Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Colombiana de Ciencias Pecuarias
Print version ISSN 0120-0690
Rev Colom Cienc Pecua vol.28 no.2 Medellín Apr./June 2015
https://doi.org/10.17533/udea.rccp.v28n2a06
ORIGINAL ARTICLE
doi: 10.17533/udea.rccp.v28n2a06
Presence of paratuberculosis in dairy cows culled in Tizayuca (Hidalgo, Mexico)¤
Presencia de paratuberculosis en vacas lecheras de desecho en Tizayuca (Hidalgo, México)
Presença de paratuberculose em vacas leiteiras de descarte no Tizayuca (Hidalgo, México)
Uziel Castillo-Velázquez1, Med Vet Zoot, MSci, PhD; Ricardo Gómez-Flores1, Microbiol, MSci, PhD; Gilberto Chávez- Gris2, MSci, PhD; Lucía C Favila-Humara3, Med Vet Zoot, MSci; Rafael Soto-Castor4, Med Vet Zoot; Edith Maldonado-Castro2, Med Vet Zoot, MSci; Patricia Tamez-Guerra1*, Microbiol, MSci, PhD.
1Departamento de Microbiología e Inmunología (DEMI), Facultad de Ciencias Biológicas (FCB), Universidad Autónoma de Nuevo León (UANL), Av. Universidad s/n AP 46-F, Cd. Universitaria, San Nicolás de los Garza N.L., México. CP 66450.
2Unidad de Servicios de Diagnóstico y Constatación, Centro de Enseñanza, Investigación y Extensión en Producción Animal en Altiplano, Facultad de Medicina Veterinaria y Zootecnia, Universidad Nacional Autónoma de México. Km. 8.5 Carr. Federal. Tequisquiapan-Ezequiel Montes. Tequuiapan, Querétaro, México. CP 76790.
3CENID Microbiología Animal. Km 15.5 carretera México Toluca. Delegación Cuajimalpa, México D.F. México. CP 05110.
4Complejo Agropecuario Industrial de Tizayuca, S.A. de C.V. Poniente 2 Ciudad Industrial, Tizayuca, Hidalgo, México. CP 43800.
*Corresponding author: Patricia Tamez Guerra. Departamento de Microbiología e Inmunología (DEMI), Facultad de Ciencias Biológicas (FCB), Universidad Autónoma de Nuevo León (UANL), Av. Universidad s/n AP 46-F, Cd. Universitaria, San Nicolás de los Garza N.L., México. CP 66450. Tel: +0115281- 83294000 ext. 2918. Email: patricia.tamezgr@uanl.edu.mx; patamez@hotmail.com
Received: October 5, 2013; accepted: June 8, 2014
Summary
Background: paratuberculosis (PTB) is clinically characterized by diarrhea and progressive weight loss in ruminants, and causes economic losses due to decreased milk production, premature animal disposal, and increased veterinary treatment costs. The presence of PTB has been increasing in milk producing bovine farms in Mexico, yet epidemiology data from specific farms remains scarce. Objective: to determine the proportion of cows positive for Mycobacterium avium subsp. paratuberculosis (Map) among culled cows. Methods: in the present study, 40 culled cows from 25 farms located at the complejo agropecuario industrial de Tizayuca (CAITSA) in Hidalgo (Mexico) were evaluated for PTB presence by histopathological and microbiological studies. Tissue and rectal scraping samples were taken from all cows, and studied for presence of histopathology PTB-compatible lesions. Samples were processed for Map isolation on selective agar culture media; typical Map colonies were confirmed by polymerase chain reaction (PCR). Results: potential PTB infection was found in 25% (10/40) of the animals evaluated by histopathology and acid-fast bacilli (AFB) staining, but only 7.5% (3/40) were positive for type 2 Map (C cattle strain) by PCR. Conclusions: the prevalence of PTB in cattle farms at CAITSA was confirmed, which may be valuable epidemiological information in Mexico.Keywords: cull cattle, Mycobacterium avium subsp. paratuberculosis, paratuberculosis diagnosis, ruminant farms.
