Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Colombiana de Ciencias Pecuarias
Print version ISSN 0120-0690
Rev Colom Cienc Pecua vol.28 no.2 Medellín Apr./June 2015
https://doi.org/10.17533/udea.rccp.v28n2a03
LITERATURE REVIEW
doi: 10.17533/udea.rccp.v28n2a03
Gastroesophageal reflux in anesthetized dogs: a review¤
Reflujo gastroesofágico en perros anestesiados: revisión de literatura
Refluxo gastroesofágico em cães anestesiados: revisão de literatura
Carlos A Rodríguez-Alarcón1, DVM, PhD; Diana M Beristain-Ruiz1*, DVM, PhD; Ramón Rivera-Barreno1, DVM, MSc, PhD; Guadalupe Díaz2, DVM; Jesús M Usón-Casaús3, DVM, PhD; Ricardo García-Herrera4, DVM, PhD; Eva M Pérez-Merino3, DVM, PhD.
1 Cuerpo Académico de Medicina y Cirugía Veterinaria, Departamento de Ciencias Veterinarias, Universidad Autónoma de Ciudad Juárez, Anillo Envolvente del PRONAF y Estocolmo S/N. Ciudad Juárez, Chihuahua, México.
2Hospital Veterinario Universitario, Universidad Autónoma de Ciudad Juárez, Anillo Envolvente del PRONAF y Estocolmo S/N. Ciudad Juárez, Chihuahua, México
3Hospital Clínico Veterinario, Facultad de Veterinaria, Universidad de Extremadura, Av. Universidad S/N. Cáceres, España.
4División Académica de Ciencias Agropecuarias, Universidad Juárez Autónoma de Tabasco. Zona de la Cultura S/N, Villahermosa, Tabasco, México.
*Corresponding Author: Diana M Beristain Ruiz. Departamento de Ciencias Veterinarias, Universidad Autónoma de Ciudad Juárez, Chihuahua, México. Email: diana.beristain@uacj.mx
Received: March 21, 2013; accepted: July 22, 2014
Summary
Gastroesophageal reflux can be a catastrophic illness in small animals during anesthesia since its complications can cause serious pathologies, such as esophagitis, esophageal stenosis and aspiration pneumonia. With an incidence from 12 to 78.5% in anesthetized dogs, gastroesophageal reflux is normally silent during anesthesia and will be notices only if regurgitation occurs and stomach acid is present in the nasal or oral cavities. In humans, gastroesophageal reflux disease (GERD) is a well-defined pathology where the lower esophageal sphincter has a sustained weakness. However, in dogs, gastroesophageal reflux disease as such is not well established, if at all; it has only been described as gastroesophagic reflux, occurring principally in anesthetized animals. There are several factors influencing the presentation of reflux in anesthetized dogs, which may be inherent to the patient (e.g. age, sex, breed, weight, or body condition), medications used prior to and during anesthesia, type of surgery or position of the animal during surgery. The objective of this review is to discuss a series of conditions that could predispose dogs to gastroesophageal reflux during anesthesia and to assist in the prevention and diagnosis of this condition.
Keywords: anesthesia, aspiration pneumonia, canine, esophagitis.
Resumen
El reflujo gastroesofágico durante la anestesia puede ser una entidad catastrófica en la clínica de pequeñas especies, ya que sus complicaciones derivan en entidades realmente graves como esofagitis, estenosis esofágica y neumonía por aspiración. Con una incidencia del 12 al 78.5% en perros anestesiados, el reflujo gastroesofágico durante la anestesia es generalmente silencioso y sólo se observa cuando existe regurgitación y el reflujo pasa a cavidad oral o nasal. En el humano, la enfermedad por reflujo gastroesofágico (ERGE) es una patología bien definida, donde el esfínter esofágico inferior presenta una debilidad sostenida. Sin embargo, en el perro esta enfermedad como tal no está bien establecida, si acaso se describe el reflujo gastroesofágico, que ocurre principalmente en animales anestesiados. Existen diversos factores que influyen en la presentación del reflujo en los perros anestesiados. Estos pueden ser inherentes al paciente (por ejemplo: edad, sexo, raza, peso o condición corporal), a medicamentos utilizados previamente y durante la anestesia, al tipo de cirugía o a la posición del animal durante la cirugía. El objetivo de esta revisión es discutir una guía de las condiciones que predisponen a la aparición de reflujo gastroesofágico durante la anestesia en perros con el fin de facilitar el diagnóstico y la prevención de esta condición.
Palabras clave: anestesia, canino, esofagitis, neumonía por aspiración.
Resumo
O refluxo gastroesofágico durante a anestesia pode ser uma entidade catastrófica na clínica de pequenas espécies, já que suas complicações resultam em entidades realmente graves, como esofagite, estenose esofágica e pneumonia por aspiração. O refluxo gastroesofágico em cães anestesiados é geralmente silencioso, com uma incidência de 12 até 78.5% e só é observada quando há regurgitação e o refluxo passa até a cavidade oral ou nasal. Nos humanos, a doença pelo refluxo gastroesofágico (ERGE) é uma patologia bem definida, onde o esfíncter esofágico inferior apresenta uma debilidade continua. Porém, esta doença em cães não está bem estabelecida, pelo qual só se descreve o refluxo gastroesofágico, que ocorre principalmente em animais anestesiados. Existem diversos fatores que influenciam na apresentação do refluxo em cães anestesiados. Estes podem ser inerentes ao paciente (por exemplo: idade, sexo, raça, peso ou condição corporal), a medicamentos utilizados previamente e durante a anestesia, ao tipo de cirurgia ou a posição do animal durante a cirurgia. O objetivo da revisão foi discutir uma guia das condições que predispõem à aparição de refluxo gastroesofágico durante a anestesia em cães com o fim de facilitar o diagnóstico e a prevenção dessa condição.
Palavras chave: anestesia, cão, esofagite, pneumonia por aspiração.
Gastroesophageal reflux in dogs
In dogs, esophagitis can be caused by a variety of reasons, such as ingestion of caustic substances (including prescribed medications), chronic vomiting, muscular diseases, improper placement of feeding tubes, tumors, or gastroesophagic reflux (GER; Willard, 2004). Hiatal hernias cause GER in some breeds, such the Shar-Pei, however, anesthesia is the most common, worrisome and probably thoroughly studied cause of GER in dogs (Willard, 2004; Glazer and Walter, 2008). Most of the drugs used during anesthesia, such as pre-anesthetics (sedatives or tranquilizers) or anesthetics have the ability to relax the lower esophageal sphincter (LES), allowing the passage of gastric contents into the esophagus (Wilson et al., 2005; Wilson et al., 2006; Wilson et al., 2007).
