Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Colombiana de Ciencias Pecuarias
Print version ISSN 0120-0690
Rev Colom Cienc Pecua vol.28 no.2 Medellín Apr./June 2015
https://doi.org/10.17533/udea.rccp.v28n2a07
ORIGINAL ARTICLE
doi: 10.17533/udea.rccp.v28n2a07
Coxiella burnetii in bulk tank milk and antibodies in farm workers at Montería, Colombia¤
Coxiella burnetii en leche de tanque y anticuerpos en trabajadores rurales en Montería, Colombia
Coxiella burnetii em leite de tanque bovina e anticorpos em trabalhadores rurais de Montería, Colômbia
Verónica Contreras1, Biol, MSc; Salim Máttar1*, Ph.D; Marco González1, MV, MSc; Jaime Álvarez1, MV, MSc; José A Oteo2 MD, Ph.D.
1Instituto de Investigaciones Biológicas del Trópico (IIBT), Universidad de Córdoba, Montería, Colombia.
2Centro de Investigación Biomédica de La Rioja (CIBIR), La Rioja, España.
*Corresponding Author: Salim Máttar. Instituto de investigaciones Biológicas del Trópico (IIBT). Universidad de Córdoba. Km. 26 vía Cereté - Ciénaga de Oro, Berástegui, Córdoba. Tel: +57 (4) 7569027. Email: mattarsalim@hotmail.com
Received: March 4, 2014; accepted: August 20, 2014
Summary
Background: Q fever is a zoonotic disease caused by Coxiella burnetii. In Colombia it is not a notifiable disease in humans and is most likely under diagnosed. There are no studies about its prevalence in important reservoir species, such as cattle. Objective: the aim of this study was to investigate the frequency of C. burnetii infection in cattle farms and determine the frequency of antibodies in farm workers at risk in rural areas of Montería, Córdoba (Colombia). Methods: eleven cattle farms were randomly chosen to investigate the infection by C. burnetii. Bulk tank milk samples of each farm were analyzed by conventional PCR for DNA detection of transposase gene IS1111 of C. burnetii. Serum samples from 61 apparently healthy people living in eight farms were analyzed by indirect inmunofluorescence against phase II IgG antibodies to C. burnetii. Results: we report the presence of C. burnetii DNA in 45% of bulk tank milk samples from cattle farms and a 61% frequency of antibodies (IgG phase II ≥1/64) in farm workers at risk. Conclusion: our results demonstrate the circulation of this bacterium in the studied farms in Montería, Colombia, showing that at-risk farm workers have a high antibody frequency.
Keywords: cattle, prevalence of diseases, Q fever, serology, zoonoses.
Resumen
Antecedentes: la fiebre Q es una zoonosis causada por Coxiella burnetii. En Colombia no es una enfermedad notificable en humanos y probablemente es subdiagnosticada. De otro lado, no se han realizado estudios acerca de su prevalencia en importantes reservorios como los bovinos. Objetivos: el objetivo de este estudio fue determinar la frecuencia de infección por C. burnetii en fincas de ganado bovino y determinar la frecuencia de la presencia de anticuerpos en trabajadores de fincas en riesgo en áreas rurales del municipio de Montería, Córdoba (Colombia). Métodos: once fincas de ganado bovino fueron aleatoriamente seleccionadas para investigar la frecuencia de infección por C. burnetii. Muestras de leche de tanque de cada finca fueron analizadas mediante PCR convencional para detección del gen transposasa IS1111 de C. burnetii. Asimismo, se colectaron muestras de suero sanguíneo de 61 personas aparentemente saludables que vivían en ocho de las fincas estudiadas, las cuales fueron analizadas mediante el ensayo de inmunofluorescencia indirecta para detección de anticuerpos IgG contra fase II de C. burnetii. Resultados: en este estudio se reporta la presencia de ADN de C. burnetii en 45% de las muestras de leche de tanque de las fincas ganaderas estudiadas y una frecuencia de anticuerpos contra C. burnetii (IgG Fase II ≥1/64) del 61% en trabajadores de fincas en riesgo. Conclusiones: los resultados de este estudio demuestran la circulación de C. burnetii en las fincas estudiadas de un área de Montería, Colombia. También, los trabajadores de fincas en situación de riesgo presentan una alta frecuencia de anticuerpos contra este patógeno.Palabras clave: bovinos, fiebre Q, prevalencia de enfermedades, serología, zoonosis.
