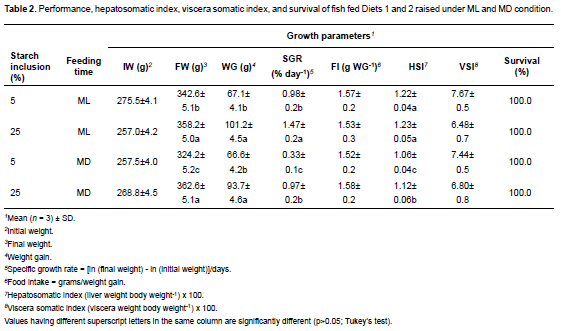
Fish fed Diet 2 showed a significant effect (p<0.05) on growth parameters such as final weight (FW) and weight gain (WG; Table 2).
The highest SGR was found (p<0.05) in fish fed Diet 2 under ML. The lowest SGR was for groups fed with Diet 1 and MD (Table 2). No difference was observed between treatments for food intake. On the other hand, dietary starch and lipid level and fixed feeding time did not influence VSI (p>0.05), but had a significant effect on HSI (p<0.05) with higher values in fish under ML condition. Survival rate was 100% in all groups.
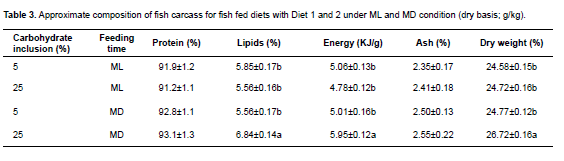
Concerning whole body composition, at the end of the growth experiment, protein content and ash were not significantly different in fish fed Diet 1 or 2, both in ML and MD. However, lipid, energy content, and dry weight were significantly higher (p<0.05) in fish fed Diet 2 under MD (Table 3).
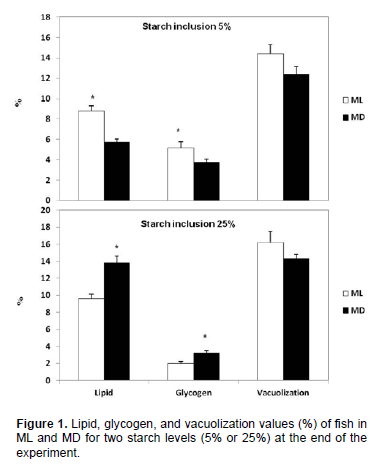
Regarding the fixed feeding time, a significant effect was observed on liver lipid and glycogen with values being generally higher in the ML than in the MD groups occurred in fish fed 5.0/11.0 (CHO/L, %; Figures 1 and 2). Surprisingly, the opposite occurred with fish fed 25.0/2.2 (CHO/L, %), with significantly lowest lipid and glycogen content (p<0.05) in the liver of fish under ML condition (Figura 1 and 3). No significant effect of fixed feeding time was observed for liver vacuolization.
Discussion
In summary, diurnal feeding improved performance of juvenile fish. However, night or light phases could be used, taking into consideration its relation to carbohydrate levels. Ultimately, feeding time effects over metabolism may provide a better understanding of fish nutrition.
It is known that carbohydrate or lipid utilization (as well as energy utilization) is affected by circadian rhythms. However, no exact data exist on hybrid catfish. It has been suggested that freshwater fish species are more sensitive to photoperiod than marine and diadromous species (Imsland et al., 1995). The studied fish were clearly affected by dietary starch levels, but also by the fixed feeding time conditions, displaying different growth and liver alterations under ML or MD condition. Thereby, light and dark cycles are required for the normal establishment of rhythmic circadian outputs and are thought to regulate the temporal coordination of many physiological processes (Vallone et al., 2007).
According to our results, the studied fish tend to be omnivorous. FW, WG and SGR responses indicate that the 25% starch diet had a growth effect, primarily for fish fed in ML (Table 2). The opposite was observed by Fu (2005) for carnivorous catfish (Silurus meridionalis) fed carbohydrates. According to Booth et al. (2013), there is a moderate proteinsparing effect of wheat and wheat starch for Seriola lalandi. The discrepancy with our results may be due to feeding habits, since diet of parental jandiá (Leiarius marmoratus) in natural environments includes plants. Additionally, large-size fish were used in the present study. Body size was found to have a positive relation with utilization of dietary carbohydrates (Tung and Shiau, 1993). In general, the optimal dietary starch level should not exceed 20% due to poor digestibility and decreased glucose tolerance at higher carbohydrate levels (Stone, 2003).
