Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Colombiana de Ciencias Pecuarias
Print version ISSN 0120-0690
Rev Colom Cienc Pecua vol.28 no.2 Medellín Apr./June 2015
https://doi.org/10.17533/udea.rccp.v28n2a02
LITERATURE REVIEW
doi: 10.17533/udea.rccp.v28n2a02
Enteric methane mitigation strategies in ruminants: a review¤
Estrategias de mitigación de metano entérico en rumiantes: revisión de literatura
Estratégias de mitigação de metano entérico em ruminantes: revisão de literatura
Luiz Gustavo Ribeiro Pereira1*, Med Vet, PhD; Fernanda S Machado1, Med Vet, MSci, PhD; Mariana M Campos1, Med Vet, MSci, PhD; Roberto Guimaraes Júnior2, Med Vet, MSci, PhD; Thierry R Tomich1, Med Vet, MSci, PhD; Larissa G Reis3, Pharm; Cassius Coombs4, Zoot, Med Vet St.
1 Embrapa Dairy Cattle and members of Rumen Gases Network. Embrapa/CNPq/FAPEMIG).
2 Embrapa Cerrados.
3 CNPq- UFJF ''Science Without Borders'' program.
4 University of Sydney.
*Corresponding author: Luiz Gustavo Ribeiro Pereira. Embrapa Gado de Leite, Rua Eugênio do Nascimento, 610, Dom Bosco, CEP 36038-330. Juiz de Fora, Minas Gerais, Brasil. Tel: +55 32 33117529. Email: luiz.gustavo@embrapa.br
Received: August 29, 2013; accepted: May 7, 2014
Summary
Livestock farming in Latin America has been criticized because of its large greenhouse gas (GHG) production resulting from the use of degraded forage and low-efficiency production performance. Agriculture contributes a significant amount of the three main greenhouse gases: methane (CH4), carbon dioxide (CO2), and nitrous oxide (N2O). Methane has a global warming potential 25 times greater than CO2. Enteric methane is an important greenhouse gas responsible for approximately 15% of global warming. The trend and legal obligation of mitigating greenhouse gas emissions will likely directly influence improved efficiency of livestock systems, including animal nutrition and handling. The development of mitigation strategies and the viability of their practical applications have been researched around the world. Various nutritional strategies to mitigate enteric methane have been studied and developed. All of them differ in terms of viability, cost, and acceptance by the producers. Their adoption should be based on the capacity to reduce methane emissions in association with economic viability and animal performance. Animal performance improvement will be achieved in production systems (mainly those related to efficient forage use) associated with good management of nutrition, health and reproduction. These are important strategies to consolidate Brazil as a food producer to the world, respecting the demands regarding land, water, biodiversity conservation and emission of greenhouse gases.
Keywords: climate change, global warming, greenhouse gas, livestock, sustainability.
Resumen
La industria pecuaria latinoamericana ha sido criticada por la emisión significativa de gases con efecto invernadero (GHG). Dicha crítica se fundamenta en los bajos indicadores zootécnicos observados en los sistemas de producción animal basados en pasturas degradadas o que se encuentran por debajo de su potencial de producción. La industria agropecuaria contribuye de manera significativa con la emisión de los tres principales GHG: metano (CH4), dióxido de carbono (CO2) y óxido nitroso (NO2). El gas metano tiene un potencial de calentamiento global 25 veces mayor que el de CO2. El metano entérico es un importante gas de efecto invernadero, que es responsable de aproximadamente el 15% del calentamiento global. La tendencia o la obligación legal de mitigar las emisiones de GHG tendrá una influencia directa sobre la necesidad del aumento de la eficiencia zootécnica en los sistemas pecuarios relacionado con el manejo nutricional de los animales que deberá ser adoptado. El desarrollo de estrategias de mitigación y la viabilidad de su aplicación práctica representan áreas de investigación alrededor del mundo. Existen diversas estrategias nutricionales que se han estudiado y desarrollado con el fin de mitigar el metano entérico. Dichas estrategias presentan diferentes viabilidades, costos y posibilidades para que sean aceptadas por los productores. La elección de la estrategia de mitigación a ser adoptada deberá estar centrada en la capacidad de reducción de las emisiones de metano asociada con la viabilidad económica y el mantenimiento del desempeño animal. El aumento de los indicadores zootécnicos que se obtendrán en los sistemas de producción (principalmente aquellos que utilicen de manera eficiente el forraje) asociado a una buena nutrición, salud y manejo reproductivo, son estrategias importantes para la consolidación de Brasil como un importante productor de alimentos para el mundo, teniendo en cuenta las demandas relacionadas con el uso del suelo, del agua, la conservación de la biodiversidad y de la emisión de gases con efecto invernadero.
Palabras clave: calentamiento global, cambios climáticos, ganadería, gases de efecto invernadero, sostenibilidad.
Resumo
A pecuária da América Latina tem sido criticada por emitir quantidades significativas de gases de efeito estufa (GHG). Tal crítica tem sido fundamentada nos baixos índices zootécnicos verificados em sistemas de exploração animal baseados em pastagens degradadas ou que se encontram abaixo do seu potencial de produção. A agropecuária contribui de forma significativa com a emissão dos três principais GHG: metano (CH4), dióxido de carbono (CO2) e óxido nitroso (NO2). O gás metano apresenta potencial de aquecimento global 25 vezes maior que o CO2. O metano entérico é um importante gás de efeito estufa, que é responsável por aproximadamente 15% do aquecimento global. A tendência ou obrigação legal de mitigar as emissões de GHG influenciará diretamente a necessidade de aumento da eficiência zootécnica nos sistemas pecuários, atrelado ao manejo nutricional dos animais a ser adotado. O desenvolvimento de estratégias de mitigação e a viabilidade da aplicação prática dessas estratégias são áreas atuais de pesquisa em todo o mundo. Existem várias estratégias de nutrição para mitigar metano entérico que têm sido estudados e desenvolvidos. Todos estes têm diferentes viabilidades, custos e possibilidades de serem adotadas pelos produtores. A escolha de qual vai ser utilizado deve basear-se na capacidade de reduzir as emissões de metano associadas com viabilidade econômica e a manutenção do desempenho do animal. O aumento nos índices zootécnicos que serão obtidos em sistemas de produção (principalmente os relacionados ao uso de forragem eficiente) associada a uma boa nutrição, saúde e manejo reprodutivo são estratégias importantes para consolidar o Brasil como um importante produtor de alimentos para o mundo, respeitando as demandas relacionadas ao uso da terra, da água, da conservação da biodiversidade e da emissão de gases de efeito estufa.
Palavras chave: aquecimento global, gases de efeito estufa, mudanças climáticas, pecuária, sustentabilidade.
Introduction
Growth of global population and increased purchase power has promoted a rapid increase in the demand for food from animal sources. The world population will have reached 9 billion by 2050, while the demand for meat and milk products is expected to increase to 465 million tons and 1.043 million tons, respectively (FAO, 2006). Latin America has a prominent position as an animal protein provider for the world (FAO, 2010).
Despite the importance of agriculture in food production and revenue, there is a lot of discussion about the environmental impact of livestock and agricultural activities in relation to climate change. Latin American livestock industries have been criticized for their large greenhouse gas production as a result of using degraded forage with performance below production potential. The inefficiencies of these low-production meat and milk systems cause large amounts of greenhouse gas emissions (IPCC, 2007).
Agriculture contributes a significant amount of the three main greenhouse gases: methane (CH4), carbon dioxide (CO2) and nitrous oxide (N2O). Methane has a global warming potential 25 times greater than CO2, persists 9 to 15 years in the atmosphere, and increases 7.0% each year (IPCC, 2006). Atmospheric methane results from anaerobic fermentation of organic matter in wetland environments, rice fields cropped by flood irrigation, enteric fermentation, anaerobic treatment of animal residues, and biomass burning.
Cattle produce methane from enteric fermentation (85 to 90%) and fecal excretion. A total of 95% of rumen methane is excreted via eructation and from the intestines, 89% of methane produced is exhaled and around 1% excreted via the anus (Murray et al., 1976). Methane from enteric fermentation represents 25% of methane anthropogenic emissions (Wuebbles and Hayhoe, 2002).
Beef cattle and sheep produce 107 to 300 g and 17.8 to 39.3 g CH4/day, respectively (Czerkawski, 1969; Holter and Young, 1992; McAllister et al., 1996), resulting in 39.1 to 109.5 kg and 6.5 to 14.4 kg annual emissions, respectively. India and Brazil are the highest emitters of enteric methane, with 14.5 and 10.3 (x 1012) g/year, respectively. Brazil is the greatest emitter of beef cattle methane followed by India and the U.S. (9.6, 8.6, and 5.1 x1012 g/year, respectively; Thorpe, 2009). By 2005, agriculture was responsible for 22% of methane emissions in Brazil (MCT, 2009).
Enteric methane, responsible for 15% of global warming, is directly related with rumen fermentation efficiency because of the loss of carbon and consequent loss of energy, which affects animal performance (Cotton and Pielke, 1995; Bell et al., 2011). It is important to understand methane synthesis mechanisms. The challenge is to develop diets and handling strategies to minimize methane production (CH4/kg of milk, meat, or wool), increase production efficiency and decrease livestock contribution to global warming.
Beef cattle have been labelled as the greatest culprit of climate change, yet most of the criticism is not scientifically based. We need to develop and validate accurate methodologies to measure methane emissions and create specific databases for the production systems in each region (Lima et al., 2006; Grainger et al., 2007). Misguided media information regarding this issue could be used as an excuse to create non-tariff obstacles to exporting Brazilian livestock products.
Discussions on how to reduce greenhouse gas emissions have focused on production and supply chain modifications of food through significant changes in consumption patterns. Significant reductions in the consumption of food from animal sources have been proposed to decrease greenhouse emissions; however, the nutritional value of different foods needs to be considered to evaluate the impact of their production on the climate (Machado et al., 2011).
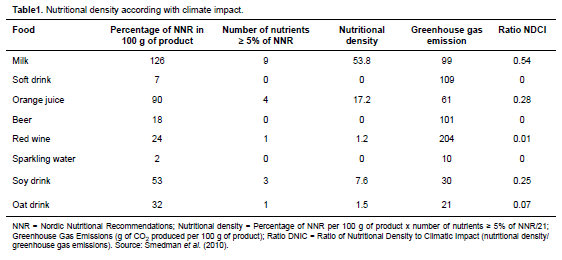
NDCI unit (nutrient density/greenhouse emission) was proposed by Smedman et al. (2010) and is comprised of the ratio between nutritional density and climate impact by combining the nutrient density of food with the gas emissions from its production. The authors compared greenhouse gas emissions from the production of milk, soft drinks, orange juice, beer, wine, sparkling water, soy drinks and oat drinks. A total of 99 g of CO2 were produced per 100 g of milk—much greater compared to the other drinks. However, when emissions were compared using NDCI values, milk had an advantage because of its high nutritional value (Table 1). This result represents a good argument to be presented in media discussions, which sometimes encourage reducing consumption of animal products to decrease the environmental impact associated with animal production.