In animals, primary gastroesophageal reflux disease (GERD) has been reported in cats (Han et al., 2003); however, it is not well established in dogs. Some of the characteristics of the etiology, pathophysiology, and treatments of GERD in humans can be applied to GER during anesthesia in dogs. The anatomy, defense mechanisms and relaxation factors of the LES serve as a basis to the understanding of GER during anesthesia, which is why we will discuss some aspects of human GERD in this review (Jergens, 2004; Vlasin et al., 2004).
The LES plays an important role in the anatomy of the digestive tract, preventing gastroesophageal reflux. There are various correlated factors that allow the LES to work efficiently as an anti-reflux barrier (Miller et al., 2009; Hyun and Bak, 2011); a) muscle layers around the esophagus and diaphragmatic crura; b) the acute angle formed at the union between the esophagus and the cardia of the stomach (a flap valve); c) the closure produced by the esophageal mucosal folds when the esophagus is collapsed; and d) the intra-abdominal portion of the esophagus.
Any alteration in these mechanisms will cause reflux in dogs, subsequently producing damage in the esophageal mucosa and causing esophagitis. The damage to the esophageal mucosa can be attributed to the prolonged contact with gastric acid, pepsin, bile salts, and trypsin. Chronic esophagitis can cause esophageal stenosis due to damage of the submucosa and muscular layers, which form an intraluminal fibrosis that causes scarring (Gualtieri, 2005). In general, it is assumed that GER precedes esophagitis, though other factors must be considered since not all dogs with GER will necessarily develop esophagitis (Wilson and Walshaw, 2004). The factors that determine the development of esophagitis after an episode of GER in small animals are similar to those found in humans: a) incompetence of the antireflux system; b) acidity and type of enzymes present in the gastric content in contact with the mucosa; c) self cleaning ability of the esophagus; and d) resistance of the esophageal mucosa. However, such factors studied in humans are not well defined in domestic animals (Pratschke et al., 1998). The pH of the gastric content, along with the length of time that it was in contact with the mucosa can be a triggering factor for esophagitis. In dogs, it has been demonstrated that a pH of less than 2.5 sustained for 20 minutes or more is capable of producing severe damage to the esophageal mucosa (Wilson and Walshaw, 2004).
Gastroesophageal reflux during anesthesia
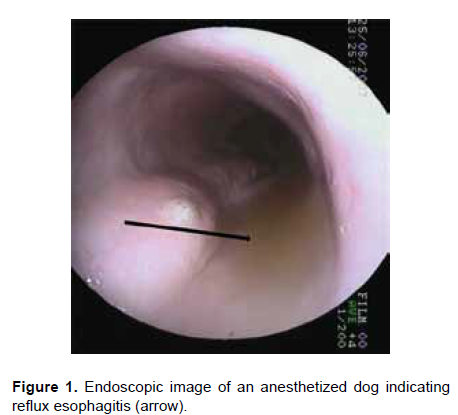
During anesthesia, GER (Figure 1) occurs when the reflux pH falls below 4.0 (gastric acid reflux) or increases above 7.5 (bile reflux) for 30 seconds or more (Wilson et al., 2005). GER during anesthesia has been correlated with various factors, including the type of surgery, patient position and the pre-anesthetic and anesthetic drugs administered. There have also been studies regarding how to prevent GER during anesthesia in small animals (Strombeck and Harrold, 1985; Roush et al., 1990; Galatos and Raptopoulos, 1995a, 1995b; Raptopoulus and Galatos, 1997; Chacon, 1998; Raptopoulus and Savvas, 2004; Rodríguez, 2010). Also, during anesthesia there is a reduction of peristalsis and a lack of saliva that neutralizes acid pH, contributing to the development of esophagitis (Jergens, 2004).
It is possible that animals presenting GER during anesthesia had an existing problem of the cardia, predisposing them to reflux, especially with the use of medications that relax the LES (Vlasin et al., 2004). In order for GER to occur in dogs, three defense components must fail: a) an external mechanism formed by the diaphragmatic crura that wraps like a sling around the abdominal esophagus augments sphincteric pressure during inspiration; b) an internal mechanism constituted by the intrinsic muscle of the distal portion of the esophageal wall; and c) the circular muscle fibers of the stomach. The internal component of the esophagus is considered the first line of defense against gastric reflux. When intragastric pressure exceeds the internal components of the LES, the external components take over, providing the next level of protection (Brasseur et al., 2007).
Influence of the type of surgery on GER in anesthetized dogs
It is difficult to establish which type of surgery causes more reflux during anesthesia. It has been found that abdominal surgeries predispose to a higher incidence of reflux because of an increase in the intra-abdominal pressure. Uterine surgery, on the other hand, is described as the most common cause of GER in dogs (Galatos and Raptopoulos, 1995b). Recent findings showed that gastrointestinal surgery, diagnostic imaging procedures, or a combination of both during the same anesthetic procedure increase the risk of regurgitation. It is possible that a change in the depth of the anesthesia, patient handling, and changes in body position may cause GER (García et al., 2013). However, another study has shown that GER occurred in 13% of anesthetized dogs, none of which had undergone abdominal surgery (Rodríguez, 2010). It is also described that placing the dog in the Trendelenburg position (head down) predisposes to reflux. This position is used when the viscera requires cranial movement. Patients that have undergone previous surgery also show a higher incidence of GER (Galatos and Raptopoulos, 1995a,b). Other studies have reported that dogs that undergo orthopedic surgery have a higher risk of presenting GER during anesthesia (Rodríguez, 2010, Lamata et al., 2012).
Influence of sex on GER in anesthetized dogs
It could be possible that the high number of females with GER during surgery of the reproductive system is due to the high number of interventions performed in small animal clinics, but other factors must be considered. For example, in humans, it is the elevation of progesterone and not the pressure of the fetus that causes reflux in pregnant women. In the same way it has been described that in female dogs progesterone and estrogen cause the LES to relax, which predisposes to reflux (Nilsson et al., 2003). Another study showed that postoperative benign esophageal stricture developed in hysterectomized dogs should probably not be attributed to the effects of increased concentrations of female sex steroid hormones. In that study, animals were evaluated under the influence of different concentrations of endogenous progesterone and estradiol-concentrations, and were premedicated with acepromazine and thiopental and maintained with halothane. Lower esophageal pH was monitored continuously for 1 hour after induction, and GER was detected in only one animal (Anagnostou et al., 2009). In another recent study on anesthetized dogs, none of the dogs with GER had undergone an ovariohysterectomy, concluding that neither gender, nor hormones, was an important factor in the predisposition to GER during anesthesia (Rodríguez, 2010).