Resumen
Antecedentes: la paratuberculosis (PTB) se caracteriza clínicamente por diarrea y pérdida de peso progresiva en rumiantes y causa pérdidas económicas por la disminución de la producción de leche, eliminación prematura de animales y el aumento de los costos de tratamiento veterinario. La PTB se ha incrementado en las granjas productoras de leche de bovinos en México, pero se carece de información epidemiológica específica de granjas locales. Objetivo: determinar el porcentaje de infección por Mycobacterium avium subsp. paratuberculosis (Map) entre las vacas de descarte. Métodos: en este estudio fueron evaluadas 40 vacas de descarte o de 25 granjas del complejo agropecuario industrial de Tizayuca (CAITSA), en el estado de Hidalgo (México), donde se evaluó la presencia de la PTB mediante estudios histopatológicos y microbiológicos. Se tomaron muestras de tejido y raspado rectal de todos los animales buscando lesiones compatibles con PTB. Las muestras fueron procesadas para el aislamiento de Map en medios de cultivo de agar selectivo, y las colonias identificadas como típicas fueron confirmadas por la prueba de reacción en cadena de la polimerasa (PCR). Resultados: en este estudio se observó positividad para PTB por histopatología y presencia de bacilos ácido alcohol resistentes (AFB) en el 25% de los animales (10/40), pero solo en el 7,5% (3/40) de las vacas de descarte se confirmó la cepa tipo 2 (cepa C) de Map mediante PCR. Conclusiones: se confirma la prevalencia de PTB en las vacas del CAITSA, lo cual podría tener valor como información epidemiológica en México.
Palabras clave: bovinos de descarte, diagnóstico de paratuberculosis, granjas de rumiantes, Mycobacterium avium subsp. paratuberculosis.
Resumo
Antecedentes: a paratuberculose (PTB) em vacas leiteiras caracteriza-se clinicamente pela presença de diarreia e perda de peso progressiva as quais provocam perdas económicas pela redução da produção de leite, abate prematuro dos animais e aumento dos custos de tratamento veterinário. A PTB aumentou-se em fazendas leiteiras no México, mas ainda não há informações epidemiológicas especificas das fazendas locais. Objetivo: determinar a percentagem de infecção por Mycobacterium avium subsp. paratuberculosis (Map) em vacas de descarte. Métodos: se avaliaram por estudos histopatológicos e microbiológicos 40 vacas de descarte de 25 fazendas do complexo agropecuário industrial de Tizayuca (CAITSA), no estado de Hidalgo (México). Tomaram-se amostras de tecido e raspado anal de todos os animais procurando por lesões compatíveis com PTB. As amostras foram processadas para isolamento de Map em meios de ágar seletivos e as colônias classificadas como típicas foram confirmadas pelo teste da reação em cadeia da enzima polimerase (PCR). Resultados: observou-se positividade para PTB pelos estudos histopatológicos e microbiológicos e a presença de bacilos ácido álcool resistente (AFB) no 25% dos animais (10/40), mas só no 7,5% (3/40) confirmaram-se a cepa tipo II (cepa C) de Map por PCR. Conclusões: confirma-se a presença de PTB em vacas do complexo CAITSA, o qual poderia ter valor epidemiológico no México.
Palavras chave: bovinos de descarte, diagnóstico de paratuberculose, granjas de ruminantes, Mycobacterium avium subsp. paratuberculosis.