Livestock production is likely to be increasingly affected by carbon emissions limits and environmental laws. The trend and legal obligation to mitigate greenhouse gas emissions is likely to have a direct influence on the efficiency of livestock systems, including animal nutrition and handling. Improvement of food practices can reduce methane emissions per kg of food intake or per kg of product (McAllister, 2011). Some alternatives to reduce methane production include specific agents and diet additives. Development of mitigation strategies and their viability have been researched around the world (Thornton, 2010).
Enteric methane production and its function in rumen ecosystem
Fermentation of diet components by rumen microbiota results in the production of short chain fatty acids (SCFAs)—an energy source for ruminants—and gases (CO2 and CH4) excreted via eructation (Martin et al., 2009a). Rumen fermentation involves an oxidation process, generating reduced co-factors (NADH, NADPH, and FADH), which are then re-oxidized (NAD+, NADP and FAD+) by dehydrogenation reactions, releasing hydrogen in the rumen. As an electron acceptor process, methanogenesis removes hydrogen gas (H2) from the rumen. Methane production is therefore essential for obtaining a high-performing rumen ecosystem because H2 accumulation, which could inhibit dehydrogenase activity in re-oxidation co-factors, is avoided. An efficient H2 capture in the rumen contributes to increase the rate of fermentation by the lack of its inhibitory effect on the microbial degradation of vegetative material (Wolin, 1979; McAllister and Newbold, 2008).
Enteric methane is derived from the activity of the methanogen Archaea, a microbial group distinct from eukaryotes (protozoa and fungi), bacteria with its own co-factors (coenzymes M, F420, and F430), and fat (isoprene-glycerol esters). Despite the central function of H2 in the metabolism, methanogenesis is important to rumen function and animal nutrition although methanogens comprise only a small part of the rumen's microbial biomass (Janssen and Kirs, 2008). Archaea methanogens are responsible for methane production in ruminants. Therefore, considerable research efforts have been made to gather more information about them (Attwood et al., 2008; Attwood et al., 2011). Identification of their metabolic activities and diversity is required for developing strategies to mitigate enteric methane emissions. Sequencing of their genomes will provide important information to develop such strategies (Buddle et al., 2010). Other microorganisms provide an appropriate environment to facilitate methanogen survival or produce substrates that would be available for methanogens. Metabolic pathway for H2 production and interspecies relationships between methanogens and other microorganisms of the ruminal ecosystem should be considered in the strategies to control methane emission by ruminants. The H2 produced by microbial fermentation is an energy source to Archaea methanogens for methane production. Formate can be used to produce methane by methanogens; however, it is a less important methane precursor than H2 and is responsible for approximately 18% of the methane produced (Hungate et al., 1970). Ruminal fermentation products are not equivalent in terms of H2 production; their amount depends on short chain fatty acid (SCFA) concentration and the relative ratio between acetate, propionate and butyrate (Owens and Goetsch, 1988; Eun et al., 2004; Martin et al., 2009a). Quantitative mathematic models consider fermentation stoichiometric calculations to balance formation of H2, SCFAs and other products for predicting methane production (Bannink et al., 2006; Ellis et al., 2008a).
Enteric methane and energy losses
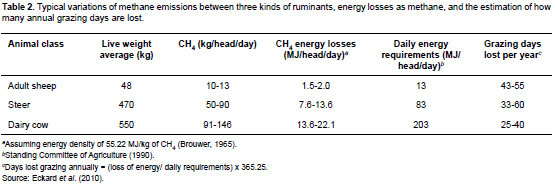
At an energetic content of 55.22 MJ/kg (Brouwer, 1965), methane represents a significant amount of energy in a production system (Table 2). Approximately 5.5 to 6.5% of raw energy ingested is converted to methane (Johnson and Ward, 1996). However, measurements in respiratory chambers (indirect calorimetry) show greater methane emissions: from 2 to 12% of raw energy ingested (Johnson and Johnson, 1995). Generally, as digestibility increases variation in methane production also increases. According to Johnson and Johnson (1995), there are two causes of methane production variation: the amount of carbohydrates fermented in the rumen, and the ratio of propionate to acetate produced.
While evaluating methane production of steers fed forage-based diets or diets with 80% concentrate, Harper et al. (1999) observed that 8.1% and 2.1% of raw energy was lost as methane, respectively. According to Kaharabata et al. (2000), a dairy cow weighing 600 kg can produce 268 to 450 g CH4 per day. This energy loss (13,344 kcal/g) would be enough to produce between 4.55 and 7.65 kg of milk containing 4% fat. Johnson et al. (1994) reported 256 L/day methane produced by steers (9.1% of raw energy ingested), 193.9 L/day by heifers (5.6% of raw energy), and 548.2 L/day (5.7% of raw energy) by lactating cows.
It is important to consider the enteric methane production per unit of animal product produced (kg of milk, meat, or wool). A balance can be established between the necessity of food produced for the growing population and the emission of greenhouse gases. A reduction of enteric methane production without compromising animal productivity is thus desired to mitigate greenhouse gas emissions and improve ruminant feed conversion efficiency.
Improving product quality through reduction of greenhouse gas emission levels can enhance efficiency of systems in Latin America. According to Barioni et al. (2007), increasing birth rate of cows from 55 to 68%, reducing slaughter age from 36 to 28 months, and reducing mortality from 7 to 4.5% in animals younger than one year of age could reduce methane emissions by 18% in relation to the equivalent level of carcass production in Brazil by 2025. This could be possible even with a 25.4% increase in meat production. This means that actions oriented to improve production efficiency would proportionally reduce methane emissions because more products (meat, milk, or wool) will be obtained using the same resources (Guimaraes Jr. et al., 2010).
Yan et al. (2010) evaluated data from 20 energy metabolism studies in open flow respiratory chambers involving 579 lactating cows with varied genetic merit, lactation number, lactation phase, and live weight. The authors studied enteric methane emissions using energy, efficiency, and productivity. Results indicate that methane losses in relation to raw energy ingested or milk energy are negatively related to milk yield, metabolizable energy (q), and efficiency to utilize the metabolizable energy for lactation (Kl). Therefore, selection of highly producing lactating cows and a more efficient use of energy represent an effective methane-mitigation strategy.
Nutritional strategies to mitigate enteric methane
The H2 produced in the rumen is critically important to the rumen ecosystem, mainly during the fermentation process. H2 should remain reduced, allowing for the reoxidation of NADH, in order to degrade nutrients for SCFA production. In this methanogenesis process, H2 manipulation in the rumen is the key to controlling methane emissions (Joblin, 1999).
According to Martin et al. (2009a), the metabolic pathways involved in H2 production and use and methanogen populations should be considered in strategies to control methane emissions. Strategies need to focus on reducing H2 production without spoiling digestion, stimulating H2 use through alternative production pathways for ruminants, and/ or Archeaea methanogenic inhibition (number and/or activity), associated with stimulation of pathways that consume H2 to avoid the negative effects of increasing partial H2 pressure in the rumen.
Diet composition and quality
Concentrate. Increasing the amount of concentrate in the diet reduces the proportion of dietary energy that is converted to methane (Blaxter and Clapperton, 1965). In other words, the addition of concentrate promotes the methane emission reduction as a proportion of ingested energy or expressed as per unit of animal product (milk and/or meat).
Fibrous carbohydrate substitution (cellulose and hemicellulose) for non-fibrous carbohydrates (starch and sugar) results in significant modifications in both the physiochemical conditions in the rumen and microbial populations. Increase of amylolytic bacteria results in a change in SCFA production, promoting a proportional increase of propionate and a reduction of acetate. Consequently, methane production is reduced because of low H2 availability in the rumen.
However, according to Martin et al. (2009a), the low acetate to propionate ratio does not always occur when animals are fed concentrate-rich diets. In this situation, the reduction of methane emissions can be explained by the reduction in both pH and ciliate protozoa. The low rumen pH can inhibit growth and/ or activity of methanogens and cellulolytic bacteria.
In high concentrate diets, the factors that induce methane reduction are: increasing propionate, which reduces H2 in the rumen, methanogenic (Hegarty, 1999), cellulolytic bacteria (Brossard et al., 2004), and ciliate protozoa inhibition via pH reduction. According to Clarke (1977) rumen ciliated protozoa are sensitive to pH changes and they cannot survive if pH increases above 7.8 or decreases below 5.0. Dehority (2005) reported death of in vitro protozoa at pH values below 5.4 and bacteriocin production by lactic bacteria, which inhibits methanogenic activity (Rodriguez and Campos, 2007).
Methane losses are relatively constant in diets with 30 to 40% concentrate (6 to 7% of raw energy ingested) while methane losses decrease rapidly to low values in diets containing 80 to 90% concentrate (2 to 3% of raw energy ingested; Lovett et al., 2003; Beauchemin and McGinn, 2005; Martin et al., 2007).
Berchielli et al. (2003) reported a quadratic relationship to methane production in beef cattle fed different dietary forage to concentrate ratios. According to the authors, the results suggest that concentrate addition in low amounts offers favorable conditions for microorganisms by providing energy to degrade fiber fractions in the rumen. However, when 60% concentrate is added to the diet the rumen environment becomes spoiled by microbial methanogenesis, evidenced by a lower rumen pH. Primavesi et al. (2004) also reported that substituting forage with concentrate results in the maximum methane emission when concentrate was added as 40% of DM.
Concentrate addition to reduce methane emission has economic and environmental limits. Possible metabolic consequences of diets rich in non-fibrous carbohydrates include ruminal acidosis, reduced milk fat and shorter productive life of animals. The economic viability of production systems with high levels of concentrate is questionable in climates more conducive to forage-based production, as in Brazil and other tropical countries.
In addition, the consequences of the increased energy density of diets should be analyzed. Greenhouse gas emissions, such as as CO2 and nitrous oxide (N2O), originating from grain production, harvesting and transportation can overcome the reduction of enteric methane emissions caused by its inclusion in ruminant diets. Johnson et al. (2002b) and Lovett et al. (2006) have reprted the flow of greenhouse gases in production systems.