Influence of breed, body condition, and age on GER during anesthesia
Among all dog breeds, it has been found that Poodles commonly present this problem (Wilson et al., 2004, Rodríguez, 2010). Another study reported that Labrador Retrievers and German Shepherds presented more reflux during anesthesia than controls (Lamata et al., 2012).
A relationship between chronic obstructive respiratory syndrome and GER has been observed in dogs (Lecoindre and Richard, 2004; Poncet et al., 2005). This is possibly because an anomalous inspiratory effort might induce an abnormally low negative intrathoracic pressure that could worsen or even induce GER. Endoscopic examination of the upper digestive tract in 30 dogs with chronic obstructive respiratory syndrome showed that 25 of these dogs presented reflux esophagitis in different stages (Lecoindre and Richard, 2004). However, brachycephalic dogs predisposed to chronic obstructive respiratory syndrome are not predisposed to GER during anesthesia.
A study showed that the abdominal esophagus has different lengths depending on the breed (Pratschke, 2004). In that study, the esophagus in most of the Greyhounds and Beagles was contained entirely within the thoracic cavity such that no portion of the esophagus could be subject to abdominal pressure, which is likely associated with GERD (Pratschke et al., 2004). If this is true, then it could be possible that Poodles, Labrador Retrievers and German Shepherds also have a short abdominal esophagus, predisposing them to reflux, though this has not been proven. The position and segment of the terminal esophagus within the abdominal cavity in dogs are very important. This portion of abdominal esophagus acts as a reflux barrier according to the law of LaPlace. This law stipulates that the pressure inside a hollow viscus is inversely proportional to its radius. The esophageal radius is consistently shorter than the gastric radius. Consequently, when both are exposed simultaneously to the positive intra abdominal pressure, the pressure within the segment of abdominal esophagus always exceeds the internal gastric pressure, which helps prevent GER (Pratschke et al., 2004). It has been documented that dogs regurgitate as a normal physiological event (Patrikios et al., 1986). Interestingly, in this species a consistent and identifiable abdominal esophagus has not been documented. In contrast, the rat, a species with a separation of the high pressure anti-reflux barrier in two distinct regions with a clear abdominal esophagus (Soto et al., 1997), does not have the ability to vomit and the phenomenon of GER has not been observed in this species (Pratschke et al., 2004). To elucidate this higher predisposition to GER during anesthesia, additional studies similar to that of Pratschke's should be conducted to address the conformation of LES in other breeds.
One study showed that there was no significant association between GER and body weight (Wilson et al., 2005). In addition, other research showed that 76.92% of the patients that presented GER during anesthesia had a normal body condition, while 23.08% were underweight and 0% were obese; there was an increase of GER in large breed dogs (33 kg to 49 kg; Rodríguez, 2010). Similarly, another study shows that dogs weighting more than 40 kg are more likely to present passive regurgitation (Lamata et al., 2012). There is very little veterinary literature on this issue; however, there have been studies regarding the role of obesity and the risk of GER in humans during anesthesia (Zacchi et al., 1991; Esquide et al., 2004; Freid, 2005; de Leon et al., 2010). In human anesthesia, it has been proven that the combination of a high intra-abdominal pressure, a high volume of gastric content with a low pH, delayed gastric emptying and a high incidence of hiatal hernias would put an obese patient at higher risk of GER (Zacchi et al., 1991). In addition, anesthetized obese patients are more likely to present Mendelson's syndrome. This is a chemical pneumonitis characterized by a bronchopulmonary reaction after aspiration of gastric contents during general anesthesia (Kamalipour et al., 2012).
A mechanism called barrier pressure (BrP) assists in avoiding aspiration during anesthesia. The BrP is the difference between LES pressure and gastric pressure. This gradient should always be positive and works as a barrier between the stomach and esophagus (de León et al., 2010). In a study that compared the pressures of the LES and BrP with high-resolution solid-state manometry in obese and non-obese patients, both pressures decreased in both types of patients during induction of anesthesia with remifentanil. In obese patients, the BrP was significantly lower, but it still remained positive in all patients (de León et al., 2010). However, other studies state that the pressure gradient between the stomach and the LES is similar between obese and non-obese patients in different surgical positions (Esquide et al., 2004; Freid, 2005). The low relationship between humans with GER and obesity was proven in a study (n = 44) in which seven obese patients were anesthetized and only one developed GER. GER did, however, develop in five out of 37 non-obese patients (Illing et al., 1992). Even though obesity itself has no influence in the presentation of GER, it does create an intra-abdominal condition that favors reflux when the gastroesophageal barrier is weakened (Zacchi, 1991). A human study of 256 fasted surgical patients found that in comparison to obese patients, lean patients that underwent anesthesia had a larger amount of gastric content with a lower pH (Harter et al., 1998). It has been described that a higher acid stomach content will predispose the patient to reflux and aspiration pneumonitis during anesthesia (Schreiner, 1998; Ng and Smith, 2001). In dogs, Rodríguez (2010) described a high incidence of GER in thinner animals that were anesthetized. Unfortunately, the results of different studies addressing the relation of GER and body condition of dogs are not conclusive. This condition is presented in both lean and obese dogs; however, it seems to be more common in large dogs, regardless of their corporal condition. Therefore, it would be important for future research to establish a correlation between dogs' body condition, pressure of the LES, development of GER during anesthesia, and GER.