Introduction
Paratuberculosis (PTB) or Johne's disease is a deadly chronic intestinal infection that affects both domestic and wild ruminants. Paratuberculosis is caused by Mycobacterium avium subsp. paratuberculosis (Map), which is distributed worldwide. These mycobacteria infect macrophages and interfere with phagosome maturation, thereby evading the host's first line of defense. The host immune system reacts to the infection by activating γδ-T cells, CD4+ T cells, and cytolytic CD8+ T cells. Map persists despite the immune system efforts. Release of cytokines and other cytotoxic mediators causes important damage to the intestinal epithelial cells (Coussens, 2001). PTB has an impact on dairy farms by decreasing milk production, inducing animal miscarriage, stillbirth, and premature delivery (Tiwari et al., 2008). In Mexico, Map was first isolated in 1979 from bovines (Ramírez et al., 1979). Recent reports have demonstrated the presence of Map in cattle, sheep, and goats in several Mexican states (Estévez- Denaives et al., 2007). An immunological analysis was conducted in 2005 at complejo agropecuario industrial de Tizayuca (CAITSA) in Hidalgo (Mexico), to establish the prevalence of this disease among 28 dairy farms. Results showed that 25/28 (89.28%) of the tested farms had at least one bovine seropositive to Map infection, whereas seroprevalence among animal herds ranged between 1.82 and 24.07% (Miranda, 2005). To better understand the correlation between Map transmission and cow infection, our objective was to determine the relationship of culled cows with symptomatic Map and macroscopic and microscopic lesions compatible with PTB within the 28 CAITSA farms with a history of this disease.
Material and methods
Animals
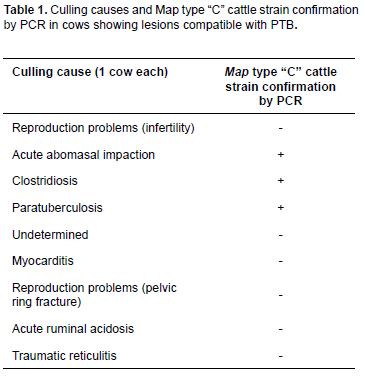
Animals used in the present study were 3.5-yearsold <i>Bos taurus</i> (Holstein Friesian) females. To determine the amount of cows positive for Map, a pathologic examination was performed in 40 culled cows referred to the CAITSA necropsy room between October, 2004 and June, 2005. Animals originated from 25 CAITSA dairy farms previously identified as seropositive to PTB (range: 1.82% to 24.07%; Miranda, 2005). All animals evaluated from these 25 farms with positive prevalence for Map infection were discarded for various reasons (Table 1).
Following Mexican regulations, all bovines were necropsied at CAITSA veterinary medical services before leaving the facilities. After general inspection of each animal was conducted, samples from the terminal ileum, ileocecal valve, and mesenteric lymph nodes were taken and submitted to both histopathological and bacteriological analysis.
Histopathological examination
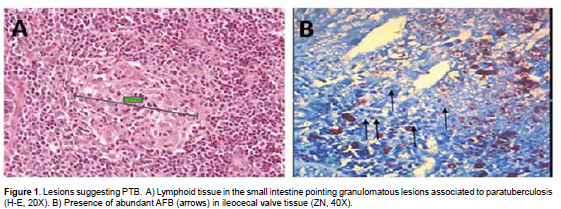
Some of the samples were fixed in 10% buffered formalin at pH 7.2 to 7.6, embedded in paraffin for subsequent 4-μm thick cuts, and stained with Ziehl-Neelsen for microscopic view of acid- fast bacilli (AFB), and hematoxylin and eosin staining (Gilardoni et al., 2012).
Bacterial isolation
Selected samples from small intestine and ileocecal valve tissues that showed lesions compatible with PTB by histopathology were then analyzed for Map identification. For this, samples were thawed for processing following the protocol described by Juste et al. (1991). In brief, each sample consisted of intestinal mucosal scraping using a sterile scalpel blade to obtain approximately 1 g of tissue, which was homogenized in 3 mL saline solution using a glass Tenbroek tissue grinder (Omni International, Kennesaw, GA). After this, samples were placed into 50 mL glass beaker and 30 mL 0.75% (v/v) hexadecylpyridinium chloride (HPC) was added and mixed in a stirring plate for one hour, and then let stand for 16 h at 25 oC. Next, 4 mL were taken from the intermediate phase and the supernatant was centrifuged at 1000 x g for 20 min. Then, the supernatant fluid was discarded. The pellet was suspended in 5 mL of an antibiotic-antimycotic solution (50 μg/mL vancomycin, amphotericin B, and nalidixic acid; Sigma-Aldrich, St. Louis, MO, USA) and incubated for 16 h at 25 oC. After this, 8 drops from the intermediate layer were inoculated in three Petri dishes with Löwenstein-Jensen (LJ) medium; only two of the dishes were supplemented with mycobactin J (2 mg/L; Allied Monitor (Fayette, MO, USA). For Map identification, inoculated dishes were incubated at 37 °C and microscopically screened from week 10 to week 14, recording growth rate, colony morphology, bacillary morphology, and staining affinity.