Concentrate composition also influences methane production. Lovett et al. (2006) evaluated the effect of pasture supplementation with concentrate composed of fiber by-products (32.8% insoluble neutral detergent fiber-NDF) on enteric methane emissions. An increase of daily methane production (from 346 to 399 g/cow/day) was observed when concentrate was added (due to its high fiber and low starch levels). However, the authors observed a tendency for methane emission/kg of milk produced to reduce because concentrate promotes milk yield.
Forage. Methane emission (g/kg dry matter ingested) is influenced by the type of forage the animal has been ingesting. Usually, animals fed legumes have less methane emissions than animals fed grasses. According to Benchaar et al. (2001), the substitution of Timothy-grass hay (Phleum pratense) for lucerne (Medicago sativa) reduced methane emission by 21% (expressed as percentage of digestible energy). McCaughey et al. (1999) observed 10% reduction in methane production per unit of product in beef cattle at pasture, when a grass-exclusive diet was substituted for alfalfa and grass (ratio 70:30). The effect of legume use on methane emission can be explained by the presence of condensed tannins (Waghorn, 2007), different levels of fiber, increased ingestion of dry matter (DM) and consequent increased rate of passage in the rumen (O´Mara et al., 2004).
There are many differences among the composition of carbohydrates in forage, which influence their methanogenic potential. C4 grasses can produce more methane per kg of DM ingested than grasses with C3 photosynthesis (Ulyatt et al., 2002; Archimède et al., 2011). Corroborating this, Primavesi et al. (2004) observed 121 to 147 kg CH4/animal/year emissions in lactating cows under tropical conditions. These values were higher in comparison to those in North America (118 kg of CH4/animal/year for animals weighing 600 kg, lactation of 6,700 kg of milk/year, and ingestion of 2.7% live weight of DM), and Eastern Europe (100kg of CH4/animal/year for cows weighing 550 kg, lactation of 4200 kg of milk/year and ingestion of 2.5% live weight of DM; IPCC, 1995; Johnson and Ward, 1996). The authors attribute this difference to the lower quality of tropical forage compared to temperate forage, especially because of higher fiber content and lower digestibility. Archimède et al. (2001) reported that methane emissions (L/kg of DM ingested) were 17% higher when ruminants were fed C4 grasses as compared to C3 grasses.
Another factor that lowers methane production by lactating cows grazing on temperate pastures is grain inclusion higher than 50%. The percentage of CH4 produced in relation to raw energy ingested ranges from 5.5 to 6.5% in North America and Eastern Europe (United States, 1990). Primavesi et al. (2004) obtained 8.3%, and 10.6% in crossbred Dutch lactating cows kept in fertilized tobiatã grass pastures (Panicum maximum cv. Tobiatã) and brachiaria (Brachiaria decumbenses Stapf.), respectively.
Conservation methods and forage processing should also be considered. According to Beauchemin et al. (2008), methanogenesis tends to be lower in silage compared to hay and lower in finely-ground feed or pellets compared to roughly picked feed. Forage milling and pelleting reduce methanogenesis
(methane production decreases 20 to 40% per unit of diet; Blaxter, 1989) due to increased passage rate. However, these effects are not apparent when feed consumption is restricted. Ammoniation or protein supplementation of low quality forage increases methane losses in proportion to digestibility, although methane production per unit of product is reduced (Johnson and Johnson, 1995).
Handling practices that improve forage quality increase animal performance and productivity per unit of area. Associated with performance increments, an increase in methane emission is expected as a result of greater ruminal fermentation. However, the amount of methane per unit of product (milk or meat) is reduced when animal production or growth increases.
Wins et al. (2010) evaluated the effects of two DM levels in pre-pasture forage (low: 1000 kg/ha and high: 2200 kg/ha) on methane emissions, voluntary DM intake (VDMI), and milk yield of cows. Methane emissions were measured in two experiments through a SF6-tracer technique. The authors concluded that low mass of pre-pasture forage improved the pasture nutritional quality and consequently reduced methane emissions (g/day; g/kg of milk; g/kg of milk solids and g/kg of DM ingested). These results are in agreement with Blaxter and Clapperton (1965) who observed that CH4 decreases while digestibility increases with higher intakes (two or three times the maintenance level). Despite intake being the most important factor in methane production, Wins et al. (2010) showed that other factors are involved in methane emissions.
Robertson and Waghorn (2002) observed that methane production per lactating cow increases as forage matures (5 and 6.5% of raw energy ingested for spring and summer, respectively). The relatively low methane emissions observed for young forage can be explained by higher levels of soluble carbohydrate and linoleic acid. Hegarty (2001) analyzed the effect of nutritional improvement of pasture on methane production in Merino, sheep, finding that the proportion of ingested energy losses in the form of methane decreased from 6.6 to 6.0% with increasing forage digestibility.
Therefore, implementation of adequate pasture handling increases feed quantity and quality and is a suitable strategy to mitigate enteric methane by increasing energy efficiency, reducing livestock's impact on the environment, and improving feed efficiency and profit (Chaves et al., 2006).
Lipid addition. Dietary supplementation with nonprotected lipids reduces methane emissions through multiple mechanisms: reduction of fermentable organic matter (lipids are not a source of energy for rumen bacteria); reduction of methanogenic activity due to the presence of medium-chain fatty acids; toxic effects on cellulolytic bacteria (Nagajara et al., 1997) and protozoa (Doreau and Ferlay, 1995) due to the effect of polyunsaturated fatty acids (PUFAs) and biohydrogenation of PUFAs.
Toxic effects of long chain fatty acids occur through their action on cell membranes, particularly gram-positive bacteria. Linoleic acid is toxic to cellulolytic bacteria (F. succinogenes, R. albus, and R. flavelasciens) because it affects cell integrity and fungus Neocallimastix frontalis growth (Maia et al., 2007). Changes in rumen microbial populations favor propionate production, increasing H2 captured in the process.
Despite PUFAs biohydrogenation resulting in H2 capture, they have little influence on methanogenesis because a complete hydrogenation of 1 mol of linolenic acid prevents the production of 0.75 mol of CH4 (Martin et al., 2009). The use of metabolic hydrogen during unsaturated fatty acids biohydrogenation is lower (1%) compared to the reduction of CO2 (48%), SCFAs synthesis (33%), and bacterial cell synthesis (12%; Czerkawski, 1986).
The effectiveness of lipid addition on reducing methane emissions depends on supplementation level, lipid source, method of supply (e.g. refined oil, oil seeds) and diet type (Beauchemin et al., 2008).
Despite the possibility of a methane reduction greater than 40% when high levels of lipids are added (Machmuller and Kreuzer, 1999; Jordan et al., 2006b), a reduction from 10 to 25% is more likely to be obtained (Beauchemin et al., 2008). It is recommended that lipid supplementation does not exceed 6 to 7% DM to avoid the decrease of VDMI. Multiple action of lipid supplementation can affect the number and activity of rumen microbes, which can compromise digestion when toxic effects promote H2 accumulation.
Beauchemin et al. (2008) revised 17 studies on beef cattle and sheep and established a connection between levels of lipid addition (% of VDMI) and methane emission (g/kg of DM intake) in different oil and fat sources. Methane emissions would be reduced by 5.6% per 1% lipid addition. The authors found considerable variation among lipid sources on methanogenesis. A sharp methane decline was observed (g/kg of DM intake) in studies with coconut oil (63.8% reduction with 7% addition; Machmuller and Kreuzer, 1999) and myristic acid (58.3% reduction with 5% addition; Machmuller et al., 2003).
Martin et al. (2009a) summarized in vivo studies (67 diets supplemented with lipids, from 28 publications) evaluating the effect of lipid sources on methane emissions from beef cattle and sheep. The results were 3.8% methane reduction (g/kg of DM ingested) per 1% fat added to the diet (% of VDMI).
It is evident that fatty acid effects on methanogenesis depend on their chemical nature. Lipid supplements rich in medium chain fatty acids (12 to 14 carbons) such as coconut, palm or canola oils (rich in lauric acid), or purified myristic acid, are more effective in depressing methane emissions in diets rich in concentrate and low in Ca (Machmuller et al., 2003). According to Dohme et al. (2001), lauric acid (C 12:0), and myristic acid (C 14:0) showed similar effects when provided separately, but when combined they promoted a synergistic sharp-reducing effect on methane emissions (Soliva et al., 2004).
Few studies have evaluated the effects of monounsaturated fatty acids (such as oleic acid in canola), and saturated fatty acids (SFA, such as palmitic and stearic in tallow) on rumen methanogenesis. A 30% decline in methane production was observed when 12% tallow was added to the diet of lactating dairy cows (Van der Honing et al., 1983). However, this effect was not observed in other cow (Johnson et al., 2002a; Woodward et al., 2006) and sheep studies (Cosgrove et al., 2008).
The mechanism of action of saturated fatty acids has been related to their ability to damage cell membranes, leading to K+ leakage—an indicator of damaged membrane—followed by cell death. Among SFA, the most toxic to bacteria membrane is lauric acid (C 12:0), followed by myristic (C 14:0) acid, and both are used as antibacterial agents (Zhou et al, 2013). The aforementioned authors tested the effects of SFA on methanogenesis and Methanobrevibacter ruminantium viability and observed higher toxicity for lauric and myristic acids, which caused a greater decrease on methane production.
Grainger et al. (2010b) evaluated methanogenesis when cottonseed was added to dairy cow diets over 12 weeks. They observed a lasting methane emissions reduction (mean 3.5 g CH4/kg of DM ingested) over 12 weeks as a result of cottonseed addition (2.61 kg of DM/cow/day). This effect increased from 5.1% in the first week to 14.5% in the twelfth week.
A compulsory inclusion of 5% biofuel to diesel fuel since 2010 is driving Brazilian agriculture to adapt oil seed production for non-food purposes. Some options of raw materials have been studied (soybean, castor bean, cotton, jatropha, palm kernel, licuri palm, babassu palm, macauba palm, radish, peanut, sunflower, canola, and coconut). Consequently, many byproducts have been produced (milled meals, pressed meals, and glycerin) and there is increased availability of a variety of oils used in biodiesel production, which have potential for ruminant diets, possibly contributing to mitigating enteric methane.
Quantifying methane-mitigation potential by using biodiesel byproducts is important because the benefits of byproduct and oil inclusions in ruminant diets can be combined with the benefits of biodiesel as energy source (reduction of CO2 emission) and thereby contribute to consolidate Brazil as a global reference in biofuels.
Additives. Another strategy to mitigate enteric methane is the use of additives. Ruminal ecosystem manipulation is an important tool used by nutritionists to increase feed conversion efficiency and animal performance. In the past, research was focused on antimicrobial use (e.g. monensin). However, the growing societal pressure against the use of this additive in animal feed has encouraged the search for other alternative methods to manipulate the rumen environment.