Few studies have correlated the age of anaesthetized patients and the development of GER or aspiration pneumonia in veterinary medicine. In some studies, no correlation was found between an increase in patient age and the risk of GER (Wilson et al., 2005; Lamata et al., 2012; García et al., 2013). Nevertheless, in a study performed in 270 dogs divided randomly in groups according to age (2 to 5 months, 6 months to 9 years, and 10 to 15 years) geriatric canine patients had a higher risk of developing GER during anesthesia and their stomach content was more acidic in the case of reflux (Galatos and Raptopoulos, 1995b). Similarly, Rodríguez (2010) found that the majority of reflux during anesthesia occurred in older dogs. At present, there is no explanation for low gastric pH in dogs or humans; also, previous studies in humans failed to establish significant changes in acid secretion (Pilotto et al., 1994; Franceschi et al., 2009). On the other hand, evidence shows that elderly human patients have an increased pH in the stomach content, but their stomach shows a delay in gastric emptying, which could possibly increase the potential of GER during anesthesia (Livingstone, 2003). However, in human anesthesia, children are considered to have a higher risk of developing GER and aspiration pneumonia during anesthesia (Alvarez y Reyes, 2009). This is because most of them have a liquid stomach content of more than 4 mL/kg at the time of anesthesia, with a pH of less than two, regardless of the fasting interval (Maekawa et al., 1998). In relation to this, a metaanalysis concluded that children who preoperatively fluid fasted for more than six hours benefit in terms of intraoperative gastric volume and pH when compared to children permitted unlimited fluids up to two hours preoperatively (Brady et al., 2009). In veterinary anesthesia, the presence of liquid in the stomach of puppies has not been described. It would be of interest to take measurements of the quantity and pH of liquid stomach content during anesthesia to establish if puppies are more likely to present GER and pneumonia aspiration during anesthesia.
Influence of different drugs on GER in anesthetized dogs
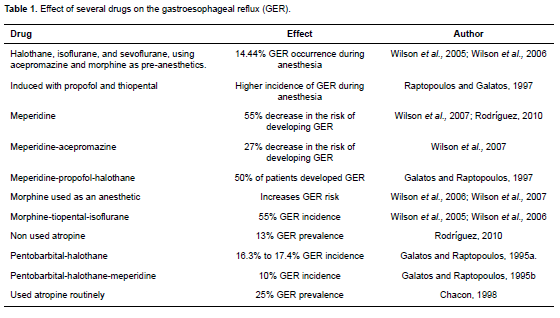
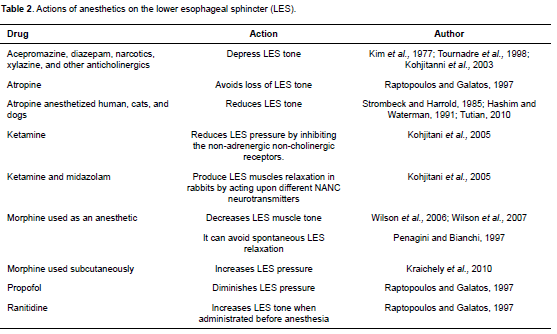
Several pre-anesthetic and anesthetic drugs, such as atropine, diazepam, acepromazine, diazepam, xylazine, morphine, halothane, and isoflurane can lead to GER by decreasing the tone of the LES (Kim et al., 1977; Strombeck and Harrold, 1985; Cox et al., 1988; Hashim and Waterman, 1993; Tournadre et al., 1998; Kohjitani et al., 2003; Epstein and Swirsky, 2009; Tables 1 and 2).
It has been shown that atropine is one of the drugs that reduce the tone of the LES in anesthetized humans, cats and dogs, thereby predisposing them to reflux (Strombeck and Harrold, 1985; Tutian, 2010). In spite of this, atropine—as well as glycopyrrolate— does not lower the pH of the esophagus or the stomach when used as a pre-anesthetic in dogs (Roush et al., 1990). The increase in the possibility of producing GER with atropine during anesthesia was confirmed in two different studies completed in the same veterinary hospital with comparable groups of anesthetized animals, and by similar anesthetic protocol. When atropine was used routinely, GER prevalence was 25% (Chacon, 1998) versus 13% when atropine was not used (Rodríguez, 2010).
In humans, diazepam has the ability to significantly lower LES pressure. In the human LES, diazepam could act as a smooth muscle relaxant since myogenic influences have been implicated in the control of LES pressure (Rushnak and Leevy, 1980). However, although dogs have striated muscle fibers in the muscle layer, Hall et al. (1987) reported that diazepam decreased LES pressure in this species.
Reports on acepromazine and its effect on GER in anesthetized dogs are inconsistent. For example, Lamata et al. (2012) found no association between the administration of acepromazine and the probability of a GER episode occurring. However, García et al. (2013) mentioned that the risk of GER was significantly lower in dogs premedicated with acepromazine in combination with an opioid in comparison to patients administered medetomidine alone. This reduction of GER in anesthetized dogs may occur because acepromazine causes a decrease in BrP compared to other drugs (Hashim and Waterman, 1993).
At present, controversy exists as to whether morphine may relax or increase LES tone. Morphine produces emesis when used as a pre-anesthetic. Vomit alone does not determine the possibility of GER during anesthesia, but it is well documented that morphine, when used as pre-anesthetic, increases the risk of GER (Wilson et al., 2005) since it decreases muscle tone in the LES (Mittal and McCallum, 1986). However, Penagini, and Bianchi (1997) stated that morphine might inhibit the spontaneous relaxation of LES. Another study claims that morphine increases LES pressure when used subcutaneously (Kraichely et al., 2010). A recent study found no association between the use of opioid drugs and GER incidence in anesthetized dogs, although opioids were not individually analyzed (García et al., 2013). Therefore, it is difficult to establish whether this drug predisposes to GER during anesthesia.
In dogs, pre-anesthetic meperidine has been associated with a reduction in the absolute risk of developing GER. Galatos and Raptopoulos (1995ab) anesthetized dogs with pentobarbital-halothane or with a combination of pentobarbital-halothanemeperidine. The group that received meperidine had lower GER incidence (10%) in comparison to the group that did not receive meperidine (16.3% to 17.4%). Wilson et al. (2008) demonstrated that administration of pre-anesthetic meperidine was associated with a 29% reduction in the absolute risk of GER in comparison to morphine.
Contractility of the LES is one of the factors that prevent regurgitation during anesthesia. Various hormones and neurotransmitters regulate this contractibility. It is known that non-adrenergic and noncholinergic (NANC) nerves regulate gastrointestinal peristalsis and relaxation mechanisms of the LES. NANC inhibitory nerves are responsible for most nerve induced relaxations of the gastrointestinal sphincteric muscle. Ketamine and midazolam produce a relaxation of LES muscles in rabbits by acting upon different NANC neurotransmitters (Kohjitani et al., 2005). N-methyl D-aspartate (NMDA) antagonists like ketamine can act differently depending on the region of the gastrointestinal tract where they are exerting its function or where they are located, and the species. It has been well established that ketamine reduces LES pressure by inhibiting non-adrenergic non-cholinergic receptors (Kohjitani et al., 2005).
