Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Colombiana de Ciencias Pecuarias
Print version ISSN 0120-0690
Rev Colom Cienc Pecua vol.28 no.2 Medellín Apr./June 2015
https://doi.org/10.17533/udea.rccp.v28n2a08
ORIGINAL ARTICLE
doi: 10.17533/udea.rccp.v28n2a08
Microstructural and physicochemical analysis of collagen in intramuscular pin bones of Bocachico fish (Prochilodus sp.)¤
Análisis microestructural y fisicoquímico del colágeno presente en espinas intramusculares del pescado Bocachico (Prochilodus sp.)
Análise microestrutural e físico químico do colágeno presente em espinhos intramusculares do peixe Bocachico (Prochilodus sp.)
Héctor Suárez1*, MVZ, PhD; Oscar Gaitán2, Qui, MSc; Consuelo Díaz1, IA PhD.
1Instituto de Ciencia y Tecnología de Alimentos ICTA, Universidad Nacional de Colombia, Bogotá, Colombia.
2Maestría en Ciencia y Tecnología de Alimentos, Universidad Nacional de Colombia, Bogotá, Colombia.
*Corresponding author: Héctor Suárez. Instituto de Ciencia y Tecnología de Alimentos ICTA, Universidad Nacional de Colombia, Bogotá, Colombia. Carrera 30 #45-03 edificio 500C ICTA; Email: hsuarezm@unal.edu.co
Received: September 18, 2013; accepted: June 7, 2014
Summary
Background: the presence of intramuscular pin bones hinders the production and commercialization of fish fillet products; however, application of physical processes, such as thermal treatments, offers alternatives for the degradation of said bones. Objective: the present study aimed to conduct a microstructural and physicochemical analysis of Bocachico intramuscular pin bones subjected to a thermal treatment. Methods: collagen extracted from intramuscular pin bones of Bocachico fillets was analyzed using SDS-polyacrylamide gel electrophoresis and viscosity. Pin bones were subjected to 1.5, 2, and 3 minutes heating time and analyzed using electron microscopy and cutting force. Results: intramuscular pin bones contain type I collagen. Threeminute thermal treatment degraded collagen components present in the internal pin bone structure, coinciding with the lowest values of the cutting force test. Conclusions: according to our results, collagen degradation initiates in the internal structure of intramuscular pin bones and moves towards the external layer which does not show the effects of thermal treatment.
Keywords: aquaculture, connective tissue, meat, microstructure.
Resumen
Antecedentes: la presencia de espinas intramusculares en filetes de peces impide la obtención y posterior comercialización de estos productos, motivo por el cual la aplicación de procesos físicos antes del tratamiento térmico podría ser una alternativa en la degradación de estas espinas. Objetivo: el alcance del presente estudio fue realizar análisis fisicoquímico y microestructural de espinas intramusculares de Bocachico sometidas a proceso térmico. Métodos: las espinas intramusculares de filetes de Bocachico fueron analizadas a través de electroforesis en gel de SDS-poliacrilamida (SDS-PAGE), temperatura de desnaturalización del colágeno, microscopia electrónica de trasmisión y fuerza de corte. Fueron utilizados tres tiempos de duración del tratamiento térmico (1,5, 2 y 3 min). Resultados: el colágeno presente en espinas intramusculares es tipo l. El tratamiento térmico durante tres minutos degrada los componentes del colágeno en las estructuras internas de la espina, coincidiendo con los valores menores para la prueba de fuerza de corte. Conclusiones: los resultados permiten concluir que la degradación del colágeno es iniciada desde el interior de la estructura de la espina intramuscular hacia la capa externa, sin que esta capa muestre efecto del tratamiento térmico.Palabras clave: acuicultura, carne, microestructura, tejido conectivo.