The ionophores are anti-methanogenic effects of ionophores are more related to the inhibition of methane precursors (formate and H2) than a direct effect on methanogen populations because methanogens are more resistant to ionophores than H2-producing bacteria. Reduction of methane precursors would be responsible for 45% of ionophore's effect on methane production while the remainder would be a consequence of decreased feed intake (Nagajara et al., 1997). The decline of methane as an effect of ionophores can be associated with growth inhibition of ciliate protozoa that produce H2 and are colonized by methanogens (McAllister et al., 1996).
Johnson and Johnson (1995) revised ionophore additions to grain-based and forage-based diets finding a great variation in rumen methanogenesis reduction (4 to 31%). They concluded that any effect is short-lasting and methane returns to normal levels after two weeks. Methane reduction was most likely associated with decreased DM rather than a direct effect on methanogenesis. Monensin effect on methane reduction is dose-dependent. Studies revised by Beauchemin et al. (2008) showed that doses lower than 15 ppm have no effect on methanogenesis (g CH4/ day or g CH4/kg DM ingested) in dairy cows. Higher doses (24 to 35 ppm) reduced methane production (between 4 and 10% g/day; and 3 to 8% g/kg DM ingested) by beef and dairy cattle (Sauer et al., 1998; McGinn et al., 2004; Van Vugt et al., 2005; Odongo et al., 2007). A 30% methane reduction was reported when 33 ppm monensin was included in low or high forage diets (Guan et al., 2006).
Grainger et al. (2010a) evaluated the use of a higher monensin dose (471 mg/day) in cows fed on ryegrass pasture supplemented with 4 kg/day of barley grain. Methane emissions were estimated in pasture animals using both SF6-tracer gas technique and respiration chambers. In both conditions, the monensin addition did not increase milk production and did not promote any effects on enteric methane emissions (g/day, g/kg of milk and g/kg of DM ingested). The authors concluded that monensin does not represent a viable strategy to mitigate methane emissions from dairy cows when they are fed concentrate-supplemented pasture.
The possible transitory effects of ionophores associated with the growing pressure to decrease antimicrobial use in animal production suggest that this strategy of methane mitigation does not represent a lasting solution for the problem.
Organic acids (malate and fumarate) represent an alternative to antimicrobial use in ruminant nutrition. These substances can stimulate lactate capture by Selenomonas ruminantium bacteria (Martin and Park, 1996) and act as a buffer to prevent rumen acidosis when the diet is high in energy-rich concentrate. In addition, organic acid supplements, which are direct precursors of propionate, demonstrate a positive dose-dependent effect on methanogenesis reduction (Asanuma et al., 1999; O´Mara, 2004).
Commercial use of organic acids is limited for ruminants because of their cost. Considering this, forage can be provided as a source of dicarboxylic acid. Intermediate components of the tricarboxylic acid cycle accumulate in plant tissue. However, according to O'Mara (2004), there is a great variation in accumulation (0.6 to 7.5% of DM). Callaway et al. (1997) conducted a study to determine malate concentrations present in hay of five alfalfa varieties at different maturity stages. At more mature stages, malate concentration was reduced from 6.5 to 7.0% in young-harvested alfalfa and 2.9 to 4.5% when it was harvested later. Martin (1998) suggested that high levels of malate in fresh forage at initial stages of growth, especially in alfalfa, can promote significant changes in rumen microbial fermentation.
There is growing interest in the use of plant secondary compounds (plant extracts) to mitigate methane since this natural alternative avoids the use of chemical additives. Some plants produce secondary metabolites to protect them from fungi, bacteria, insects and herbivores. The effects of these molecules on rumen methanogenesis are highly variable. Most studies have focused on tannins, saponins, and essential oils. When high levels of these substances are ingested, adverse effects on animal performance and health can occur, but in low concentrations they can improve rumen fermentation (Morais et al., 2006; Beauchemin et al., 2008).
Tannins are polyphenolic substances with varied molecular weight and complexity, and are classified as either hydrolysable or condensed. Antimethanogenic activity of tannins found in plants has been associated with condensed tannins.
Dairy cows presented lower methane emissions when fed Lotus corniculatus (26.9 g CH4/kg of DM ingested and 378 g CH4/kg of milk solids) compared to ryegrass silage-fed cows (35.23 g CH4/kg of DM ingested and 434 g CH4/kg of milk solids) (Woodward et al., 2001). Oliveira et al. (2006) did not observe any effect on methanogenesis when low and high tannin levels in sorghum silage diets were fed to beef cattle.
Saponins in Brachiaria decumbes and alfalfa (Medicago sativa) are glucosides with a direct effect on rumen microorganisms. Saponins reduce protein degradation and simultaneously favor protein synthesis and microbial biomass synthesis; both processes result in reduced H2 availability to methanogenesis (Martin et al., 2009a). The main antimethanogenic mechanism of saponins is related to its toxic effects on ciliate protozoa. This compound emulsifies the lipid cell membranes of protozoa, altering permeability and consequently causes cell death (Wallace et al., 2002).
Hess et al. (2004) observed a 54% decrease in protozoa numbers and 20% reduction in in vitro methane production when saponins were used in high levels (12 mg/g of DM). Guo et al. (2008) observed methanogenesis reductions of 8% and protozoa reductions of 50% when saponins were used in vitro. The authors reported a decline in methanogenic activity (76%), measured through mcrA (methyl coenzyme-M reductase) gene expression with no effect on methanogen numbers.
Essential oils are secondary metabolites responsible for the smell and color of some plants. Some molecules present in essential oils have antimicrobial activities that act on gram-positive and gram-negative bacteria. Among the essential oils studied, garlic oil (Allium sativa) extracted through vaporization and distillation showed some effect on in vitro methanogenesis. Busquet et al. (2005) evaluated the effect of garlic oil and four of its components (diallyl sulphide, diallyl disulphide, allyl mercaptan, and allicin) on in vitro ruminal fermentation. Methane production after 17 hours of fermentation was reduced significantly by garlic oil, allyl mercaptam, and diallyl disulfide.
McAllister et al. (2008) studied a commercially available allicin product, finding no effect on daily SCFAs or ammonia (N-NH3) production at levels of 0, 2 and 20 μg/mL. However, at 20 μg/mL, methane production was reduced significantly; this can be related to the reduction in methanogen populations in relation to total bacteria.
Watabane et al. (2010) evaluated cashew nut shell liquid (CNSL). CNSL contains phenolic compounds (e.g. anarcadic acid) that selectively inhibit Grampositive bacteria. The authors carried out in vitro experiments using a concentrate-rich diet (30:70 forage to concentrate) to evaluate different doses of raw and thermal processed CNSL. Results indicated that raw CNSL could be used for rumen manipulation, increasing propionate production and reducing methane emissions.
Mitigation strategies via alternative pathways to use H2
Redirection of H2 towards processes that produce beneficial products to ruminants is another strategy to mitigate methane. Some examples of these processes are the addition of substrate, which can stimulate propionate production and the attempts to insert bacteria, which express reductive acetogenesis in the rumen. These processes increase propionate and acetate production, respectively, as well as reduce H2 availability for methanogenesis (Van Zijderveld et al., 2010). Nitrate and/or sulphate salts have also been evaluated because they provide an alternative pathway for H2 use.
Acetogenic probiotics. In hindgut fermentation species (such as humans, hamsters, rabbits and rats), reductive acetogenesis is a natural mechanism to use H2 in the gastrointestinal tract. It is known that acetogenesis occurs in the rumen, but the hydrogenotrophic capacity and environmental significance are not well understood.
Eubacterium limosum was the first acetogenic microorganism discovered in the rumen. It was isolated in sheep fed a molasses-based diet (Gethner et al., 1981). It demonstrated an ability to grow in a medium with CO2 and H2 and produce acetate. Due to the difficulty of isolating acetogenic bacteria, it was concluded that these microorganisms were foreign to the rumen and acetogenesis was not considered a relevant ruminal process. However, with the increasing discussion about the influence of methane on global warming, the acetogenesis process is starting to be considered as a potential methane mitigation strategy.
In addition, acetate (final product of the reaction) has an advantageous characteristic because it is an additional source of energy to the host animal. However, when comparing acetogenesis and methanogenesis as competitors for reduction equivalents in the rumen, acetogenesis is less efficient than methanogenesis because it requires a higher concentration of H2 to reduce CO2 to acetate than methanogens need to reduce CO2 to CH4. The latter reaction is thermodynamically favorable (Weimer, 1998).
Recent studies indicate that ruminants have at least a small population of acetogenic bacteria, the density of which is influenced by the diet. Acetogenic presence in the rumen is a defense mechanism to avoid H2 accumulation when methanogenesis is inhibited; therefore, these microorganisms do not compete with methanogens (Hegarty, 2001). Acetogenic bacteria are present in high numbers when methanogenesis is not established in newborn calves (Morvan et al., 1994) and when cattle are fed on low forage : concentrate diets (Leedle and Greening, 1988). Recent isolation of new gastrointestinal bacteria species using H2 (Klieve e Joblin, 2007) represents a new perspective for this mitigation strategy.
Nitrate and sulphate salts. The use of nitrate as an alternative to H2 is not recommended because of the toxic effects of nitrite, an intermediate compound from the reduction of nitrate to ammonia. Nitrate to nitrite reduction (ΔGT = -130 kJ/mol H2) and subsequent reduction from nitrite to ammonia (ΔGT = -124 kJ/mol H2) releases more energy than reduction from CO2 to CH4 (ΔGT = -16.9 kJ/mol H2; Ungerfeld and Kohn, 2006). This process could be the main pathway to eliminate H2 if sufficient nitrate was available in the rumen. Reduction of nitrate to ammonia consumes eight electrons and each mol of reduced nitrate can reduce 1 mol of methane. The ammonia produced could be available to other anabolic processes and would be an important source of fermentable N in diets deficient in crude protein, where lower concentrations of ammonia in the rumen limit microbial protein synthesis (van Zijderveld et al., 2010).
For animals not adapted to the use of nitrate in the diet, the ability of rumen microorganisms to reduce nitrate to nitrite is greater than the ability to reduce nitrite to ammonia. This nitrate compound is absorbed in the ruminal epithelium and promotes the conversion of blood hemoglobin from ferrous form (Fe2+) to ferric form (Fe3+), which inhibits hemoglobin's ability to carry O2 to tissues (methemoglobin), resulting in general anoxia, decreasing animal performance, and in severe cases, leading to fatality (Ozmen et al., 2005). Supplementation with sulphur or cysteine can decrease nitrite accumulation in the rumen because sulphate is a reducer (ΔGT = - 21.1 kJ/mol of H2) that competes for electrons, which decrease methane production (Ungerfiel and Kohn, 2006).