Likewise, an increase of dietary lipids can improve growth and protein utilization. The diet containing 42% protein and 19% lipid would be suitable for optimum growth and effective protein utilization of catfish fingerlings (Pseudobagrus fulvidraco; Kim and Lee 2005). Li et al. (2014) suggested that excessive carbohydrates (30%) lower the lipid content of juvenile giant croaker (Nibea japonica). However, changes in dietary carbohydrate levels from 12% to 23% (substituting the dietary lipid) did not alter growth performance in other South American catfish (Pseudoplatystoma coruscans; Martino et al., 2005). Catfish are generally nocturnal eaters, and carbohydrate utilization tests have been usually performed during the light phase of the light/dark cycle. In our experiment, no difference concerning food intake was observed between treatments, suggesting an adaptation of fish to the feeding schedule. Food availability in the wild is not usually random but cyclic. Thus, foraging behavior is confined to a period of the day when food is abundant and the presence of predators is reduced (López-Olmeda et al., 2009). The fixed feeding time can generate an anticipatory activity that confers an adaptive advantage as it may improve food utilization by preparing the animals physiologically, e.g. entraining the rhythms of gut motility (Sánchez- Vázquez and Madrid, 2001), digestive enzymes, such as amylase (Vera et al., 2007), and glucose utilization (López-Olmeda et al., 2009). This may explain why catfish are known for their ability to feed at varying light intensities.
Differences in body fat, energy and dry weight were only observed for fish fed in MD, with 25.0/2.2 (CHO/L, %). ML and MD results could be partially explained by glucose tolerance of fish, taking time of day into account. According to López-Olmeda et al. (2009) goldfish (Carassius auratus) display a daily rhythm of dextrin tolerance with a peak of blood glucose around ML. Interestingly, when the carbohydrate provided was glucose, administered either orally or intraperitoneally, an inverse tolerance pattern was observed, with higher blood glucose at MD. In humans, studies show the best tolerance to glucose occurs in the morning and the worst tolerance in the evening (La Fleur et al., 2001). This mechanism is not well understood, but the interaction results between carbohydrate level and feeding schedule in our study may be originated by factors such as glucose metabolism or glucose uptake in peripheral organs due to hormonal rhythms involved in glucose regulation by insulin or glucagon (Van Cauter et al., 1997). Both photoperiod regime and diet influence the glucose regulatory capacity of Atlantic salmon following a high-dose glucose injection (Hemre et al., 2002). In rats, the increased in vivo insulin responsiveness after high CHO feedings is most likely due to post receptor increases in various aspects of glucose metabolism (Olefsky and Saekow, 1978). This is consistent with the findings of Polakof et al. (2010).
For omnivorous warm-water fish such as catfish (Ictalurus punctatus; Wilson, 1994) and cachama (Piaractus brachypomus; Vásquez-Torres and Arias- Castellanos, 2013), dietary carbohydrates, mainly starch, constitute the main dietary source of energy. Fish fed 25% starch in MD condition tended to be fatter, indicating that they may be able to better use carbohydrates by lipogenesis (Table 3). It is known that improved utilization of highly digestible carbohydrates may be due to increased lipogenesis. According to Bergot (1979), increased carbohydrate utilization from starch results in increased lipid deposition in viscera and liver glycogen. Dietary dextrin concentration above 15.70% (CHO:L = 5.3) is not well utilized by jundiá (Rhamdia quelen), an omnivorous fish (Moro et al., 2010).
The present study provides some interesting results on carbohydrate/lipid level and feeding time in catfish. It has been reported that a high carbohydrate diet tends to yield a comparatively high HSI in hybrid striped bass (Morone crysops ?× M. saxatilis ?; Nematipour et al., 1992) and African catfish (Clarias gariepinus; Ali and Jauncey, 2004).
However, the effect of carbohydrate on high HIS and low lipid was observed only in fish fed under ML condition, but not for MD. Also, fish fed 5% starch and 11% soybean oil in ML showed a higher liver lipid and glycogen content, but the opposite occurs for fish fed in MD, where the highest lipid and glycogen content was observed when fed 25% starch and 2.2 soybean oil during the night period. According to Hemre et al. (2002), liver glycogen values and hepatosomatic index in Atlantic salmon reflect dietary starch levels, and are also significantly influenced by light regime. An explanation for this higher lipid and glycogen content of MD-fed catfish compared with ML fish fed 25% starch may have resulted from a metabolic capacity overloading in the night period. This overload leads to high lipogenesis, as shown by the high lipid content in the fish fed 25% starch under MD condition. However, histology showed that starch offered in ML or MD did not caused significant changes, such as vacuolization of liver parenchyma. These alterations are often associated with a degenerative-necrotic condition.
Acknowledgements
This study was supported by PPM-00238-09/ FAPEMIG projects. We are also grateful to Grupo Águas Claras-Colpani® for kindly providing the animals.