Resumo
Antecedentes: a presença de espinhos intramusculares impede a obtenção de filetes de peixe e posterior comercialização destes produtos, razão pela qual a aplicação de processos físicos antes do tratamento térmico pode ser uma alternativa na degradação desses espinhos. Objetivo: realizar analise físico-químico e microestrutural em espinhos intramusculares do peixe Bocachico submetidos a processo térmico. Métodos: os espinhos intramusculares de filetes do Bocachico foram analisados por eletroforese em gel de SDSpoliacrilamida (SDS-PAGE), temperatura de desnaturação do colágeno, microscopia electrónica de transmissão e força de corte. Foram utilizados três tempos de duração do tratamento térmico (1,5, 2 e 3 min). Resultados: o colágeno presente em espinhos intramusculares é do tipo l. O tratamento térmico por três minutos degrada os componentes do colágeno nas estruturas internas do espinho, coincidindo com os menores valores para o teste de resistência ao corte. Conclusões: os resultados mostram que a degradação do colágeno é iniciado desde o interior do espinho intramuscular até a capa externa, sem que essa capa externa seja afetada pelo tratamento térmico.Palavras chave: aquicultura, carne, microestrutura, tecido conjuntivo.
Introduction
Bocachico fish is the common name of Prochilodus sp. It is native to the watersheds of various South American countries. In Colombia, it is traditionally farmed and consumed, although production is low. The presence of intramuscular pin bones hindered the production and commercialization of Bocachico fillets until new technologies were developed, facilitating fillet production from native fish species with intramuscular pin bones. These bones are subjected to cross sectioning; allowing for collagen degradation during the cooking process. (Mesa- Granda et al., 2006; Suarez et al., 2008; Suárez- Mahecha et al., 2009). Cuts in intramuscular pin bones expose their internal components. However, it is unknown what type of collagen is associated with intramuscular pin bones of Bocachico. The effect that thermal treatment could have on collagen degradation is also unknown.
The molecular structure of fish collagen is influenced by the amount of amino acids present in cold water (temperate) and warm water (tropical) species (Regenstein et al., 2007). Furthermore, hydroxyproline content is much higher in collagen of tropical fish when compared to temperate fish. Comparatively, mammals have higher hydroxyproline content in collagen. These differences are important for collagen stability.
Pin bones are formed by collagen and hydroxyapatite [Ca10 (PO4)3 (OH)2; also known as bioapatite, hydroxyl apatite, carbonate of apatite] among other components, such as lipids and water. Type I collagen represents approximately 20% of bone mass, 35% of bone volume and more than 90% of the organic bone matrix (Pasteris et al., 2008). The principal difference between type I collagen in different tissues (e.g., skin and bone) is the arrangement and reticulation of collagen fibers (Hanson and Eyre, 1996; Bailey et al., 1998). Collagen is a triple helix molecule composed of three chains. In mammals, collagen has two identical helixes (α1; α2) and a third helix [(α1)2 α2 heterotrimeric].
A third of the total amino acids in collagen are glycines. Proline and hydroxyproline are also present in collagen. This high hydroxyproline content is unusual among proteins and is directly related to the stability and insolubility of the molecule. In comparison with collagen, the chemical and mechanical properties of the mineral phase of bone (hydroxyapatite) are not well known. Hydroxyapatite has two functions in mineralized tissues: one is largely mechanical (rigidity and resistance of the structure); and the other is physiological (serving as an ion reservoir; Glimcher, 1998). Hydroxyapatite is a mineral phase characterized by small crystals (Noto, 2011). The size and conformation of hydroxyapatite crystals is similar in fish and mammal bones (Kim et al., 1995) but there is large variability in the size of hydroxyapatite crystals between tissues and taxonomic groups (Fratzl et al., 2004; Olszta et al., 2007). Mineralized bone contains approximately 66% in weight and 50% in volume of hydroxyapatite, with little variation. Fish, contrary to other vertebrates, contain fewer minerals (Biltz and Pellegrino, 1969), reflected by the low bone density of some fish species (Smith, 2008). The relative proportions of organic and inorganic components, as well as the interactions between these components, are largely responsible for the physical properties of bone, such as strength, hardness and rigidity (Pasteris et al, 2008). The objective of this study was to obtain intramuscular pin bones from thermal-treated Bocachico fillets and determine their ultrastructural and physiochemical variations.
Material and methods
Intramuscular bones were obtained from Bocachico fillets (Prochilodus sp.) weighing between 400 and 500 g. Fillets were cut into 4-mm sections. Fish were obtained from an aquaculture market.