Van Zijderveld et al. (2010) evaluated the effects of nitrate and sulphate addition on methane emissions with sheep diets (2.6% DM) in respiratory chambers. Methane production was reduced while the supplements were used (nitrate: 32% decrease; sulphate: 16% decrease; nitrate and sulphate: 47% decrease). The reduction in methane emissions due to nitrate use was more pronounced when it was used after feeding, while the sulphate effect was observed throughout the day. The authors concluded that when these compounds were provided in a safe way, nitrate and sulphate salts are potential agents to mitigate enteric methane.
Vaccination against rumen methanogens. The efficiency of vaccination depends on the connection between saliva antibodies and the methanogen surface resulting in their inactivation or removal. Therefore the vaccine's primary targets in the methanogens are surface proteins or proteins associated with membranes (Buddle et al., 2010). This strategy involves vaccination of animals to induce production of saliva antibodies that are released in the rumen to neutralize methanogen effects or reduce methane emissions.
Cook et al. (2008) utilized a passive immunization technique using chicken egg yolk as a quick, economical and non-invasive source of antibody production (IgY) from a bird vaccine prepared from integral cells of three ruminal methanogen strains. The authors observed that the addition of high levels of bird antibody (IgY) reduced methane production in cultured rumen liquid in vitro. However, these results were not permanent; this was attributed to either the possible instability of antibodies in rumen liquid or to the presence of methanogens not grown in a prepared bird vaccine and therefore unaffected by IgY antibodies.
A large amount of rumen methanogens cannot be cultivated in a laboratory (Wright et al., 2006); therefore, it is possible that these non-cultivable bacteria, of which there are no antibodies developed for, may grow to replace the methanogens that have developed antibodies (McAllister et al., 2008). Methanogen diversity in the rumen can be influenced by diet and geographic location (Wright et al., 2007). There is a challenge to develop a vaccine with a vast action spectrum against methanogens that can be effective in different conditions and regions.
Wright et al. (2004) evaluated sheep immunization using prepared integral cells of three methanogens and observed 7.7% reduction in methane emissions. However, when the study was repeated using five methanogens the vaccine did not promote immunization, although a change occurred in rumen microbial fauna (Williams et al., 2009). These results emphasize the difficulty in producing an effective vaccine that can reduce enteric methane emissions using prepared methanogen cells (Buddle et al., 2010).
The development of a recombinant vaccine against cell surface proteins existing in several species of methanogens can improve the effectiveness of vaccination as a method to mitigate enteric methane (McAllister et al., 2008). Buddle et al. (2010) proposed the development of vaccines against proteins essential to the growth of methanogens and/ or methanogenesis, with cross-reactions to other species through the genetic sequence information of M. ruminantium.
Bacteriophages and bacteriocins. Biological control strategies, such as the use of bacteriophages and bacteriocins, can be effective to directly inhibit Archaea methanogens and redirect H2 to reductive rumen bacteria that may be propiogenic or acetogenic (McAllister et al., 2008).
Bacteriophages are present in all biological ecosystems and have the ability to penetrate and consequently cause lysis in the host cell. This effect of bacteriophages and their genes can be a potential strategy to mitigate methane (Buddle et al., 2010). Only six Archaea bacteriophages have currently been genetically sequenced and described and only two are methanogenic bacteriophages: Methanobacterium phages psi M1 and M2, and Methanothermobacter phage psi M100 (Pfister et al., 1998; Luo et al., 2001). The quick adaptation of microorganisms to bacteriophages challenges the use of this strategy and as a result, bacteriophages have to be identified, sequenced and characterized (Buddle et al., 2010). Bacteriophages are host-specific, which is another limiting factor for using this strategy to reduce methane due to the high number of methanogen species in the rumen (Janssen and Kirs, 2008; McAllister et al., 2008).
Bacteriocins, bactericidal peptides produced by bacteria, could also be used (McAllister et al., 2008). However, there is scarce information on their effects on methanogenesis. Nisine, a bactoriocin produced by Lactococcus lactis, has been studied as a tool for mitigating methane. Sar et al. (2005) evaluated the effects of different concentrations of nisine on methane production in vitro in a continuous culture system. As its concentration increased from 5 to 30 μmol/L, methane production was reduced from 14 to 40%. Cattle HC5 bacteriocin, produced by Streptococcus bovis, inhibited in vitro methanogenesis up to 50% (Lee et al., 2002).
Identification of stable bacteriocins in the rumen environment and specific to methanogenic bacteria is an area for future research. In vivo studies are necessary to establish the lasting adaptability and effectiveness to use bacteriocins as a feed additive (Boadi et al., 2004; McAliister et al., 2008).
Pasture handling and crop livestock systems
The majority of enteric methane emissions in Brazil come from extensive cattle-farming systems (Lima, 2002) and grazing on degraded pastures. This scenario generates inefficient production processes that cause more methane production per unit of animal product (Guimares Jr. et al., 2010). Among the alternatives to mitigate greenhouse gas emissions from livestock enterprises is to use forage with higher nutritional value, associated with adequate pasture handling (DeRamus et al., 2003; Lassey, 2007).
Investing in recuperating degraded pasture is another potential strategy. According to a report from FAO (2006), pasture (native and cultivated) represents the second largest source of global potential carbon (C) capture, draining 1.7 billion tons per year from the atmosphere. This is second only to forest capture, which can drain 2 billion tons of C per year. Adequate pasture handling for improving soil fertility can help accumulate soil C by a ratio of 0.3 T of C/ha/year (IPCC, 2000) and mitigate 1.1 T of equivalent CO2/ ha/year. This would be enough to offset approximately 80% of the annual methane emission from one beef cattle unit estimated at 57 kg (IPCC, 1996), which is equivalent to 1.42 T of CO2 (57 kg x 25 CH4/ year global warming potential of the gas = 1.42 T of equivalent CO2). Productive and well-handled forage can therefore provide favourable conditions to significantly increase animal performance and absorb large amounts of carbon emitted from livestock, becoming an important component in the balance of greenhouse gases (Guimaraes Jr. et al., 2010).
Well-managed foraging areas can be important sites for carbon accumulation and support stocking rates of 1 to 3 animal units per ha. Recuperation of degraded areas is an option for improving animal production and to retain chemical and physical traits of the land, while it simultaneously increases carbon stock (Boddey et al., 2001).
Crop-livestock integration has been recognized as an alternative to reduce greenhouse gas emissions from agriculture. The Brazilian Government added the crop-livestock integration technology to the proposal presented at the 15th Conference of the Parties (COP 15) by the Intergovernmental Panel on Climate Change as a mitigation activity that can be applied nationally to reduce greenhouse gas emissions. The Government committed to implement this technology on 4 million hectares, expecting to reduce between 18 and 22 million T of equivalent CO2. It is therefore expected that the incentive to use this technology in Brazil in the coming years will grow through public development policies (Guimaraes Jr. et al., 2010).
Methodologies to evaluate enteric methane emissions
Before using mitigation strategies, it is necessary to have enteric methane emissions measured accurately to determine emissions from each management technique and to prepare national inventories.
Different techniques have been developed to quantify methane emissions. Validation and application in different production systems gives credibility to activities related to national inventories of greenhouse gas emissions from livestock and to develop public policies towards tending to global demands of reducing the environmental impacts of agriculture.
Methane emissions can be measured with in vivo and in vitro methodologies (McAllister, 2011). The use of experimental animals represents high costs. Consequently, in vitro methodologies are the primary option to evaluate methane reduction or inhibition. In vitro techniques are less costly and allow for rapid screening of diets and their combinations to evaluate the effects of a wide range of additives and feed ingredients on methanogenesis (Makkar and Vercoe, 2007). Diet additives and inhibitors able to reduce methane in vitro can later be evaluated in vivo with increased costs and details, addressing more practical feeding situations.
The in vivo reference method (gold standard) to quantify enteric methane production involves the use of respiratory chambers and gas collection (Rodriguez et al., 2007). McAllister (2011) indicated respiratory chambers are the reference method to compare methane-mitigation agents.
Respiratory chambers require costly investments and labor, restrict animal movement, and can only evaluate a limited number of animals. Descriptions about the conventional system of open flow respirometry can be found in studies by Yong et al. (1975), Bryant et al. (1977), McLean and Tobin (1987), and Miller and Koes (1988); modern systems are described by Grainger et al. (2007), Odongo et al. (2007), and Rodríguez et al. (2007).
Methane emissions can be measured by inserting indicators in the rumen, such as the sulphur hexafluoride (SF6) tracer gas methodology (Johnson et al., 1994), which has been adopted as a standard method for grazing animals.
Tracer-SF6 gas technique has been used to measure methane emissions in grazing animals (Johnson et al., 1994; Lassey et al., 1997; Woodward et al., 2006). A small permeation tube with SF6 of a known release rate is inserted in the rumen. Expired air is sampled through a stainless steel capillary tube (adapted to halter) connected to a vacuum yoke (built of highresistance PVC pipe), which is connected to a metal valve with a sampling septum and a quick coupling. The CH4 and SF6 concentrations are determined by gas chromatography. Methane flow emissions can be calculated from the release ratio of SF6 in the rumen and the concentration of CH4 and SF6 in the sample (Johnson and Johnson, 1995; USEPA, 2000). This technique does not require animals to be caged, allowing them to move and graze (Johnson et al., 2007).
Pinares-Patiño et al. (2011) reported that the tracer gas methodology presents larger variability when compared with respiratory chambers; therefore more animals will be necessary to detect differences among treatments.
At IPCC (2006), specific information from each country was reported; the models used to predict enteric methane emissions included data such as diet composition, enteric fermentation product composition, seasonality, characterization of animal population, feed quality and availability, and methane mitigation strategies. Enteric methane emission measurements are necessary to complete these documents. National and international inventories of greenhouse gases are based on mathematical models. Mechanistic models and regression models allow for the analysis of causes and variations in methane production (Ellis et al., 2008a). Multiple regression equations have been reported in the literature (Kriss, 1930; Axelsson, 1949; Blaxter and Clapperton, 1965; Moe and Tyrrel, 1979; Mills et al., 2003; Ellis et al., 2007; Ellis et al., 2008a). The optimal equation to predict methane production will depend upon which diet will be used and whether the equation considers the variates for each specific situation (Ellis et al., 2008a). Modeling has been applied to methane emission studies and is an important tool in developing greenhouse gas inventories and mitigation strategies.
Final considerations
Ruminant methane emissions are a consequence of gastrointestinal fermentation processes, which allow animals to transform cellulose-rich roughage into milk and meat. A survey of methane emission potential of agriculture systems and evaluation of mitigation strategies should be holistic, considering carbon dynamics and balance in the entire production system.