PCR confirmation of Map isolates
Confirmation of Map among developed colonies was performed using molecular testing by PCR analysis. Briefly, to proceed to DNA extraction, colonies from LJ culture with mycobactin J were collected with sterile loops and suspended in 250 μL sterile PBS in 2-mL conic tubes. DNA extraction included boiling each colony sample for 20 min. Definitive identification of the developed colony was performed by PCR testing using the following primers: sense 5'-CGCTGCTGGAGTTGATTG IS900-3' and antisense 5'-TTTCCTTCGGTGCGTTTTC IS900-3', which amplify an expected 973 bp product (Whittington et al., 2000). Each PCR reaction contained 10 μL of 1x buffer, 3 μΜ of MgSO4, 0.6 μL of dNTP's (10 mM), 1 nM of each primer, 5 U of Taq polymerase (Life Technologies Corporation, Carlsbad, CA), 2 ng of DNA, and molecular biology grade H2O to a 50 μL final volume. The amplification protocol consisted of an initial denaturing cycle at 95 °C for 3 min, followed by 25 cycles at 94 °C for 30 s, 60 °C for 30 s, 72 °C for 3 s, and a final extension cycle of 72 °C for 7 min. Finally, an aliquot of each PCR product was electrophoresed on 1% w/v agarose gel with 1% TAE 1x, and stained in 1% ethidium bromide solution for band visualization.
PCR strain typing of Map isolates
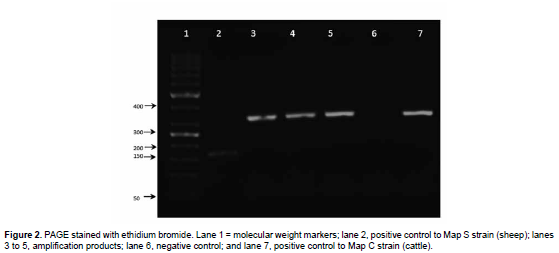
DNA extracted from bacterial colonies was used as template for PCR specific strain type test following the methodology described by Collins et al. (2002). Strain identification was performed by PCR testing, using the following primers: sense 5'-CACTTCTTACTCGGACTTC DMC531-3' and antisense 5'-TGCCCTCGATGACGAGGAGGAGTA DMC531-3', which amplify an expected 310 bp product for type C Map (cattle) strain; and sense 5'-GCTTGACAACGTCATTGAGAA DMC136-3' and antisense 5'-GCCTGGATGGATGCCCTCAATGACG DMC136-3', to amplify an expected 162 bp product for type S (sheep) strain (Collins et al., 2002). Each PCR reaction consisted of 1x reaction solution enhancer (Life Technologies Corporation), 1x buffer, 2 μM of MgSO4, 0.2 L of dNTPs, 1-pM of each primer, 5 U of Taq polymerase (Life Technologies Corporation, CA, USA), 2 ng of DNA and 50 L of molecular biology grade H2O to a 50 μL final volume. The amplification protocol consisted of an initial denaturing cycle at 95 °C for 5 min, followed by 30 cycles at 94 °C for 30 s, 60 °C for 30 s, 72 °C for 30 s, and a final extension cycle 72 °C for 5 min. Visualization of the amplification products was performed by gel electrophoresis in 1% w/v agarose gel with TAE 1X, and stained in 1% ethidium bromide solution.
Results
From all 28 selected farms (based on previous seropositive PTB results), only 25 farms discarded bovines during the evaluation period. From these farms, all with positive prevalence for the disease, a total of 40 cows were discarded and evaluated for the anatomopathological study. Those cows had various health disorders and only one cow was culled due to PTB (Table 1). According to histopathology, only 10 cows (25%) showed lesions compatible with PTB. AFB and leukocyte infiltration were also observed in such lesions (Figure 1).