Several induction agents are used in small animal anesthesia; two of the most common inducers are propofol and thiopental. Raptopoulos and Galatos (1997) showed that dogs induced with propofol had higher GER incidence during anesthesia than those induced with thiopental. It is believed that this is because propofol diminishes LES pressure. Although further research is needed to elucidate all the potential effects of anesthesia inductors on GER, thiopental should be used as an induction agent in patients with high risk of GER in anesthesia, considering aspects such as breed, body weight (> 40 kg), patient position and orthopedic surgery.
Other researchers reported 55% GER incidence using a morphine-thiopental-isoflurane protocol (Wilson et al., 2005; Wilson et al., 2006a). Wilson et al. (2007) compared the number of dogs that presented GER when using only meperidine, morphine, or meperidineacepromazine. They found that the dogs that received meperidine alone had a 55% decrease in the risk of developing GER and the group with meperdineacepromazine had a 27% decrease. Similar results were obtained in recent studies, where meperidine proved to be the drug less likely to induce GER in these patients (Rodríguez, 2010). Meperidine may reduce the possibility of GER in dogs. However, according to Wilson et al. (2005), it does not produce high quality sedation. Therefore, it is not useful to include it in the anesthetic protocol when GER prevention during anesthesia is desired (Wilson et al., 2005).
It must also be taken into consideration that halothane, nitric oxide, and sodium pentothal inhibit the transient relaxation of the esophageal sphincter (Cox et al., 1988). 14.44% of dogs anesthetized with halothane, isoflurane, and sevoflurane, and using acepromazine and morphine as pre-anesthetics, presented GER during anesthesia. This was attributed to acepromazine, since it decreases muscle tone of the LES and does not avoid morphine-induced vomit. Maintaining anesthesia with halothane, isoflurane, and sevoflurane did not have an influence on GER development during surgery (Wilson et al., 2005; Wilson et al., 2006; Lamata et al., 2012; García et al., 2013).
Influence of patient position on GER during anesthesia
There have been several studies in both human and veterinary surgery about the effect of patient position during surgery and the development of GER. For example, when dogs are positioned in ventral recumbency they present a reduction of barrier pressure in the LES during anesthesia (Pratschke et al., 2001). However, Galatos and Raptopoulos (1995b) mentioned that only the heavier patients placed in this position presented high incidence of reflux during anesthesia, and that patient weight showed no influence on reflux development when using other surgical positions. Furthermore, according to Favarato et al. (2011), decubitus position is not a predisposing factor of GER in anesthetized dogs.
A reduction of pressure of the LES in patients in ventral recumbency has been demonstrated by Pratschke et al. (2001) by placing deep-chested dogs on a flat, hard surface, and observing that thorax anatomy becomes distorted, particularly the diaphragm. This occurs as a result of the pressure placed on the sternum when the weight of the animal is in a vertical position, since body weight is normally supported by all four limbs (Pratschke et al., 2001). Similarly, this diaphragmatic distortion occurs in heavy dogs (Lamata et al., 2012). Since the diaphragm is vital in maintaining the pressure of the LES, the distortion created by the weight of the dog on the surgical table is an important part of the reduced protection by tone loss (Pratschke et al., 2001). However, Rodríguez (2010) reported that most patients that developed GER during anesthesia had been placed in right lateral recumbency. This is most likely because the right crus is larger and longer than the left; then, if the dog is placed in right lateral recumbency on top of a hard and flat surface, its weight will prevent the right crus from working adequately, favoring the appearance of reflux.
Preventing GER during anesthesia
The consequences of GER are not only dangerous, but also difficult to treat. It is better to prevent GER from occurring during anesthesia, and in the event that it does develop, the damage must be minimized (Ng and Smith, 2001). In humans, the methods utilized to decrease GER incidence and severity of the injuries consists of managing the amount of gastric content via fasting, decreasing its pH, and taking measures to maintain an adequate tone of the LES (Ng and Smith, 2001). In a review about physiological aspects of GER and aspiration in anesthetized patients, it was determined that total fasting (nil per os/NPO) is not the most suitable recommendation to decrease GER incidence. This is because patients with 24 hours of fasting produce more HCl with a more acidic pH. Adult patients can drink clear liquids up to three hours before anesthesia since this does not increase the risk of reflux. A minimum of five hours is required to evacuate a light meal, but more than nine hours are needed for a heavy meal (Ng and Smith, 2001).
In children, the minimum fasting time required of clear liquids (water, fruit juice without pulp, carbonated drinks, tea, and dextrose) to have an adequate gastric emptying without any digestive problems was two to six hours (López-Muñoz et al., 2002; Maharaj, 2009).
Prolonged fasting, generally starting the night before surgery, was traditionally recommended in veterinary medicine, though this practice has changed. Now it is recommended that adult dogs solid-food fast for five to ten hours and they may have access to water up to two to three hours before anesthesia (Raptopoulos and Galatos, 1997; Raptopoulos and Savas, 2004; Muir et al., 2007; Savas and Raptopoulos, 2009; Epstein and Swirsky, 2009). It has been proven that a fasting period of more than 18 hours decreases LES tone in anesthetized dogs, therefore favoring GER development (Galatos and Raptopoulos, 1995a).
Although oral antacids in gel form have proven effective for increasing the pH of gastric content, it is not advisable to use them before anesthesia since they can have fatal consequences if aspiration occurs. In humans, water-soluble antacids, such as sodium bicarbonate, can also lower the stomach pH without presenting this danger (Sánchez, 2002); however, safety and potential damage to respiratory system—in case of bronchoaspiration— have not been established in dogs.