Several nutritional strategies have been studied and developed to mitigate enteric methane. They have different viability and cost. The choice of which one to adopt should be based on its capacity to reduce methane emissions associated with economical viability and animal performance.
Improving production parameters related to efficient forage-use and associated with good nutritional, health and reproductive management is an important strategy to consolidate tropical countries as food producers for the world, attending the demands related to land, water, biodiversity conservation, and greenhouse gases emissions.
Conflicts of interest
The authors declare they have no conflicts of interest with regard to the work presented in this report.
Notes
¤To cite this article: Ribeiro LG, Machado FS, Campos MM, Guimaraes R, Tomich TR, Reis LG, Coombs C. Enteric methane mitigation strategies in ruminants: a review. Rev Colomb Cienc Pecu 2015; 28:124-143.
References
Asanuma N, Iwamoto M, Hino T. Effect of the addition of fumarate on methane production by ruminal microorganisms in vitro. J Dairy Sci 1999; 82:780-787. [ Links ]
Attwood GT, Altermann E, Kelly WJ, Leahy SC, Zhang L, Morrison M. Exploring rumen methanogen genomes to identify targets for methane mitigation strategies. Anim Feed Sci Technol 2011; 166-167:6575. [ Links ]
Attwood GT, Kelly WJ, Altermann EH, Leahy SC. Analysis of the Methanobrevibacter ruminantium draft genome: understanding methanogen biology to inhibit their action in the rumen. Aust J Exp Agric 2008; 48:83-88. [ Links ]
Archimède H, Eugène M, Marie Magdeleine C, Boval M, Martin C, Morgavi DP, Lecomte P, Doreau M. Comparasion of methane production between C3 and C4 grasses and legumes. Anim Feed Sci Technol 2011; 166-167:59-64. [ Links ]
Axelsson J. The amount of produced methane energy in the European metabolic experiments with adult cattle. Ann R Agric Coll Sweden 1949; 16:404-419. [ Links ]
Bannink A, Kogut J, Dijkstra J, France J, Kebreab E, Van Vuuren AM, Tamminga S. Estimation of the stoichiometry of volatile fatty acid production in the rumen of lactating cows. J Theor Bio 2006; 238:36-51. [ Links ]
Barioni LG, Lima MA de, Zen S, Guimarães Júnior R, Ferreira AC. Abaseline projection of methane emissions by the Brazilian beef sector: preliminary results. In: Greenhouse Gases and Animal Agriculture conference. Christchurch, New Zealand: Proceedings; 2007. [ Links ]
Bell MJ, Wall E, Simm G, Russel G. Effects of genetic line and feeding system on methane from dairy systems. Anim Feed Sci Technol 2011; 166-167:699-707. [ Links ]
Benchaar C, Pomar C, Chiquette J. Evaluation of dietary strategies to reduce methane production in ruminants: a modelling approach. Can J Anim Sci 2001; 81:563-574. [ Links ]
Berchielli TT, Pedreira MS, Oliveira SG, Primavesi O, Lima M, Frigueto RTS. Determinação da produção de metano e pH ruminal em bovinos de corte alimentados com diferentes relações volumoso:concentrado. Reunião anual da sociedade brasileira de zootecnia, 40th [CD-ROM]. Santa Maria SBZ, 2003. [ Links ]
Beauchemin KA, Kreuzer M, O'Mara F, McAllister TA. Nutritional management for enteric methane abatement: a review. Aust J Exp Agric 2008; 48:21-27. [ Links ]
Beauchemin KA, McGinn SM. Methane emissions from feedlot cattle fed barley or corn diets. J Anim Sci 2005; 83:653-661. [ Links ]
Blaxter KL. Energy metabolism in animals and man. New York: Cambridge University Press; 1989. [ Links ]
Blaxter KL, Clapperton JL. Prediction of the amount of methane produced by ruminants. Br J Nutr 1965; 19:511-522. [ Links ]
Boadi D, Benchaar C, Chiquette J, Massé D. Mitigation strategies to reduce enteric methane emissions from dairy cows: update review. Can J Anim Sci 2004; 830:319-335. [ Links ]
Braun M, Schoberth S, Gottschalk G. Enumeration of bacteria forming acetate from H2 and CO2 in anaerobic habitats. Arch Microbiol 1979; 120(3):201-204. [ Links ]
Brossard L, Martin C, Chaucheyras-Durand F, Michalet-Doreau B. Protozoa at the origin of butyric and non-lactic latent acidosis in sheep. Reprod Nutr Dev 2004; 44:195-206. [ Links ]
Brouwer, E. Report of subcommittee on constants and factors. In: Blaxter KL, editor. Proceedings of the 3th Symposium on energy Metabolism Academic Press; 1965; Londom. 1965; p.441-443. [ Links ]
Buddle BM, Denis M, Attwood GT, Altermann E, Janssen PH, Ronimus RS, Pinares-Patiño CS, Muetzel S, Neil Wedlock D. Strategies to reduce methane emissions from farmed ruminants grazing on pasture. Vet J 2011; 188(1):11-7. [ Links ] doi:10.1016/j. tvjl.2010.02.019.
Busquet M, Calsamiglia S, Ferret A, Carro MD, Kamel C. Effect of garlic oil and four of its compounds on rumen microbial fermentation. J Dairy Sci 2005; 88:4393-4404. [ Links ]
Callaway TR, Martin SA, Wampler JL, Hill NS, Hill GM. Malate content of forage varieties commonly fed to cattle. J Dairy Sci 1997; 80:1651-1655. [ Links ]
Chaves AV, Thompson LC, Iwaasa AD. Effect of pasture type (alfalfa vs. grass) on methane and carbon dioxide production by yearling beef heifers. Can J Anim Sci 2006; 86:409-418. [ Links ]
Clarke RTJ. Protozoa in the rumen ecosystem. In: Clarke TTJ, Bauchop T Editors. Microbial ecology of the gut. New York: Academic Press; 1977. p.251-275. [ Links ]
Cook SR, Maiti PK, Chaves AV, Benchaar C, Beauchemin KA, McAllister TA. Avian (IgY) anti-methanogen antibodies for reducing ruminal methane production: in vitro assessment of their effects. Aust J Exp Agric 2008; 48(1-2):260-264. doi:10.1071/ EA07249 [ Links ]
Cord-Ruwisch R, Sietz H J, Conrad R. The capacity of hydrogenotrophic anaerobic bacteria to compete for traces of hydrogen depends on the redox potential of terminal electron acceptor. Arch Microbiol 1988; 149:350-357. [ Links ]
Cosgrove GP, Waghorn GC, Anderson CB, Peters JS, Smith A, Molano G, Deighton M. The effect of oils fed to sheep on methane production and digestion of ryegrass pasture. Aust J Exp Agric 2008; 48:189-192. [ Links ]
Cotton WR, Pielke RA. Human impacts on weather and climate. Cambridge: Cambridge University; 1995. p.288. [ Links ]
Czerkawski JW. Methane production in ruminants and its significance. World Rev Nutr Diet 1969; 11:240-282. [ Links ]
Czerkawski JW. Degradation of solid feeds in the rumen: spatial distribution of microbial activities and its consequences. In: Milligan LP, Grovum WL, Dobson A, editors. Control of digestion and metabolism in ruminants. Englewood Cliffs (NJ): Prentice Hall; 1986. p. 158-172. [ Links ]
Dehority BA. Effect of pH on viability of Entodinium caudatum, Entodinium exiguum, Epidinium caudatum, and Ophryoscolex purkynjei in vitro. J Eukaryot Microbiol 2005; 52:339-342. [ Links ]
DeRamus HA, Clement TC, Giampola DD, Dickson PC. Methane emissions of beef cattle on forrages: efficiency of grazing management systems. J Environ Qual 2003; 32:269-277. [ Links ]
Denman SE, Tomkins NW, McSweeney CS. Quantitative and diversity analysis of ruminal methanogenic populations in response to antimethanogenic compound bromochloromethane. FEMS Microbiol Ecol 2007; 62:313-322. [ Links ]
Dohme F, Machmüller A, Wasserfallen A, Kreuzer M. Ruminal methanogenesis as influenced by individual fatty acids supplemented to complete ruminant diets. Lett Appl Microbiol 2001; 32:47-51. [ Links ]
Doreau M, Ferlay A. Effect of dietary lipids on nitrogen metabolism in the rumen: a review. Livest Prod Sci 1995; 43:97-110. [ Links ]
Eckard RJ, Grainger C, Klein CAM. Options for the abatement of methane and nitrous oxide from ruminant production: a review. Livest Sci 2010; 130:47-56. [ Links ]
Ellis JL, Kebreab E, Odongo NE, McBride BW, Okine EK, France J. Prediction of methane production from dairy and beef cattle. J Dairy Sci 2007; 90:3456-3466. [ Links ]
Ellis JL, Dijkstra J, Kebreab E, Bannink A, Odongo NE, McBride BW, France J. Aspects of rumen microbiology central to mechanistic modeling of methane production in cattle. J Agric Sci Camb 2008; 146:212-233. [ Links ]
Estados Unidos. Enviromental Protection Agency. Greenhouse gas emissions from agricultural systems. In: Workshop on Greenhouse Gas Emissions from Agriculture, 1989 Washington. Proceedings. Washington: United States Enviromental Protection Agency; 1990. v.1, p.VII-3-VII-22. Summary report. [ Links ]
Eun JS, Fellner V, Gumpertz ML. Methane production by mixed ruminal cultures incubated in dual-flow fermentors. J Dairy Sci 2004; 87:112-121. [ Links ]
FAO - Food and Agriculture Organization of the United Nations. FAO statistical databases 2006; [Access date: June 4, 2010] URL: http://faostat.fao.org [ Links ]
FAO - Food and Agriculture Organization of the United Nations. The State of Food and Agriculture. Livestock in the balance, Roma: FAO; 2009. p.166; [Access date: February 5, 2010] URL: http://www.fao.org/docrep/012/i0680e/i0680e.pdf. [ Links ]
Genthner BRS, Davis CL, Bryant MP. Features of rumen and sludge strains of Eubacterium limosum, a methanol and H2- CO2-utilising species. Appl Environ Microbiol 1981; 42:12-19. [ Links ]
Goel G, Makkar HPS, Becker K. Inhibition of microbial methanogens by bromochloromethane: effects on microbial communities and rumen fermentation using batch and continuous fermentations. Br J Nutr 2009; 101:1484-1492. [ Links ]