Conflicts of interest
The authors declare they have no conflicts of interest with regard to the work presented in this report.
¤ To cite this article: Prieto-Guevara MJ, Silva RF, Costa LS, Pereira RT, Rosa PV. Effect of fixed feeding time on growth, body composition, and hepatic histology of hybrid catfish (Pseudoplatystoma reticulatum x Leiarius marmoratus) fed with carbohydrates and lipids ratios. Rev Colomb Cienc Pecu 2015; 28:83-92.
References
Ali MZ, Jauncey K. Optimal dietary carbohydrate to lipid ratio in African catfish Clarias gariepinus (Burchell 1822). Aquacult Int 2004; 12:169-180. [ Links ]
Anthouard M, Kentouri M, Divanach P. An analysis of feeding activities of seabass (Dicentrarchus labrax, Moronidae), raised under different lighting conditions. Ictyophysiology Acta 1993; 16:59-73. [ Links ]
AOAC. Official Methods of Analysis of AOAC International. AOAC International, USA: Gaithersburg, MA, 2003. [ Links ]
Bergot F. Carbohydrate in rainbow trout diets: effects of the level and source of carbohydrate and the number of meals on growth and body composition. Aquaculture 1979; 18:157-167. [ Links ]
Biswas AK, Seoka M, Inoue Y, Takii K, Kumai H. Photoperiod influences the growth, food intake, feed efficiency and digestibility of red sea bream (Pagrus major). Aquaculture 2005; 250:666-673. [ Links ]
Booth MA, Moses MD, Allan GL. Utilization of carbohydrate by yellowtail kingfish Seriola lalandi. Aquaculture 2013; 376- 379:151-161. [ Links ]
Bucking C, Wood CM. The effect of postprandial changes in pH along the gastrointestinal tract on the distribution of ions between the solid and fluid phases of the chyme in rainbow trout. Aquacult Nutr 2009; 15:82-296. [ Links ]
Fu SJ. The growth performance of southern catfish fed diets with raw, precooked cornstarch and glucose at two levels. Aquacult Nutr 2005; 11:257-261. [ Links ]
Gélineau A, Mambrini M, Leatherland JF, Boujard T. Effect of feeding time on hepatic nucleic acid, plasma T3, T4, and GH concentrations in rainbow trout. Physiol Behav 1996; 59:1061-1067. [ Links ]
Hemre GI, Mommsen TP, Krogdahl A. Carbohydrates in fish nutrition: effects on growth, glucose metabolism and hepatic enzymes. Aquacult Nutr 2002; 8:175-194. [ Links ]
Imsland A, Folkvord AF, Steffansson SO. Growth, oxygen consumption and activity of juvenile turbot (Scophthalmus maximus) reared under different temperatures and photoperiods. Neth J Sea Res 1995; 34:149-159. [ Links ]
Juell JE, Furevik DM, Bjordal A. Demand feeding in salmon farming by hydroacoustic food detection. Aquacult Eng 1993; 12:155-167. [ Links ]
Kim LO, Lee SM. Effects of the dietary protein and lipid levels on growth and body composition of bagrid catfish, Pseudobagrus fulvidraco. Aquaculture 2005; 243:323-329. [ Links ]
La Fleur SE, Kalsbeek A, Wortel J, Fekkes ML, Buijs RM. A daily rhythm in glucose tolerance. A role for the suprachiasmatic nucleus. Diabetes 2001; 50:1237-1243. [ Links ]
Larue-Achagiotis C, Le Magnen J. Fast-induced changes in plasma glucose, insulin and free fatty acid concentration compared in rats during the night and day. Physiol Behav 1983; 30:93-96. [ Links ]
Li X, Wang J, Han T, Hu S, Jiang Y. Effects of dietary carbohydrate level on growth and body composition of juvenile giant croaker Nibea japonica. Aquac Res 2014. In press (DOI: 10.1111/ are.12437). [ Links ]
López-Olmeda JF, Egea-Álvarez M, Sánchez-Vázquez FJ. Glucose tolerance in fish: Is the daily feeding time important? Physiol Behav 2009; 96:631-636. [ Links ]
Martino RC, Cyrino JEP, Portz L, Trugo LC. Performance, carcass composition and nutrient utilization of surubim Pseudoplatystoma coruscans (Agassiz) fed diets with varying carbohydrate and lipid levels. Aquacult Nutr 2005; 11:131-137. [ Links ]
Messina M, Piccolo G, Tulli F, Messina CM, Cardinaletti G, Tibaldi E. Lipid composition and metabolism of European sea bass (Dicentrarchus labrax L.) fed diets containing wheat gluten and legume meals as substitutes for fish meal. Aquaculture 2013; 376-379:6-8. [ Links ]