Preparation of intramuscular bone collagen
Collagen was obtained using the method proposed by Nagai and Suzuki (2000). All preparation procedures were conducted at 4 °C. Bones were treated with 0.1 N NaOH to eliminate non-collagen proteins, washed with distilled water and lyophilized. Pin bones were decalcified with 0.5 M of ethylenediaminetetraacetic acid-EDTA (pH 7.4) for 5 days, changing the EDTA solution once a day. The residue was then washed with distilled water.
Fat was eliminated with 10% butyl alcohol. Then, the residue was washed with distilled water and lyophilized. The insoluble material was extracted with 0.5 M acetic acid for 3 days and the extract was centrifuged at 20,000 x g for 1 h. The residue was again extracted with the same solution for 2 days and the extract was centrifuged under the same conditions. Each viscous solution was mixed and salted with the addition of NaCl for a final concentration of 0.9 M, followed by collagen precipitation with addition of NaCl (final concentration of 2.6 M) at a neutral pH (in 0.05 M of Tris-HCL, pH 7.5). The resulting precipitate was obtained by centrifugation at 20,000 x g for 1 h and was dissolved in 0.5 M acetic acid. Afterwards, it was dialyzed with 0.1 M acetic acid and distilled water and finally lyophilized.
Determination of collagen denaturalization
Determination of denaturalization temperature was based on the method described by Kimura et al. (1988): filling a cylinder viscosimeter with a 0.1% (m/v) solution of collagen in acetic acid and immersing the cylinder in a water bath at 30 °C for 30 min to allow the solution to reach equilibrium with water temperature.
Temperature was increased by 10 oC intervals until 50 °C was reached; each temperature was maintained for 10 min. Viscosity of the collagen solution was measured at temperature intervals of 2 °C between 30 °C and 50 °C. Fractional viscosity was calculated with the following equation:

Thermal denaturalization curves were obtained by graphing fractional viscosities versus temperature. The denaturalization temperature was the temperature at which the fractional viscosity was 0.5. To determine collagen denaturalization a concentric-cylinder viscosimeter was used (rotovisco rv20 model with M5 measuring system, Haake®, Berlin, Germany). Type I porcine collagen was used (Sigma®, Sigma-Aldrich Corp., St. Louis, MO, USA) as standard to compare.
SDS-polyacrylamide gel electrophoresis (SDSPAGE)
SDS-PAGE was carried out according to the method by Weber and Osborn (1969). The collagen sample was dissolved in 0.02 M sodium phosphate (pH 7.2) containing 1% SDS and 3.5 M urea. Electrophoresis was performed at 3.5% in 0.1 M buffer phosphate gel (pH 7.2) containing 0.1% SDS.
Cooking of intramuscular bones
Bocachico intramuscular pin bones were microwaved at 2450 MHz and 1800 watts for 3 min. This procedure was based on the method by Gokoglu et al. (2004).
Microstructural analysis
Microstructural analysis was carried out using a scanning electron microscope (SEM) (Quanta 200 model, FEI®, Columbus, Ohio, USA), registering the changes occurring in uncooked samples and in samples cooked for 1.5, 2, and 3 min. Ultrastructural alterations of intramuscular pin bones were described. The samples were fractured manually. A small amount of sample was fixed to a strip of self-adhesive, carbon paper, and polarized with a fine layer of gold-platinum for microstructural observation. Samples were observed using 15 kV.
Texture analysis (fracturability)
A texturometer was used for the fracturability analysis (TA.XT Plus model, Stabel Micro Systems, Stable Micro Systems®, Scarsdale, NY, USA) with 50 kg force capacity. The doubling technique in three points was used with fracturability changes determined in the pin bones pre- and post-cooking. This determination was carried out through compression force, considering the firmness, expressed in Newton (N), defined as the required mechanical force for the deformation of the material at the point of biorupture. A 3.0 mm diameter cylinder at a rate of 3 mm/s was used.
Results
Collagen denaturalization
Collagen denaturalization temperatures as obtained from the effect of temperature on viscosity are presented in Table 1.