Studies evaluating GER in anesthetized humans and dogs have focused on the use of prokinetics and medications to reduce gastric acidity to prevent reflux (Wilson et al., 2006b; Panti et al., 2009; Favarato et al., 2012). In humans, it is also known that drugs that decrease hydrochloric acid production in the stomach, such as ranitidine and omeprazole increase pH and gastric fluids during surgery and therefore decrease the risk of developing Mendelson's syndrome (Grümberg, 2003; Moro, 2004). However, Tamhankar et al. (2004) reported that oral administration of omeprazole every 12 h for 7 days did not decrease GER. Esomeprazole is the proton pump inhibitor studied in dogs; dogs that received IV esomeprazole (1 mg/kg) alone in a prospective randomized placebo-controlled study presented significant increase in gastric and esophageal pH, but the drug did not significantly decrease GER frequency; however, combination of esomeprazole (1 mg/kg) and cisapride (1 mg/kg) IV was associated with significant decrease of reflux in anaesthetized dogs (Zacuto et al., 2012). Ranitidine has been used in dogs to reduce GER during anesthesia, but results vary. According to some reports, ranitidine increased LES tone when administered before anesthesia; and it prevented tone loss of the LES when used before atropine (Raptopoulos and Galatos, 1997; Harter et al., 1998; Burrows, 2006). It is important to consider that ranitidine has no effect on LES tone when it is administered after atropine (Harter et al., 1998); however, according to recent findings ranitidine does not reduce GER when administered to dogs as an intravenous bolus at a dose of 2 mg/kg 6 h before anesthesia (Favarato et al., 2012).
In regard to prokinetics used in human anesthesiology to prevent GER during anesthesia, it has been proven that administration of 200 mg erythromycin before surgery increases the stomach pH. This is due to the stimulation of motilin receptors that reduce gastrin levels (Anderson, 2010). Wilson et al. (2006b) showed that a low dose of metoclopramide did not have a significant effect on GER incidence in dogs; however, a high dose (bolus loading dose of 1 mg/kg, IV, followed by continuous infusion at a rate of 1 mg/kg/h) reduced the relative risk to develop GER in 54%.
Conclusion
Occurrence of GER during anesthesia is a relatively frequent problem and a risk factor for further complications, such as aspiration pneumonia, esophagitis and esophageal strictures. The main factors involved include: Poodle patients, position (Trendelenburg or right lateral recumbence), orthopedic surgeries utilizing atropine and morphine as preanesthetics, and the use of propofol as an induction agent. A complete pre-surgery clinical history will also help to identify any existing digestive and/or esophageal disease, which may increase the risk of developing GER. Proper monitoring, along with the administration of certain drugs, such as prokinetics and proton pump inhibitors, can diminish GER occurrence. Further studies are needed to establish preventive protocols during anesthetic procedures. Studies in humans have rendered a guide for pathophysiologic and epidemiologic considerations, symptom evaluation, diagnostic workup, medical therapy and surgical therapy for GERD. Studies on other triggering factors, in addition to their management and prevention, are also needed.
Conflicts of interest
The authors declare they have no conflicts of interest with regard to the work presented in this report.
Notes
¤To cite this article: Rodríguez-Alarcón CA, Berinstain-Ruiz DM, Riveira-Barreno R, Díaz G, Usón-Casaús JM, García-Herrera R, Pérez-Merino EM. Gastroesophageal reflux in anesthetized dogs: a review. Rev Colomb Cienc Pecu 2015; 28:144-155.
References
Alvarez L, Reyes RD. Ayuno preoperatorio en niños sanos de 2, 4 y 6 horas. Rev Colomb Anestesiol 2009; 37:63-70. [ Links ]
Anagnostou TL, Savvas I, Kazakos GM, Ververidis HN, Haritopoulou MR, Rallis TS, Raptopoulos D. Effect of endogenous progesterone and oestradiol-17beta on the incidence of gastro-oesophageal reflux and on the barrier pressure during general anaesthesia in the female dog. Vet Anaesth Analg 2009; 36:308-318. [ Links ] Anderson K. Gastroesophageal reflux disease. Radiol Technol 2010; 81:251-268. [ Links ]
Brady M, Kinn S, Ness V, O'Rourke K, Randhawa N, Stuart P. Preoperative fasting for preventing perioperative complications in children. Cochrane Database Syst Rev 2009; 4: DOI: 10.1002/14651858.CD005285.pub2 [ Links ]
Brasseur JG, Ulerich R, Dai Q, Patel DK, Soliman A, Miller LS. Pharmacological dissection of the human gastro-oesophageal segment into three sphincteric components. J Physiol 2007; 580:961-975. [ Links ]
Burrows C. Trastornos digestivos. In: Schaer M, editor. Medicina clínica del perro y el gato. Barcelona, España: Elsevier-Masson; 2006. p.270-281. [ Links ]
Chacón MB. Estudio prospectivo y retrospectivo endoscópico esofagogástricos en perros de la clínica con patología digestiva y sin ella. [Tesis de Grado]. Universidad de Extremadura. Facultad de Veterinaria; 1998. [ Links ]
Cox MR, Martin CJ, Dent J, Westmore M. Effect of general anaesthesia on transient lower oesophageal sphincter relaxations in the dog. Aust N Z J Surg 1988; 58:825-830. [ Links ]
de Leon A, Thörn S-E, Wattwil M. High-Resolution solid-state manometry of the upper and lower esophageal sphincters during anesthesia induction: a comparison between obese and non-obese patients. Anaesth Analg 2010; 111:149-153. [ Links ]
Epstein A, Swirsky N. Post-anesthetic esophageal dysfunction in a dog. Isr J Vet Med 2009; 64:12-13. [ Links ]
Esquide J, de Luis R, Valero C. Anestesia en la cirugía bariátrica. Cir Esp 2004; 75:273-279. [ Links ]