Grainger C, Clarke T, McGinn SM, Auldist MJ, Beauchemin KA, Hannah MC, Waghorn GC, Clark H, Eckard RJ. Methane emissions from dairy cows measured using the sulfur hexafluoride (SF6) tracer and chamber techniques. J Dairy Sci 2007; 90:27455-2766. [ Links ]
Grainger C, Williams R, Eckard RJ, Hannah MC. A high dose of monensina does not reduce methane emissions of dairy cows offered pasture supplemented with grain. J Dairy Sci 2010a; 93:5300-5308. [ Links ]
Grainger C, Williams R, Clarke T, Wright A-DG, Eckard RJ. Supplementation with whole cottonseed causes long-term reduction of methane emissions from lactating dairy cows offered a forage and cereal grain diet. J Dairy Sci 2010b; 93:2612-2619 [ Links ]
Guan H, Wittenberg KM, Ominski KH, Krause DO. Efficacy of ionophores in cattle diets for mitigation of enteric methane. J Anim Sci 2006; 84:1896-1906. doi: 10.2527/jas.2005-652 [ Links ]
Guimarães Júnior R, Marchao RL, Vilela L, Pereira LGR. Produção animal na integração lavoura-pecuária. In: 5th Simpósio Mineiro de Nutrição de Gado de Leite; Belo Horizonte 2010. Belo Horizonte: UFMG, 2010. p.111-123. [ Links ]
Guo YQ, Liu JX, Lu Y, Zhu WY, Denman SE, McSweeney CS. Effect of tea saponin on methanogenesis, microbial community structure and expression of mcrA gene, in cultures of rumen microorganisms. Lett Appl Microbiol 2008; 47:421-426. [ Links ]
Harper LA, Denmead OT, Freney JR, Byers FM. Direct measurements of methane emissions from grazing and feedlot cattle. J Anim Sci 1999; 77:1392-1401. [ Links ]
Hegarty R. Greenhouse gas emissions from the Australian livestock sector what do we know, what can we do? Canberra (NSW): Agriculture Australian Greenhouse Office; 2001. p.35. [ Links ]
Hegarty RS, Bird SH, Vanselow BA, Woodgate R. Effects of the absence of protozoa from birth or form weaning on the growth of microorganisms. Biotechnol Bioeng 2008; 39:833-858. [ Links ]
Hess H D, Beuret RA, Lotscher M, Hindrichsen IK, Machmuller A, Carulla JE, Lascano CE, Kreuzer M. Ruminal fermentation, methanogenesis and nitrogen utilization of sheep receiving tropical grass hay-concentrate diets offered with Sapindus saponaria fruits and Cratylia argentea foliage. Animal Science 2004; 79:177-189. [ Links ]
Holter JB, Young AJ. Methane prediction in dry and lactating Holstein cows. J Dairy Sci 1992; 75:2165-2175. [ Links ]
Hungate RE, Smith W, Bauchop T, Yu I, Rabinowitz JC. Formate as an intermediate in the rumen fermentation. J Bacteriol 1970; 102:384-397. [ Links ]
IBGE - Instituto Brasileiro de Geografia e Estatística. Censo Agropecuário 2006. Brasil, Grandes Regiões e Unidades da Federação. Rio de Janeiro: IBGE; 2009. 777p; [Access date: February 2010] URL: http://www.ibge.gov.br/home/default.php [ Links ]
IPCC - Intergovernamental Panel on Climate Change. Climate change 1994: radiative forcing of climate change and an evaluation of the IPCC IS92 emission scenarios. Cambridge: University Press; 1995. 339p. [ Links ]
IPCC - Intergovernamental Panel on Climate Change. Emissions from livestock and manure management.In: Eggleston HS, Buendia L, Miwa K, Ngara T, Tabane K, editors. IPCC Guideliness for nacional greenhouse gas inventories. Hayama: IGES; 2006. p.747-846. [ Links ]
IPCC - Intergovernmental Panel on Climate Change. Fourth Assessment Report (AR4): Mitigation of Climate Change. Cambridge, United Kingdom and New York, USA; 2007; [Access date: November 30, 2010] URL: http://www.ipcc.ch/publications_and_data/ar4/wg3/en/contents.html [ Links ]
Janssen PH, Kirs M. Structure of the archaeal community of therumen. Appl Environ Microbiol 2008; 74:3619-3625. [ Links ]
Joblin KN. Ruminal acetogens and their potential to lower ruminantmethane emissions. Aust J Agric Res 1999; 50(8):1321-1327. [ Links ]
Johnson KA, Kincaid RL, Westberg HH, Gaskins CT, LambBK, Cronrath JD. The effect of oilseeds in diets of lactating cowson milk production and methane emissions. J Dairy Sci 2002a;85:1509-1515. [ Links ]
Johnson DE, Phetteplace HW, Seidl AF. Methane, nitrous oxideand carbon dioxide emissions from ruminant livestock productionsystems. In: Takahashi J, Young BA, editors. Greenhouse gasesand animal agriculture. Amsterdam, The Netherlands: Elsevier;2002b. p.77-85. [ Links ]
Johnson KA, Huyler MT, Westberg HH, Lamb BK, Zimmerman P. Measurement of methane emissions from ruminant livestock usinga SF6 tracer technique. Environ Sci Technol 1994; 28:359-362 [ Links ]
Johnson KA, Johnson DE. Methane emissions from cattle. J AnimSci 1995; 73:2483-2492. [ Links ]
Johnson DE, Ward GM. Estimates of animal methane emissions. Environ Monit Assess 1996; 42:133-141. [ Links ]
Jordan E, Lovett DK, Monahan FH, Callan J, Flynn B, O'MaraFP. Effect of refined coconut oil or copra meal on methane output, and on intake and performance of beef heifers. J Anim Sci 2006;84:162-170. [ Links ]
Kaharabata SK, Schuepp P, Desjardins RL. Estimating methaneemissions from dairy cattle housed in a barn and feedlot using anatmospheric tracer. Environ Sci Technol 2000; 34(15):3296-3302. [ Links ]
Klieve AV, Joblin K. Comparison in hydrogen utilisation of ruminal and marsupial reductive acetogens. In: Kennedy R, editor. 5 year research progress report 2002-2007. Wellington, NewZealand: The Pastoral Greenhouse Gas Research Consortium;2007. p.34-35. [ Links ]
Kriss M. Quantitative relations of the dry matter of the food consumed the heat production, the gaseous outgo, and insensibleloss in body weight of cattle. J Agric Res 1930; 40:283-295. [ Links ]
Lassey KR. Livestock methane emission: from the individual grazing animal through national inventories to the global methanecycle. Agric For Meteorol 2007; 142:120-132. [ Links ]
Lassey KR, Ulyatt MJ, Martin RJ, Walker CF, Shelton ID. Methane emissions measured directly from grazing livestock in New Zealand. Atmos Environ 1997; 31:2905-2914. [ Links ]
Lima MA. Agropecuária brasileira e as mudanças climáticasglobais: caracterização do problema, oportunidades e desafios. Caderno de Ciência & Tecnologia 2002; 19:451-472. [ Links ]
Lima MA, Pessoa MCPY, Ligo MAV. Primeiro inventáriobrasileiro de emissões antrópicas de gases de efeito estufa. In: Relatórios de referência: Emissões de metano da pecuária. Brasília: Ministério da Ciência e Tecnologia; 2006. [ Links ]
Lovett DK, Shalloo L, Dillon P, O'Mara FP. A system approachto quantify greenhouse gas fluxes from pastoral dairy productionas affected by management regime. Agr Syst 2006; 88:156-179. [ Links ]
Lovett DK, Lovell S, Stack L, Callan J, Finlay M, Conolly J,O'Mara FP. Effect of forage/concentrate ratio and dietary coconut oil level on methane output and performance of finishing beefheifers. Livest Sci 2003; 84:135-146. [ Links ]
Machado FS, Pereira LGR, Guimarães Júnior R, Lopes FCF, Chaves AV, Campos MM, Morenz MJF. In: Embrapa Serie Documentos 147. Emissões de metano na pecuária: conceitos, métodos de avaliação e estratégias de mitigação. Juiz de Fora (MG): Embrapa Gado de Leite; 2011. [ Links ]
Mackie RI, Bryant M P. Acetogenesis and the rumen: syntrophicrelationships. In: Drake HL, editor. Acetogenesis. New York: Chapman and Hall; 1994. p.331-364. [ Links ]
Machmuller A, Kreuzer M. Methane suppression by coconut oiland associated effects on nutrient and energy balance in sheep. Can J Anim Sci 1999; 79:65-72. [ Links ]
Machmuller A, Soliva CR, Kreuzer M. Methane-suppressing effect of myristic acid in sheep as affected by dietary calciumand forage proportion. Br J Nutr 2003; 90:529-540. [ Links ]
Maia MRG, Chaudhary LC, Figueres L, Wallace RJ. Metabolismof polyunsaturated fatty acids and their toxicity to the microflora of the rumen. Antonie Van Leeuwenhoek 2007; 91:303-314. [ Links ]
Martin SA. Manipulation of ruminal fermentation with organicacids: a review. J Anim Sci 1998; 76:3123-3132. [ Links ]
Martin C, Rouel J, Jouany JP, Doreau M, Chilliard Y. Methane output and diet digestibility in response to feeding dairy cows crude linseed, extruded linseed, or linseed oil. J Anim Sci 2008; 86:2642-2650. [ Links ]
Martin C, Morgavi DP, Doreau M. Methane mitigation inruminants: from microbes to the farm scale. Animal 2009a; 4(3):351-365. [ Links ]
Martin C, Ferlay A, Chilliard Y, Doreau M. Decrease in methaneemissions in dairy cows with increase in dietary linseed content. In: British Society of Animal Science; 30 March-1 April 2009. Southport, UK; 2009b. p.21. [ Links ]
Martin SA, Park CM. Effect of extracellular hydrogen onorganic acid utilization by the ruminal bacterium Selenomonasruminantium. Curr Microbiol 1996; 32:327-331. [ Links ]
McAllister TA, Bae HD, Jone GA, Cheng KJ. Microbial attachment and feed digestion in the rumen. J Anim Sci 1994;72:3004-3018. [ Links ]
McAllister TA, Newbold CJ. Redirecting rumen methane to reduce methanogenesis. Aust J Exp Agric 2008; 48:7-13. [ Links ]
McAllister TA. Greenhouse gases in animal agriculture - Finding a balance between food production and emissions. Anim Feed Sci Tech 2011; 166-167:1-6. [ Links ]
McAllister TA, Okine EK, Mathison GW, Cheng KJ. Dietary, environmental and microbiological aspects of methane production in ruminants. Can J Anim Sci 1996; 76:231-243. [ Links ]