Colony development of expected Map growth on solid culture after 10 and 14 weeks of incubation was observed among samples from histopathological lesions compatible with PTB (taken from 10 cows). Macroscopically, colonies were white and smooth, and were of 1-3 mm in diameter. Smears taken from these colonies were stained with ZN staining to confirm the Map bacillary morphology and AFB staining (Gilardoni et al., 2012). PCR analysis was performed for Map molecular characterization of these colonies. Identification was confirmed in all the tested colonies, where the expected 973bp product was amplified after using primers from IS900 (Figure 2). The specific PCR test confirmed Map identification and also that culture from the three colonies belonged to type C Map cattle strain within 3 out of the 10 PTB compatible samples from the 40 initially analyzed cows (3/49; 7.25%).
Discussion
Tizayuca is a municipality of Hidalgo state located in central-eastern Mexico, where dairy farming is part of the local economy. Unfortunately, PTB represents a great threat among producers because of its high prevalence levels, strong economic impact, and public health relevance due to a possible association with Crohn's disease (Gilardoni et al., 2012).
Only 25 of 200 cow farms in CAITSA were evaluated in this study (Miranda, 2005), since authorizations to evaluate the rest of the farms were not granted. Twenty-five out of 28 dairy farms were found seropositive to Map in a previous study, where cows with lesions compatible with PTB were detected (Miranda, 2005). We decided to evaluate cows from these farms to confirm if this illness was a factor to select cows for culling. Pathology reports suggest that 80% of the causes to cull a cow from a productive farm are due to low milk production, weight loss, or illness related to decreased fertility. Nevertheless, in some cases it is recommended to perform histopathology and bacterial isolation to obtain a definitive diagnosis of the illness. Only 2.5% (1/40) of the culled cows presented macroscopic lesions compatible with PTB in the present study; histopathology resulted in diagnosing this disease in 10% of cows, which was confirmed by positive bacterial isolation and molecular identification by PCR.
Only one out of the 40 culled cows was taken to the necropsy room with presumptive PTB diagnosis. This cow presented emaciation and diarrhea, and acidfast bacilli were observed in samples obtained from smears of rectal scraping. This procedure should be considered insufficient since PTB diagnosis in this area is considered positive by associating with bacillary morphology of the clinical microscopic aggregates. However, this procedure did not detect PTB in all the infected cows, since mycobacterial excretion in feces is intermittent and AFB determination is not specific for Map. Presence of additional damage to the rectum only occurred in advanced stages; therefore it is recommended to use more sensitive and specific tests, such as ELISA and PCR (Clarke, 1997; Oble, 1980).
The frequency of positive PTB cases found in this study was greater than that reported in previous CAITSA studies (Oble, 1980). Sometimes, infected cows with Map only develop tissue lesions; thus, diagnosis after necropsy underestimates the true proportion of PTB cases (González et al., 2005). In the present study, three colonies were cultured using intestinal samples from histological lesions compatible with PTB; however, we did not find reports of Map isolates from samples without apparent tissue lesions (González et al., 2005).
We determined through specific PCR type test that the three presumptive Map isolates of developed colonies from positive histological samples corresponded to type C strain, which has already been described as the most common strain in goats and other ruminants in Mexico (Estévez-Denaives et al., 2007). As mentioned before, a large proportion of culled cows from CAITSA farms are sold to other dairy farms or local butchers and may be sub-clinically infected and undiagnosed, representing a potentially greater number of culled animals that may be infected with Map. Additionally, it is likely that Map is disseminated to other farms through this practice. Further testing is needed to determine PTB prevalence in Mexico and eventually establish control strategies. Currently there are no campaigns to limit mobilization of PTB ruminants, which allows this mycobacteriosis to silently spread across the country (Möbius et al., 2008). In addition to feces, handling of cows might increase PTB prevalence in farms where infected cows do not present evident PTB lesions (Crossley et al., 2005). The use of sensitive tests, such as histopathology and PCR, are highly recommended to better understand the epidemiology of PTB.