Favarato E, Souza M, Costa P, Favarato L, Nehme R, Monteiro B. Evaluation of metoclopramide and ranitidine on the prevention of gastroesophageal reflux episodes in anesthetized dogs. Res Vet Sci 2012; 93:466-467. [ Links ]
Favarato ES, de Souza MV, dos Santos Costa PR, Pompermayer LG, Favarato LSC, Júnior JIR. Ambulatory esophageal pHmetry in healthy dogs with and without the influence of general anesthesia. Vet Res Commun 2011; 35:271-282. [ Links ]
Franceschi M, Di Mario F, Leandro G, Maggi S, Pilotto A. Acidrelated disorders in the elderly. Best Pract Res Clin Gastroenterol 2009; 23:839-848. [ Links ]
Freid EB. The rapid sequence induction revisited: obesity and sleep apnea syndrome. Anesthesiol Clin North America 2005; 23:551-564. [ Links ]
Galatos AD, Raptopoulos D. Gastro-oesophageal reflux during anaesthesia in the dog: the effect of preoperative fasting and premedication. Vet Rec 1995a; 137(19):479-483. [ Links ]
Galatos AD, Raptopoulos D. Gastro-oesophageal reflux during anaesthesia in the dog: the effect of age, positioning and type of surgical procedure. Vet Rec 1995b; 137(20):513-516. [ Links ]
García CDM, Pinchbeck G, Dugdale A, Senior J. Retrospective study of the risk factors and prevalence of regurgitation in dogs undergoing general anaesthesia. Open Vet Sci J 2013; 7:6-11. [ Links ]
Glazer A, Walters P. Esophagitis and esophageal strictures. Compend Contin Educ Vet 2008; 5:281-292. [ Links ]
Grünberg G. Omeprazol para la reducción del riesgo de desarrollo de síndrome de Mendelson en pacientes coordinados para cirugía abdominal. Anest Analg Reanim 2003; 18. [ Links ]
Gualtieri M. Esophageal structures of the dog and cat: diagnosis and treatment. Proceedings of the 30th Congress of the World Small Animal Veterinary Association, 2005 May 11-14; Mexico City, Mexico. WSAVA; 2005. [ Links ]
Hall J, Magne M, Twedt D. Effect of acepromazine, diazepam, fentanyl-droperidol, and oxymorphone on gastroesophageal sphincter pressure in healthy dogs. Am J Vet Res 1987; 48:556-557. [ Links ]
Han E, Broussard J, Baer KE. Feline esophagitis secondary to gastroesophageal reflux disease: clinical signs and radiographic, endoscopic, and histopathologic findings. J Am Anim Hosp Assoc 2003; 39:161-167. [ Links ]
Harter RL, Kelly WB, Kramer MG, Perez CE, Dzwonczyk RR. A comparison of the volume and ph of gastric contents of obese and lean surgical patients. Anesth Analg 1998; 86:147-152. [ Links ]
Hashim MA, Waterman AE. Effects of thiopentone, propofol, alphaxalone-alphadolone, ketamine, and xylazine-ketamine on lower oesophageal sphincter pressure and barrier pressure in cats. Vet Rec 1991; 129:137-139. [ Links ]
Hyun JJ, Bak YT. Clinical significance of hiatal hernia. Gut liver 2011; 5:267-277. [ Links ]
Illing L, Duncan PG, Yip R. Gastroesophageal reflux during anaesthesia. Can J Anaesth 1992; 39:466-470. [ Links ]
Jergens AE. Enfermedades del esófago. In: Ettinger SJ, Editor. Tratado de medicina interna veterinaria. Filadelfia: Elsevier Saunders; 2004. p.1298-1310. [ Links ]
Kamalipour H, Lahsaee M, Foroutan HR, Ghoreishi M, Kamali K. Comparison of different non-pharmacological preoperative preparations on gastric fluid volume and acidity: a randomized controlled trial. Anaesth Intens Care 2012; 16:165-168. [ Links ]
Kim KC, Patdu R, Kim HW. The relationship between intragástrica and lower esophageal sphincter pressures during general anesthesia. Anesthesiology 1977; 46:424-426. [ Links ]
Kohjitani A, Funahashi M, Miyawaki T, Hanazaki M, Matsuo R, Shimada M. Peripheral N-methyl-D-aspartate receptors modulate nonadrenergic noncholinergic lower esophageal sphincter relaxation in rabbits. Anesth Analg 2005; 101:1681-1688. [ Links ]
Kohjitani A, Miyawaki T, Funahashi M, Higuchi H, Matsuo R, Shimada M. Ketamine and midazolam differentially inhibit nonadrenergic noncholinergic lower esophageal sphincter relaxation in rabbits: role of superoxide anion and nitric oxide synthase. Anesthesiology 2003; 98:449-458. [ Links ]
Kraichely RE, Arora AS, Murray JA. Opiate-induced oesophageal dysmotility. Aliment Pharmacol Ther 2010; 31:601-606. [ Links ]
Lamata C, Loughton V, Jones M, Alibhai H, Armitage, Chan E, Walsh K, Brodbelt D. The risk of passive regurgitation during general anaesthesia in a population of referred dogs in the UK. Vet Anaesth Analg 2012; 39:266-274. [ Links ]
Lecoindre P, Richard S. Digestive disorders associated with the chronic obstructive respiratory syndrome of brachycephalic dogs: 30 cases (1999-2001). Rev Med Vet-Toulouse 2004; 155:141-146. [ Links ]
Livingstone S. The older patient. Pharm J 2003; 270:862-863. [ Links ] López-Muñoz AC, Tomás BJ, Montero BR. [Guidelines for preoperative fasting and premedication to reduce the risk of pulmonary aspiration]. Rev Esp Anestesiol Reanim 2002; 49:314-323. [ Links ]
Maekawa N, Nishina K, Mikawa K, Shiga M, Obara H. Comparison of pirenzepine, ranitidine, and pirenzepine-ranitidine combination for reducing preoperative gastric fluid acidity and volume in children. Br J Anaesth 1998; 80:53-57. [ Links ]
Maharaj D. Eating and drinking in labor: Should it be allowed? Eur J Obstet Gynecol Reprod Biol 2009; 146:3-7. [ Links ]
Miller L, Dai Q, Vegesna A, Korimilli A, Ulerich R, Schiffner B, Brassuer J. A missing sphincteric component of the gastrooesophageal junction in patients with GERD. Neurogastroenterol Motil 2009; 21:813-e52. [ Links ]
Mittal RK, McCallum RW. Effects of morphine and naloxone on esophageal motility and gastric emptying in man. Dig Dis Sci 1986; 31:936-42. [ Links ]
Moro ET. Prevention of pulmonary gastric contents aspiration. Rev Bras Anestesiol 2004; 54:261-275. [ Links ]
Muir WW, Hubbell JAE, Skarda RT, Bednarski RM. Handbook of Veterinary Anesthesia. 4th ed. St. Louis (MO): Mosby; 2007. [ Links ] Ng A, Smith G. Gastroesophageal reflux and aspiration of gastric contents in anesthetic practice. Anesth Analg 2001; 93:494-513. [ Links ]