Montoya A, López-Olmeda JF, Yúfera M, Sánchez-Muros MJ, Sánchez-Vázquez FJ. Feeding time synchronises daily rhythms of behaviour and digestive physiology in gilthead seabream (Sparus aurata). Aquaculture 2010; 306:315-321. [ Links ]
Moro GV, Camilo RY, Moraes G, Fracalossi DM. Dietary nonprotein energy sources: growth, digestive enzyme activities and nutrient utilization by the catfish jundiá, Rhamdia quelen. Aquac Res 2010; 41:394-400. [ Links ]
National Research Council (NRC). Nutrient Requirements of Fish. National Academy Press, Washington, DC, (USA); 1993. [ Links ]
Nematipour GR, Brown ML, Gatlin DM. Effect of dietary carbohydrate: lipid ratio on growth and body composition of hybrid striped bass. J World Aquacult Soc 1992; 23:128-132. [ Links ]
Olefsky JM, Saekow M. The effects of dietary carbohydrate content on insulin binding and glucose metabolism by isolated rat adipocytes. Endocrinology 1978; 103:2252-2263. [ Links ]
Petit G, Beauchaud M, Attia J, Buisson B. Food intake and growth of lagermouth bass (Micropterus salmoides) held under alternated light/dark cycle (12L:12D) or exposed to continuous light. Aquaculture 2003; 228:397-401. [ Links ]
Polakof S, Moon TW, Aguirre P, Skiba-Cassy S, Panserat S. Effects of insulin infusion on glucose homeostasis and glucose metabolism in rainbow trout fed a high-carbohydrate diet. J Exp Biol 2010; 213:4151-4157. [ Links ]
Ruis JF, Talamini LM, Buys JP, Rietveld WJ. Effects of time of feeding on recovery of food-entrained rhythms during subsequent fasting in SCN- lesioned rats. Physiol Behav 1989; 46:857-866. [ Links ]
Sánchez JA, López-Olmeda JF, Blanco-Vives B, Sánchez-Vázquez FJ. Effects of feeding schedule on locomotor activity rhythms and stress response in sea bream. Physiol Behav 2009; 98:125-129. [ Links ]
Sánchez-Vázquez FJ, Madrid JA. Feeding anticipatory activity in fish. In: Houlihan DF, Boujard T, Jobling M, editors. Food intake in fish. Oxford: Blackwell Science; 2001. p. 216-232. [ Links ]
Sánchez-Vázquez FJ, Zamora S, Madrid JA. Light-dark and food restriction cycles in sea bass: effect of conflicting zeitgebers on demand-feeding rhythms. Physiol Behav 1995; 58:705-714. [ Links ]
Siikavuopio SI, Sæther BS, Aas K. Growth in juvenile Atlantic cod (Gadus morhua L.) fed continuously or during light or dark hours. Aquaculture 2008; 278:192-194. [ Links ]
Stone DAJ. Dietary carbohydrate utilization by fish. Rev Fish Sci 2003; 11:337-369. [ Links ]
Tallett AJ, Blundell JE, Rodgers RJ. Night and day: diurnal differences in the behavioural satiety sequence in male rats. Physiol Behav 2009; 97:125-130. [ Links ]
Tung PS, Shiau SY. Carbohydrate utilization versus body size in tilapia Oreochromis niloticus x O. aureus. Comp Biochem Physiol A 1993; 104:585-588. [ Links ]
Vallone D, Lahiri K, Dickmeis T, Foulkes N. Start the clock. Circadian rhythms and development. Dev Dynam 2007; 236:142-155. [ Links ]
Van Cauter E, Polonsky KS, Scheen AJ. Roles of circadian rhythmicity and sleep in human glucose regulation. Endocr Rev 1997; 18:716-738. [ Links ]
Vásquez-Torres W, Arias-Castellanos JA. Effect of dietary carbohydrates and lipids on growth in cachama (Piaractus brachypomus). Aquac Res 2013; 44:1768-1776. [ Links ]
Villamizar N, Blanco-Vives B, Migaud H, Davie A, Carboni S, Sánchez-Vázquez FJ. Effects of light during early larval development of some aquacultured teleosts: a review. Aquaculture 2011; 315:86-94. [ Links ]
Wang L, Liu W, Lu K, Xu W, Cai D, Zhang C, Qian Y. Effects of dietary carbohydrate/lipid ratios on non-specific immune responses, oxidative status and liver histology of juvenile yellow catfish Pelteobagrus fulvidraco. Aquaculture 2014; 426:41-48. [ Links ]
Wilson RP. Utilization of dietary carbohydrate by fish. Aquaculture 1994; 124:67-80. [ Links ]