McCaughey WP, Wittenberg K, Corrigan D. Impact of pasturetype on methane production by lactating beef cows. Can J Anim Sci 1999, 79:221-226. [ Links ]
McGinn SM, Beauchemin KA, Coates T, Colombatto D. Methaneemissions from beef cattle: effect of monensin, sunflower oil,enzymes, yeast, and fumaric acid. J Anim Sci 2004; 82:3346-3356. [ Links ]
MCT - Ministério da Ciência e Tecnologia. Inventário Brasileiro das Emissões e Remoções Antrópicas de Gases de Efeito Estufa.Informações Gerais e Valores Preliminares, 2009; [Access date:February 18, 2010] URL: http://www.mct.gov.br [ Links ]
Mills JAN, Kebreab E, Yates CM, Crompton LA, Cammell SB, Dhanoa MS, Agnew RE, France J. Alternatives approaches top redicting methane emissions from dairy cows. J Anim Sci 2003;81:3141-3150. [ Links ]
Moe PW, Tyrrel HF. Methane production in dairy cows. J Dairy Sci 1979; 62:1583-1586. [ Links ]
Morais JAS, Berchielli TT, Reis RA. Aditivos. In: Berchielli TT, Pires AV, Oliveira SG, editors. Nutrição de ruminantes. Jaboticabal: FUNEP; 2006. p.111-140. [ Links ]
Morvan B, Doré J, Rieu-Lesme F, Foucat L, Fonty G, Gouet P. Establishment of hydrogen-utilizing bacteria in the rumen of thenew born lamb. FEMS Microbiol Lett 1994; 117:249-256. [ Links ]
Murray RM, Bryant AM, Leng RA. Rates of production ofmethane in the rumen and large intestines of sheep. Br J Nutr1976; 36:1-14. [ Links ]
Nagaraja TG, Newbold CJ, Van Nevel CJ, Demeyer DI. Manipulation of ruminal fermentation. In: Hobson PN, StewartCS, editors. The rumen microbial ecosystem. London: Blackie Academic & Professional; 1997. p.523-632. [ Links ]
Odongo NE, Bagg R, Vessie G, Dick P, Or-Rashid MM, Hook SE, Gray JT, Kebreak E, France J, McBride BW. Long-term effects of feeding monensin on methane production in lactating dairy cows. J Dairy Sci 2007; 90:1781-1788. [ Links ]
Oliveira SG, Berchielli TT, Pedreira MS, Primavesi O, Frighettoe R, Lima MA. Effect of tannin levels in sorghum silage and concentrate supplementation on apparent digestibility and methane emission in beef catle. Anim Feed Sci Tech 2006; 135(2007):236-248. [ Links ]
O'Mara F. Greenhouse gas production from dairying: reducing methane production. Adv Dairy Technol 2004; 16:295-309. [ Links ]
Ozmen OF, Sahinduran S, Unsal A. Pathological and toxicological investigations of chromic nitrate poisoning in cattle. Toxicol Envoron Chem 2005; 87:99-106. [ Links ]
Owens FN, Goetsch AL. Ruminal fermentation. In: Church DC, editor. The ruminant animal: digestive physiology and nutrition.Waveland: Press; 1988. p.145-171. [ Links ]
Pinares-Patiño CS, Lassey KR, Martin RJ, Molano G, Fernandez M, MacLean S, Sandoval E, Luo D, Clark H. Assessment of thesulphur hexafluoride (SF6) tracer technique using respiration chambers for estimation of methane emissions from sheep. Anim Feed Sci and Tech 2011; 166-167:201-209. [ Links ]
Pressman BC. Ionophorus antibiotics as model for biological transport. Fed Proc 1976; 27:1283-1288. [ Links ]
Primavesi O, Pedreira MS, Frighetto RST, Lima MA, Berchielli TT, Oliveira SG, Rodrigues AA, Barbosa PF. Manejo alimentar de bovinos leiteiros e sua relação com produção de metano ruminal. In: Embrapa Pecuária Sudeste. Circular Técnica 39. São Carlos: Embrapa Pecuária Sudeste; 2004. [ Links ]
Robertson LJ, Waghorn GC. Dairy industry perspectives on methane emissions and production from cattle fed pasture or totalmixed rations in New Zealand. Proceedings of the New Zealand Society Animal Production 2002; 62:213-218. [ Links ]
Rodriguez NM, Campos WE. Manipulação ruminal para redução da emissão de metano. In: 1th Simpósio Nacional sobre Produção Animal e Ambiente; Belo Horizonte 2007. Anais Belo Horizonte: Universidade Federal de Minas Gerais, 2007, p.1-28. [ Links ]
Sauer FD, Fellner V, Kinsman R, Kramer JKG, Jackson HA, Lee AJ, Chen S. Methane output and lactation response in Holstein cattle with monensina or unsaturated fat added to the diet. J Anim Sci 1998; 76:906-914. [ Links ]
Smedman A, Mânsson-Lindmark H, Drewnowski A, Edman AM. Nutrient density of beverages in relation to climate impact. Food Nutr Res 2010; 54:5170-577. [ Links ]
Soliva CR, Meile L, Cieslak A, Kreuzer M, Machmuller A. Rumen simulation technique study on the interactions of dietary lauric and myristic acid supplementation in suppressing ruminal methanogenesis. Br J Nutr 2004; 92: 689-700. [ Links ]
Stumm CK, Gijzen HJ, Vogels GD. Association of methanogenic bacteria with ovine rumen ciliates. Br J nutr 1982; 47:95-99. [ Links ] Thorpe A. Enteric fermentation and ruminant eructation: the role (and control?) of methane in the climate change debate. Climatic change 2009; 93:407-431. [ Links ]
Thornton PK. Livestock production: recent trends, future prospects. Philos Trans R Soc Lond B Biol Sci 2010; 365:2853-2867. [ Links ]
Ulyatt MJ, Lassey KR, Shelton ID, Walker CF. Methane emission from dairy cows and wether sheep fed subtropical grass-dominant pastures in midsummer in New Zealand. New Zeal J Agr Res 2002; 45:227-234. [ Links ]
Ungerfeld EM, Kohn RA. The role of thermodynamics in the control of ruminal fermentation. In: Sejrsen K, Hvelplund T, Nielsen MO, editors. Ruminant physiology. digestion, metabolism and impact of nutrition on gene expression, immunology and stress. Wageningen: Wageningen Academic Publishers; 2006.p.55-85. [ Links ]
USEPA. United States Environmental Protection Agency. Evaluating Ruminant Livestock Efficiency Projects and Programs In: Peer Review Draft. Washington: DC; 2000. 48p. [ Links ]
USDA. United States Department of Agriculture. Foreign Agricultural Service (FAS). Market and Trade Data: trade reports archives. [Access date: February 18, 2010] URL: http://www.fas.usda.gov/livestock_arc.asp [ Links ]
Van der Honing Y, Tamminga S, Wieman BJ, Steg A, vanDonselaar B, Van Gils LGM. Further studies on the effect of fat supplementation of concentrates fed to lactating cows, 2: total digestion and energy utilization. Neth J Agr Sci 1983; 31:27-36. [ Links ]
Van Vugt SJ, Waghorn GC, Clark DA, Woodward SL. Impact of monensin on methane production and performance of cows fed forage diets. Proceedings of the New Zealand Society of Animal Production 2005; 65:362-366. [ Links ]
Van Zijderveld SM, Gerrits WJJ, Apajalahti JA, Newbold JR, Dijkstra J, Leng RA, Perdok HB. Nitrate and sulfate: Effective alternative hydrogen sinks for mitigation of ruminal methane production in sheep. J Dairy Sci 2010; 93:5856-5866. [ Links ]
Wallace RJ, Czerkawski JW, Breckenridge G. Effect of monensin on the fermentation of basal rations in the simulation technique (Rusitec). Br J Nutr 2002; 114:101. [ Links ]
Waghorn GC. Beneficial and detrimental effects of dietary condensed tannins for sustainable sheep and goat production: progress and challenges. Anim Feed Sci Techn 2007; 147:116-139. [ Links ]
Watanabe Y, Suzuki R, Koike S, Nagashima K, Mochizuki M, Forster RJ, Kobayashi Y. In vitro evaluation of cashew nut shell liquid as a methane-inhibiting and propionate-enhancing agent of ruminants. J Dairy Sci 2010; 93:5258-5267. [ Links ]
Weimer PJ. Manipulating ruminal fermentation: a microbial ecological perspective. J Anim Sci 1998; 76:3114-3122. [ Links ]
Williams YJ, Popovski S, Rea SM, Skillman LC, Toovey AF, Northwood KS, Wright A-DG. A vaccine against rumen methanogens can alter the composition of Archaea populations. Appl Environ Microbiol 2009, 75:1860-1866. [ Links ]
Wims CM, Deighton MH, Lewis E. Effect of pregrazing herba gemass on methane production, dry matter intake and milk production of grazing dairy cows during the mid-season period. J Dairy Sci 2010; 93:4976-4985. [ Links ]
Wolin MJ. The rumen fermentation: a model for microbial interactions in anaerobic ecosystems. Adv Microb Ecol 1979; 3:49-77. [ Links ]
Woodward, S. L. Waghorn GC, Ulyatt MJ, Lassey KR. Early indications that feeding Lotus will reduce methane emissions from ruminants. Proceedings of New Zealand Society of Animal Production 2001; 61:23-26. [ Links ]
Woodward SL, Waghorn GC, Thomson NA. Supplementing dairy cows with oils to improve performance and reduce methane -does it work? Proceedings of the New Zealand Society of Animal Production 2006; 66:176-181. [ Links ]
Wright A-DG, Kennedy P, O'Neill C, Toovey AF, Popovski S, Rea SM, Pimm CL, Klein L. Reducing methane emissions in sheep by immunization against rumen methanogens. Vaccine 2004; 22:3976-3985. [ Links ]
Wright A-DG, Toovey AF, Pimm CL. Molecular identification of methanogenic archaea from sheep in Queensland, Australia reveal more uncultured novel Archaea. Anaerobe 2006;12:134-139. [ Links ]
Wright A-DG, Auckland CH, Lynn DH. Molecular diversity of methanogens in feedlot cattle from two Ontario and Prince Edward Island. Appl Environ Microbiol 2007; 73:4206-4210. [ Links ]
Wuebbles DJ, Hayhoe K. Atmospheric methane and globalchange. Earth-Sci Rev 2002; 57:177-210. [ Links ]
Yan T, Mayne CS, Gordon FG. Mitigation of enteric methane emissions through improving efficiency of energy utilization and productivity in lactating dairy cows. J Dairy Sci 2010;93:2630-2638. [ Links ]