Denaturalization temperature was around 31 °C. This value is lower than that reported for porcine skin (37 °C) and higher than that of skin collagen in other marine fish species, such as the Japanese sea-bass (Lateolabrax japonicus) 26.5 °C; chub mackerel (Scomber japonicus) 25.6 °C; and bullhead shark (Carcharhinus leucas) 25 °C (Nagai and Suzuki, 2000). However, Pati et al. (2010) reported 30 °C denaturalization temperature for sea bass pin bones. That is to say, various studies have reported collagen denaturalization temperature of cold-water fish lower than that of tropical-water fish (Nagai and Suzuki, 2000).
Determination of collagen in intramuscular pin bones
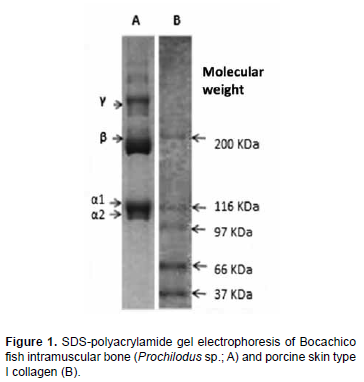
Figure 1 presents SDS-PAGE electrophoresis from Bocachico samples of intramuscular bone and porcine skin type I collagen. The results indicate the existence of two different subunits of α chain in intramuscular bones, confirming its type I collagen classification. Intramuscular bone had two different chains (α1 and α2), around 116KDa and 110KDa, respectively, coinciding with other studies on pin bones of tropical and temperate water fish (Ogawa et al., 2003; Ogawa et al., 2004). Figure 1 also shows the molecular chains of γ and β forms present in type I collagen in fish, with the β component present in the cross-links of the dimer chains and γ in the cross-links of the trimer chains.
Microstructural analysis
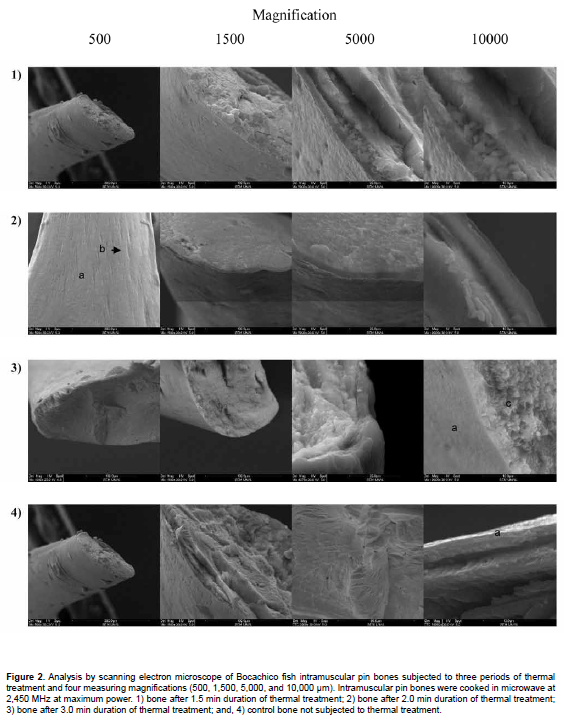
These results are presented in Figure 2. Microphotographs of the control sample show the structures in intramuscular pin bones. The external layer (a), easily discernible in all microphotographs, where the bone trabeculae are present (b), permits the passage of blood vessels that supply nutrients to the intramuscular bones. This layer does not show alterations due to the thermal treatment. Different layers of collagen and hydroxyapatite crystals (c) can also be seen, which were affected by the thermal treatment and are indistinguishable as a result of collagen degradation and possible alteration of the hydroxyapatite molecular structure. As duration of the thermal treatment increased, the structures began to lose their architectural arrangement and differentiation between layers. The results indicate that degradation of the structures formed by organic and inorganic components is predominant in the internal layers of the bones, while this is not readily discernible in the external layer.
Texture analysis (fracturabilty)
Results of the force analysis test resulted in statistical differences (p<0.05), with the lowest value for the three-minute thermal treatment (Table 2).
Discussion
Collagen denaturalization
Differences in denaturalization temperatures can be attributed to the amount of collagen and inorganic components present. It is estimated that collagen represents approximately 30% of pin bone components and the rest are inorganic substances such as calcium, phosphorus, sulfur and nitrogen (Mori et al., 2012).