Resumo
Antecedentes: a febre Q é uma zoonose causada por Coxiella burnetii. Na Colômbia não é uma doença de notificação obrigatória em seres humanos e é provavelmente subdiagnosticada. Além disto, não há estudos sobre sua prevalência nas principais espécies de reservatórios, como os bovinos. Objetivos: determinar a frequência de infecção por C. burnetii em fazendas de gado de leite e determinar a frequência de anticorpos em trabalhadores rurais em risco do município de Montería, Córdoba (Colômbia). Métodos: 11 fazendas de gado leiteiro foram selecionadas aleatoriamente para investigar a frequência de infecção por C. burnetii. Amostras de leite do tanque de cada fazenda foram analisadas por PCR convencional para a detecção do gene IS1111 transposase de C. burnetii. Além disso, amostras de soro de 61 pessoas aparentemente saudáveis que vivem em oito das propriedades estudadas foram analisadas por imunofluorescência indireta para a detecção de anticorpos IgG contra C. burnetii fase II. Resultados: neste estudo, o DNA de C. burnetii foi encontrado em 45% das amostras de leite do tanque, e uma frequência de anticorpos contra C. burnetii (fase II IgG ≥ 1/64) de 61% em trabalhadores rurais em risco. Conclusões: os resultados deste estudo demonstram a circulação de C. burnetii em algumas fazendas de gado em uma área de Montería, Colômbia. Além disso, os trabalhadores rurais em situação de risco têm uma alta frequência de anticorpos contra este patógeno.Palavras chave: bovinos, febre Q, prevalência de doenças, sorologia, zoonose.
Introduction
Q fever is a zoonotic disease caused by Coxiella burnetii that concerns public health throughout the world. Domestic ruminants such as cattle, goats and sheep are known to be the principal source of human infection (Maurin and Raoult, 1999). Bacterial shedding occurs in mammals trough placenta, birth fluids, vaginal mucus, feces, and milk (Berri et al., 2001; Arricau-Bouvery et al., 2003; Guatteo et al., 2007a; Rodolakis et al., 2007). C. burnetii is a frequent cause of reproductive disorders in these hosts (Berri et al., 2002; Guatteo et al., 2007b). In goats, C. burnetii has been reported as a cause of abortions and stillbirths in 19% of 211 cases reported in California, USA (Moeller, 2001). It was identified in Switzerland as a cause of abortions in 10% of 144 cases (Chanton-Greutmann et al., 2002). These reports are more abundant than those found for cattle and sheep (approximately 1% or less; Kirkbride, 1993; Muskens, et al., 2012). Metritis and infertility are clinical signs of infection in cattle; however the infection is frequently subclinical (Rodolakis, 2009) despite the occurrence of C. burnetii shedding (Rodolakis et al., 2007).
Inhalation of dust contaminated with the bacteria from feces, urine or birth products from infected animals is the main source of infection in humans, resulting in several clinical manifestations; however, 60% of infections could lead to asymptomatic seroconversion (Maurin and Raoult, 1999). Most human patients experience a nonspecific febrile illness during acute infection. Atypical pneumonia or febrile hepatitis may also occur. The disease can become chronic, manifested as endocarditis, chronic hepatitis, and osteomyelitis, or as a chronic vascular infection (Anderson et al., 2013).