Nilsson M, Johnsen R, Ye W, Hveem K, Lagergren J. Obesity and estrogen as risk factors for gastroesophageal reflux symptoms. JAMA 2003; 290:66-72. [ Links ]
Panti A, Bennett R, Corletto F, Brearley J, Jeffrey N, Mellanby R. The effect of omeprazole on oesophageal pH in dogs during anaesthesia. J Small Anim Pract 2009; 50:540-544. [ Links ]
Patrikios J, Martin CJ, Dent J. Relationship of transient lower esophageal sphincter relaxation to postprandial gastroesophageal reflux and belching in dogs. Gastroenterology 1986; 90:545-551. [ Links ]
Penagini R, Bianchi PA. Effect of morphine on gastroesophageal reflux and transient lower esophageal sphincter relaxation. Gastroenterology 1997; 113:409-414. [ Links ]
Pilotto A, Vianello F, Di Mario F, Plebani M, Farinati F, Azzini F. Effect of age on gastric acid, pepsin, pepsinogen group A, and gastrin secretion in peptic ulcer patients. Gerontology 1994; 40:253-259. [ Links ]
Poncet C, Dupre G, Freiche V, Bouvy B. Long-term results of upper respiratory syndrome surgery and gastrointestinal tract medical treatment in 51 brachycephalic dogs. J Small Anim Pract 2006; 47:137-42. [ Links ]
Pratschke KM, Bellenger CR, McAllister H, Campion D. Barrier pressure at the gastroesophageal junction in anesthetized dogs. Am J Vet Res 2001; 62:1068-1072. [ Links ]
Pratschke KM, Fitzpatrick E, Campion D, McAllister H, Bellenger CR. Topography of the gastro-oesophageal junction in the dog revisited: possible clinical implications. Res Vet Sci 2004; 76:171-177. [ Links ]
Pratschke KM, Hughes JM, Skelly C, Bellenger CR. Hiatal herniation as a complication of chronic diaphragmatic herniation. J Small Anim Pract 1998; 39:33-38. [ Links ]
Raptopoulos D, Galatos AD. Gastro-oesophageal reflux during anaesthesia induced with either thiopentone or propofol in the dog. Vet Anaesth Analg 1997; 24:20-22. [ Links ]
Raptopoulos D, Savvas I. Pre-operative fasting: ''nil per os after midnight''- Time to change? Proceedings of the 29th Congress of the World Small Animal Veterinary Association, 2004 Oct 6-11; Rhodes, Greece. ,WSAVA, 2004. [ Links ]
Rodríguez-Alarcón CA. Estudio retrospectivo y prospectivo de las endoscopias del tracto digestivo en pequeños animales. [Tesis de Doctorado]. Universidad de Extremadura. Facultad de Veterinaria; 2010. [ Links ]
Roush JK, Keene BW, Eicker SW, Bjorling DE. Effects of atropine and glycopyrrolate on esophageal, gastric, and tracheal pH in anesthetized dogs. Vet Surg 1990; 19:88-92. [ Links ]
Rushnak M, Leevy C. Effect of diazepam on the lower esophageal sphincter. A double-blind controlled study. Am J Gastroenterol 1980; 73:127-130. [ Links ]
Sánchez D. Aspiración pulmonar del contenido gástrico. Rev Venez Anestesiol 2002; 7:253-259. [ Links ]
Savas I, Raptopoulos D. Incidence of gastro-oesophageal reflux during anaesthesia, following two different fasting times in dogs. Vet Anaesth Analg 2009; 27:54-62. [ Links ]
Schreiner MS. Gastric fluid volume: is it really a risk factor for pulmonary aspiration? Anaesth Analg 1998; 87(4):754-756. [ Links ]
Soto C, Qi B, Diez-Pardo JA, Tovar JA. Identification of diaphragmatic crural component of gastroesophageal barrier in the rat. Dig Dis Sci 1997; 42:2420-2425. [ Links ]
Strombeck DR, Harrold D. Effects of atropine, acepromazine, meperidine, and xylazine on gastroesophageal sphincter pressure in the dog. Am J Vet Res 1985; 46:963-965. [ Links ]
Tamhankar AP, Peters JH, Portale G, Hsieh CC, Hagen JA, Bremner CG, DeMeester TR. Omeprazole does not reduce gastroesophageal reflux: New insights using multichannel intraluminal impedance technology. J Gastrointest Surg 2004; 8:890-897. [ Links ]
Tournadre JP, Barclay M, Bouletreau P, Chassard D. Lower oesophageal sphincter tone increases after induction of anaesthesia in pigs with full stomach. Can J Anaesth 1998; 45:479-482. [ Links ]
Tutian R. Adverse effects of drugs on the esophagus. Best Pract Res Clin Gastroenterol 2010; 24:91-97. [ Links ]
Vlasin M, Husnik R, Fichtel T, Rauserová L. Acquired esophageal stricture in the dog: a case report. Vet Med - Czech 2004; 49:143-147. [ Links ]
Washabau R. Efficacy of gastrointestinal drugs: what works in the dog?-SOTAL. Proceedings of the 26th Congress of the World Small Animal Veterinary Association, 2001 Vancouver. Canada. Aug 8-11; WSAVA; 2001. [ Links ]
Willard MD. Recognizing and treating esophageal disorders in dogs and cats. Vet Med 2004; 99:448-454. [ Links ]
Wilson DV, Boruta DT, Evans AT. Influence of halothane, isoflurane, and sevoflurane on gastroesophageal reflux during anesthesia in dogs. Am J Vet Res 2006; 67:1821-1825. [ Links ]
Wilson DV, Evans AT, Mauer WA. Influence of metoclopramide on gastroesophageal reflux in anesthetized dogs. Am J Vet Res 2006; 67:26-31. [ Links ]
Wilson DV, Evans AT, Miller R. Effects of preanesthetic administration of morphine on gastroesophageal reflux and regurgitation during anesthesia in dogs. Am J Vet Res 2005; 66:386-390. [ Links ]
Wilson DV, Evans TA, Mauer WA. Pre-anesthetic meperidine: associated vomiting and gastroesophageal reflux during the subsequent anesthetic in dogs. Vet Anaesth Analg 2007; 34:15-22. [ Links ]
Wilson DV, Walshaw R. Postanesthetic esophageal dysfunction in 13 dogs. J Am Anim Hosp Assoc 2004; 40:455-460. [ Links ]
Zacchi P, Mearin F, Humber P, Formiguera X. Effect of obesity on gastroesophageal resistance to flow in man. Dig Dis Sci 1991; 36:1473-1480. [ Links ]
Zacuto AC, Marks SL, Osborn J, Douthitt KL, Hollingshead KL, Hayashi K, Kapatkin AS, Pypendop BH, Belafsky PC. The influence of esomeprazole and cisapride on gastroesophageal reflux during anesthesia in dogs. J Vet Intern Med 2012; 26:518-525. [ Links ]