Acknowledgements
This study was supported by PAPIIT IT225211 grant to GCG. We would like to thank Luis Antonio Morales Arreola for histological and photography assistance, and Jaime Eugenio Cordova for his support to this study.
Conflicts of interest
The authors declare they have no conflicts of interest with regard to the work presented in this report.
Notes
¤To cite this article: Castillo-Velásquez U, Gómez-Flores R, Chávez-Gris G, Favila-Humara LC, Soto-Castor R, Maldonado-Castro E, Tamez-Guerra P. Presence of paratuberculosis in dairy cows culled in Tizayuca (Hidalgo, México). Rev Colomb Cienc Pecu 2015; 28:174-180.
References
Clarke CJ. The pathology and pathogenesis of paratuberculosis in ruminants and other species. J Comp Pathol 1997; 116:217-261.
Collins DM, De Zoete M, Cavaignac SM. Mycobacterium avium subsp. paratuberculosis strains from cattle and sheep can be distinguished by a PCR test based on a novel DNA sequence difference. J Clin Microbiol 2002; 40:4760-4762.
Coussens PM. Mycobacterium paratuberculosis and the bovine immune system. Anim Health Res Rev 2001; 2:141-161.
Crossley BM, Zagmutt-Vergara FJ, Fyock TL, Whitlock RH, Gardner IA. Fecal shedding of Mycobacterium avium subsp. paratuberculosis by dairy cows. Vet Microbiol 2005; 107:257-263.
Estévez-Denaives I, Hernández-Castro R, Trujillo-García AM, Chávez-Gris G. Detection of Mycobacterium avium subsp. paratuberculosis in goat and sheep flocks in Mexico. Small Rum Res 2007; 72:209-213.
Gilardoni LR, Paolicchi FA, Mundo SL. Bovine paratuberculosis: a review of the advantages and disadvantages of different diagnostic tests. Rev Argent Microbiol 2012; 44:201-215.
Gonzalez J, Geijo MV, Pariente CG, Verna A, Corpa JM, Reyes LE, Ferreras MC, Juste RA, Marin JFG, Perez V. Histopathological classification of lesions associated with natural paratuberculosis infection. J Comp Pathol 2005; 133:184-196.
Juste RA, Marco JC, Saez de Ocaris C, Adúriz JJ. Comparison of different media for isolation of small ruminant strains of Mycobacterium paratuberculosis. Vet Microbiol 1991; 28:385-390.
Miranda BMV. Evaluación del impacto económico de la paratuberculosis en ganado bovino lechero (Sistema intensivo), en el Complejo Agropecuario Industrial de Tizayuca, Hidalgo. [M Sci thesis]. México D. F.: Facultad de Medicina Veterinaria y Zootecnia, UNAM; 2005.
Möbius P, Hotzel H, Rassbach A, Köhler H. Comparison of 13 single-round and nested PCR assays targeting IS900, ISMav2, f57 and locus 255 for detection of Mycobacterium avium subsp. paratuberculosis. Vet Microbiol 2008; 126:324-333.
Oble HI. Análisis de las causas de mortalidad de bovinos adultos en el Complejo Agropecuario Industrial de Tizayuca, Hidalgo, de 1980 a 1984. [Tesis de licenciatura]. México D. F.: Facultad de Medicina Veterinaria y Zootecnia, UNAM; 1980.
Ramírez C, Trigo E, Suárez F, Merkal R. Aislamiento e identificación de Mycobacterium paratuberculosis en México. Téc Pec Méx 1979; 36:74-75.
Tiwari A, VanLeeuwen JA, Dohoo IR, Keefe GP, Weersink A. Estimate of the direct production losses in Canadian dairy herds with subclinical Mycobacterium avium subspecies paratuberculosis infection. Can Vet J 2008; 49:569-576.
Whittington RJ, Hope AF, Marshall DJ, Taragel CA, Marsh I. Molecular epidemiology of Mycobacterium avium subsp. paratuberculosis: IS900 restriction fragment length polymorphism and IS1311 polymorphism analyses of Isolates from animals and a human in Australia. J Clin Microbiol 2000; 38:3240-3248.