Only few cases of Q fever have been reported in Colombia, though it could be more prevalent. A human case of endocarditis caused by C. burnetii was reported in Medellín, Colombia in 2012 (Betancur and Múnera, 2012) and at the end of that year a case of pneumonia caused by Coxiella was reported in Cali, Colombia (Cardona, 2012). There are no previous studies conducted in domestic ruminants. It is not known if people in contact with cattle are at risk of infection. The aim of this study was to investigate the frequency of C. burnetii infection in cattle farms and to determine the frequency of antibodies in farm workers from rural areas of Montería, Colombia.
Material and methods
Ethical statement
The research ethics committee of the Institute of Tropical Biological Research at Universidad de Córdoba approved the study through Act 026-2011. Samples were taken after participants signed an informed consent.
Sampling
Eleven cattle farms in rural areas of Montería, Colombia (8º 48´4694'' N; 75º 54´5415'' W) were randomly chosen for C. burnetii infection research during July and August, 2012. These farms were chosen from a dairy area, which receives milk from 60% of the 3341 registered farms in Montería. The farms studied had a dual-purpose production system (milk and meat). All farms had between 150 and 600 animals and initial C. burnetii infection status was unknown. Cases of sporadic abortions and mastitis were common in all farms tested. 50 ml samples of bulk tank milk (BTM) per farm were collected into sterile plastic tubes and screened using PCR methods. Serum samples from 61 apparently healthy people who lived in eight farms were analyzed by indirect inmunofluorescence assay (IFA). Epidemiological data was gathered from all participants who were asked about the possibility of Q fever occurrence in the past. InfoStat software (Version 2013; Group InfoStat, FCA, Argentina) was used for statistical analysis (Di Rienzo et al., 2013). A Chi square was conducted to establish dependence between variables obtained from humans and frequency of antibodies against C. burnetii.
DNA extraction and molecular detection of Coxiella burnetii
Milk samples and a negative control (sterile water) were subjected to DNA extraction following the manufacturer's instructions (Purelink, DNA mini kit, Invitrogen, CA, USA). DNA extraction was performed directly from 400 μl of homogenized whole milk and eluted in a final volume of 100 μl. To ensure there was no contamination, a negative control (sterile water) was included for DNA extraction. Purified DNA was stored at -20 ºC until use as a template for polymerase chain reaction (PCR). A conventional PCR was conducted with primers CoxP4 (5´-GGCTGCGTGGTGATGG; Genbank accession number: M80806) and CoxM9 (GTCCCGGTTCAACAATTCG), which amplify a 435 bp product of the transposase gene of C. burnetii (IS1111; Panning et al., 2008). Positive control was not used to avoid contaminations. Amplification was carried out in 50 μl total reaction volume with 1 PCR buffer 1X, 4 μl MgCl2 (25mM), 2 μl DNTPs (10 mM), 25 μl Taq Polymerase (made at home), 1.5 μl of each primer (5 μM), and 2 μl total DNA. The products were separated by electrophoresis on agarose gel (1.5%), stained with ethidium bromide and examined using an ultraviolet (UV) transilluminator.
Indirect inmunofluorescence assay (IFA)
Blood serum samples were used to detect IgG antibodies against phase II of C. burnetii using an indirect inmunofluorescence kit according to manufacturer's instructions (Ref. PCOBUI+II, Vircell, Granada, Spain). Sensitivity and specificity of this particular IgG test are 97 and 99%, respectively (Vircell, Granada, Spain). A negative and positive control was included in each assay, and the positive samples were confirmed. A title ≥ 1:64 was taken as suggestive of past or present infection. Positive sera with IgG titers ≥ 1:64 were diluted at 1:128, 1:256, 1:512, and 1:1024.