Denaturalization temperature of collagen from different tissues, such as skin, pin bones and fins, is directly correlated with the content of amino acids such as proline and hydroxyproline (Burjanadze and Kisiriya, 1982). In addition to the spatial conformation of the triple helix in collagen, the quantity of these amino acids determines intraand intermolecular stability. On the other hand, denaturalization temperature of collagen from pig and heifer skin is 37 °C and 40.8 °C, respectively (Ikoma et al., 2003), both presenting high amino acid content. Meanwhile, cold water fish collagen has low denaturalization temperature because proline and hydroxyproline contents are low (Sadowska et al., 2003). Furthermore, differences in denaturalization temperatures have been reported for variations in habitat temperatures; for example, deep water fish have lower denaturalization temperature compared to fresh water fish and terrestrial mammals (Pati et al., 2010).
Collagen determination in intramuscular pin bones
Kimura et al. (1991) reported the presence of (α1)2 and α2 molecular forms of collagen 1 in carp as the principal components, and α1, α2, and α3 as secondary components. However, the present study did not find α3 chains, coinciding with Nagai and Suzuki (2000).
As previously mentioned, thermal stability of collagen is directly related to the abundance of proline and hydroxyproline, where high contents of these amino acids increase thermal stability due to a higher density of the cross-links. Furthermore, age can also affect collagen stability due to formation of Schiff bases that confer higher stability to the molecule (Suárez et al., 2002). However, fish used in this study were obtained from aquaculture and were not older than 8 months of age.
Microstructural analysis
Collagen strength and bone mineralization can be affected by age, so culture time of fish is an important factor that must be considered when contemplating fillet production using species with intramuscular bones. According to the previous descriptions (Norman et al., 1996; Zioupos et al., 1999), collagen concentration with aging, for example in human bone, contributes to porosity and resistance to fractures, while pin bone mineralization gives rigidity and resistance (Wang et al., 2000). In this sense, the relationship between mineral and organic phases of pin bones is not well understood (Katti et al., 2010). Various studies have suggested that most hydroxyapatite crystals in pin bones are produced within the collagen fibrils (Weiner et al., 1999; Jäger and Fratzl, 2000), while other authors (Hellmich and Ulm, 2002; Sasaki et al., 2002; Fritsch and Hellmich, 2007) suggest hydroxyapatite is produced outside the fibrils. In this manner, resistance of the collagen-mineral interaction is conferred by different molecular links (i.e., hydrogen links, Van der Waals interactions, and hydrophobic interactions; Walsh et al., 1994).
Collagen and hydroxyapatite arrangements appear to be similar in mammals and fish (Bigp et al., 2000). Fish, however, are characterized by a higher proportion of non-calcified collagen in the skeleton (Neuman and Mulryan, 1968). On the other hand, collagen fibrils in fish pin bones have lower density compared to mammals (Lee and Glimcher, 1991).
Texture analysis (fracturabilty)
This value results from the high degradation of collagen and hydroxyapatite crystals, although no alterations were observed in the external layer of intramuscular bones during microstructural analysis. This did not influence the cutting force results, possibly because it also contained degraded components. It is possible that the amount of mineral per length unit, as determined in studies on bone density, is only partly responsible for physical properties, mechanical resistance and chemical degradation. Finally, when collagen degradation occurs, collagen changes into gelatin. So, bone strength, consisting of collagen fibers and hydroxyapatite, decreases.
These results lead to the conclusion that collagen degradation initiates in the internal structure of intramuscular pin bones and moves towards the external layer, which does not show the thermal treatment effects. In this sense, collagen degradation is promoted from within pin bones, also affecting the outer layer. Thus, it is necessary to fracture the pin bones to promote heat degradation of collagen present in intramuscular pin bones.
Conflicts of interest
The authors declare they have no conflicts of interest with regard to the work presented in this report.
Notes
¤To cite this article: Suárez H, Gaitán O, Díaz C. Microstructural and physicochemical analysis of collagen in intramuscular pin bones of Bocachico fish (Prochilodus sp.). Rev Colomb Cienc Pecu 2015; 28:188-196.