Results
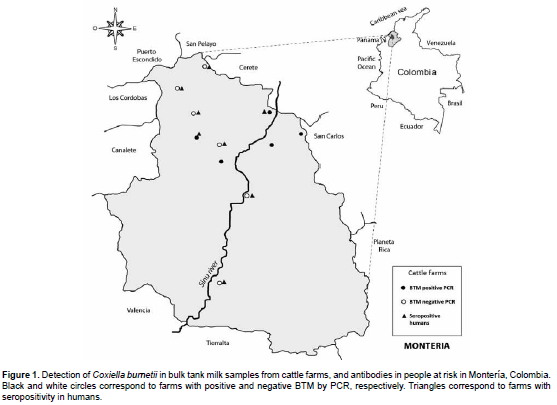
Coxiella burnetii DNA was detected in 5 out of 11 BTM samples (45%). After sequencing positive samples, all of them were 100% identical to the sequence of C. burnetii strain CbuK 154Q (Genbank accession number CP001020). Location of farms with positive and negative bulk milk samples is indicated in Figure 1.
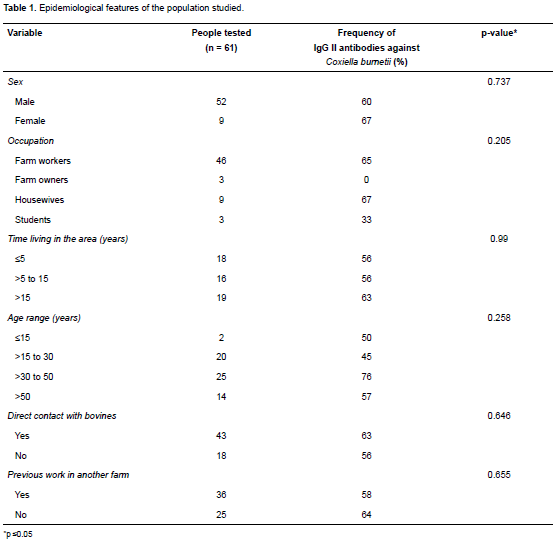
Of the 61 people involved in the serological study, 52 were male and 9 female; 84% of men were farm workers and their mean age was 38.26 years (SD 13, 63). 61% (37/61) had IgG antibodies against C. burnetii phase II. End titers were determined for all seropositive participants: IgG II ≥1:64 (n = 24); ≥1:128 (n = 3), ≥1:256 (n = 7), and ≥1:1024 (n = 3). The epidemiological features of the studied population are summarized in Table 1. No differences were found between antibody frequency according to gender, age and time living in the area.
Discussion
We report the presence of C. burnetii DNA in BTM of cattle from Montería, Northern Colombia, and have not found similar reports in this country. These results demonstrate the circulation of C. burnetii in some cattle herds of an area of Montería. PCR analysis of BTM is a useful tool that could be used to detect, at large scale, infection by C. burnetii in cattle considering the low cost of the technique and the easiness of sample collection (Guatteo, 2007). In this study, the use of CoxP4 and CoxM9 primers (Panning et al., 2008) for amplification of a fragment (435 pb) from IS1111gene allowed to increase assay sensitivity, because this gene is present at multiple copy number (7 to 110 copies), depending on C. burnetii strains (Klee et al., 2006).
According to our findings, although the number of samples analyzed was low, BTM infection frequency was high (45%). These results are similar to a report from the United States, where 9 of 21 raw milk samples (42%) from seven states contained C. burnetii DNA (Loftis et al., 2010). Other studies based on BTM testing showed that more than 90% of US dairy herds were infected with C. burnetii (Kim et al., 2005). PCR detection of C. burnetii in bovine milk has not been totally standardized because bacterium excretion patterns by cattle can be intermittent or continuous (Rodolakis, 2007). We cannot conclude that the farms with negative results were totally free of C. burnetii infection (Guatteo et al., 2007). Although small ruminants (sheep and goats) are also an important source of C. burnetii (Rodolakis, 2009), presence of these animals in the farms was not investigated in this study. Further epidemiological studies are necessary to establish the frequency of C. burnetii shedding in cattle and small ruminants.
People sampled worked for eight of the studied farms. Antibody presence was detected in people belonging to all BTM-negative farms and two farms with positive BTM (Figure 1). We were not able to obtain human blood samples from the remaining three BTM-positive farms. A high frequency (61%) of IgG antibodies against C. burnetii phase II was found, but we cannot conclude that the infection of these individuals was acquired in the studied farms because 59% of them had previously worked in other farms. However, no differences were found in antibody frequencies in people with or without history of working in other premises (Table 1). All individuals tested in our study were clinically healthy and did not have a previous Q fever diagnosis, although febrile episodes were frequently recorded. According to these data, it is very feasible that human cases of Q fever are underdiagnosed in the studied area. Accordingly, physicians should be informed and look for this condition.
These results are higher than those informed in a previous study where 24% overall seroprevalence was found in rural workers from five villages of Córdoba and Sucre, Colombia (Máttar, 2006). Population-proportion differences of both studies were highly significant (p<0.001). However, seroprevalence among the five villages in the previous study varied from 0% to 61.5%, which is similar to our study. On the other hand, our results are higher than reports from other countries where Q fever is prevalent. A 3% seroprevalence was reported in Dane farmers in close contact with cattle (Bosnjak, et al., 2012), and 19.5% seroprevalence was reported in healthy Turkish farm workers (Seyitoglu, et al., 2006). The results of the present study are similar to a report from the Netherlands where overall seroprevalence in cattle farm residents was 72%. Of these, 87%, 54%, and 44% of farm workers, housewives and children had antibody titers, respectively. Antibody frequency in housewives and farm workers was similar in our study (Table 1).
In conclusion, our study suggests that C. burnetii is frequent in some Montería cattle farms. At-risk farm workers had high antibody frequency; all of them were clinically healthy and were not previously diagnosed with Q fever.
Acknowledgements
The authors gratefully acknowledge the farms owners and rural workers who joined the project. Thanks also to Dr Ana L. García-Pérez and Diva Santana. To Universidad de Córdoba, Project CIUC Code 1-2-08-110-26, Numeral FMV 04-11, who funded this research.
Conflicts of interest
The authors declare they have no conflicts of interest with regard to the work presented in this report.
Notes
¤To cite this article: Contreras V, Máttar S, González M, Álvarez J, Oteo JA. Coxiella burnetii in bulk tank milk and antibodies in farm workers at Montería, Colombia. Rev Colomb Cienc Pecu 2015; 28:181-187.
References
Anderson A, Bijlmer H, Fournier P, Graves S, Hartzell J, Kersh GJ, Limonard G, Marrie TJ, Massung RF, McQuiston JH. Diagnosis and Management of Q Fever. Recommendations from CDC and the Q Fever Working Group. MMWR 2013; 62:1-23. [ Links ]
Arricau-Bouvery N, Souriau A, Lechopier P, Rodolakis A. Experimental Coxiella burnetii infection in pregnant goats: excretion routes. Vet Res 2003; 34:423-433. [ Links ]
Berri M, Souriau A, Crosby M, Crochet D, Lechopier P, Rodolakis A. Relationships between the shedding of Coxiella burnetii, clinical signs, and serological responses of 34 sheep. Vet Rec 2001; 148:502-505. [ Links ]
Berri M, Souriau A, Crosby M, Rodolakis A. Shedding of Coxiella burnetii in ewes in two pregnancies following an episode of Coxiella abortion in a sheep flock. Vet Microbiol 2002; 85:55-60. [ Links ]
Betancur CA, Múnera AG. Coxiella burnetii endocarditis: Q fever. Acta Médica Colombiana 2012; 37:31-33. [ Links ]
Bosnjak E, Hvass AMSW, Villumsen S, Nielsen H. Emerging evidence for Q fever in humans in Denmark: role of contact with dairy cattle. Clin Microbiol Infec 2010; 16:1285-1288. [ Links ]
Cardona, JC. Neumonía por Coxiella burnetii: presentación de un caso y revisión de la literatura/Coxiella burnetii pneumonia: case report and literature review. CES Medicina 2012; 26:201-208. [ Links ]
Chanton-Greutmann H, Thoma R, Corboz L, Borel N, Pospischil A. Abortion in small ruminants in Switzerland: investigations during two lambing seasons with special regard to Chlamydiae. SAT, Schweiz Arch Tierh 2002; 144(9):483-492. [ Links ]
Di Rienzo JA, Casanoves F, Balzarini MG, Gonzalez L, Tablada M, Robledo CW. InfoStat versión 2013. Grupo InfoStat, FCA, Universidad Nacional de Córdoba, Argentina. URL: http://www. infostat.com.ar [ Links ]
Guatteo R, Beaudeau F, Joly A, Seegers H. Coxiella burnetii shedding by dairy cows. Vet Res 2007a; 38:849-860. [ Links ]
Guatteo R, Beaudeau F, Joly A, Seegers H. Assessing the withinherd prevalence of Coxiella burnetii milk-shedder cows using a real-time PCR applied to bulk tank milk. Zoonoses Public Hlth 2007b; 54:191-194. [ Links ]
Kim SG, Kim EH, Lafferty C, Dubovi E. Coxiella burnetii in bulk tank milk samples, United States. Emerg Infect Dis 2005; 11:619-621. [ Links ]
Kirkbride CA. Diagnoses in 1,784 ovine abortions and stillbirths. J Vet Diagn Invest 1993; 5:398-402. [ Links ]
Klee SR, Tyczka J, Ellerbrok H, Franz T, Linke S, Baljer G, Appel B. Highly sensitive real-time PCR for specific detection and quantification of Coxiella burnetii. BMC Microbiology 2006; 6(1):2. [ Links ]
Loftis AD, Priestley RA, Massung RF. Detection of Coxiella burnetii in commercially available raw milk from the United States. Foodborne Pathog Dis 2010; 7:1453-1456. [ Links ]
Máttar S, Parra M. Detection of antibodies to Anaplasma, Bartonella and Coxiella in rural inhabitants of the Caribbean area of Colombia. Rev. MVZ Córdoba 2006; 11:781-789. [ Links ]
Maurin M, Raoult D. Q fever. Clin Microbiol Rev1999; 12: 518-553. [ Links ]
Moeller RB. Causes of caprine abortion: diagnostic assessment of 211 cases (1991-1998). J Vet Diagn Invest 2001; 13:265-270. [ Links ]
Muskens J, Wouda W, von Bannisseht-Wijsmuller T, Van Maanen C. Prevalence of Coxiella burnetii infections in aborted fetuses and stillborn calves. Vet Rec 2012; 170:260. [ Links ]
Panning M, Kilwinski J, Greiner-Fischer S, Peters M, Kramme S, Frangoulidis D, Meyer H, Henning K, Drosten C. High throughput detection of Coxiella burnetii by real-time PCR with internal control system and automated DNA preparation. BMC Microbiology 2008; 8:77. [ Links ]
Rodolakis A. Q Fever in dairy animals. Ann NY Acad Sci 2009; 1166:90-93. [ Links ]
Rodolakis A, Hechard C, Caudron C, Souriau A, Bodier CC, Blanchard B, Camuset P, Devillechaise P, Natorp JC. Comparison of Coxiella burnetii shedding in milk of dairy bovine, caprine, and ovine herds. J Dairy Sci 2007; 90:5352-5360. [ Links ]
Seyitoglu S, Özkurt Z, Dinler U, Okumus B. The seroprevalence of coxiellosis in farmers and cattle in Erzurum district in Turkey. Turk J Vet Anim Sci 2006; 30(1). [ Links ]