References
Bailey AJ, Paul RG, Knott L. Mechanisms of maturation and ageing of collagen. Mech Ageing Dev 1998; 106:1-56. [ Links ]
Bigi A, Koch MH, Panzavolta S, Roveri N, Rubini K. Structural aspects of the calcification process of lower vertebrate collagen. Connect Tissue Res 2000; 41:37-43. [ Links ]
Biltz RM, Pellegrino E. The chemical anatomy of bone I. A comparative study of bone composition in sixteen vertebrates. J Bone Joint Surg Am 1969; 51:456-466. [ Links ]
Burjanadze TV, Kisiriya EL. Dependence of thermal stability on the number of hydrogen bonds in water-bridged collagen structure. Biopolymers 1982; 21:1695-1701. [ Links ]
Fratzl P, Gupta HS, Paschalisb EP, Roschgerb P. Structure and mechanical quality of the collagen-mineral nano-composite in bone. J Mater Chem 2004; 14:2115-2123. [ Links ]
Fritsch A, Hellmich C. Universal microstructural patterns in cortical and trabecular, extracellular and extravascular bone materials: micromechanics-based prediction of anisotropic elasticity. J Theor Biol 2007; 244:597-620. [ Links ]
Glimcher MJ. The nature of the mineral phase in bone: biological and clinical implications. In: Avioli LV, Krane SM, editors. Metabolic bone disease and clinically related disorders. San Diego: Academic Press; 1998. p. 23-50. [ Links ]
Hanson DA, Eyre DR. Molecular site specificity of pyridinoline and pyrrole cross-links in type I collagen of human bone. J Biol Chem 1996; 271:26508-26516. [ Links ]
Hellmich Ch, Ulm FJ. Are mineralized tissues open crystal foams reinforced by crosslinked collagen? some energy arguments. J Biomech 2002; 35:1199-1212. [ Links ]
Ikoma T, Kobayashi H, Tanaka J, Walsh D, Mann S. Physical properties of type I collagen extracted from fish scales of Pagrus major and Oreochromis niloticas. Int J Biol Macromol 2003; 32:199-204. [ Links ]
Jäger I, Fratzl P. Mineralized collagen fibrils: a mechanical model with a staggered arrangement of mineral particles. Biophy J 2000; 79:1737-1746. [ Links ]
Katti DR, Pradhan SM, Katti KS. Directional dependence of hydroxyapatite-collagen interactions on mechanics of collagen. J Biomech 2010; 43:1723-1730. [ Links ]
Kim H, Rey C, Glimcher, Melvin J. Isolation of calciumphosphate crystals of bone by non-aqueous methods at low temperature. J Bone Miner Res 1995; 10:1589-1601. [ Links ]
Kimura S, Miyauchi Y, Uchida N. Scale and bone type I collagens of carp (Cyprinus carpio). Comp Biochem Physiol B 1991; 99:473-476. [ Links ]
Lee DD, Glimcher M J. Three-dimensional spatial relationship between the collagen fibrils and the inorganic calcium phosphate crystals of pickerel (Americanus americanus) and herring (Clupea harengus) bone. J mol biol 1991; 217:487-501. [ Links ]
Neuman W, Mulryan B. The discrepancy in the carbonate found in fish bone and blood. Calcif Tissue Res 1968; 2:237-241. [ Links ]
Mori H, Tone Y, Shimizu K, Zikihara K, Tokutomi S, Ida T, Ihara H, Haraet M. Studies on fish scale collagen of the Pacific saury (Cololabis saira). Mater Sci Eng 2012; 33:174-181. [ Links ]
Mesa-Granda M, Cerón-Muñoz M, Olivera M, Botero-Aguirre M. Rayos x: una herramienta para la cuantificación de algunas estructuras óseas en cachama blanca, Piaractus brachypomus (Cuvier, 1818). Actual Biol 2006; 28:67-73. [ Links ]
Nagai T, Suzuki N. Isolation of collagen from fish waste materialskin, bone, and fins. Food Chem 2000; 68:277-281. [ Links ]
Norman TL, Nivargikar SV, Burr DB. Resistance to crack growth in human cortical bone is greater in shear than in tension. J Biomech 1996; 29:1023-1031. [ Links ]
Noto CR. Hierarchical control of terrestrial vertebrate taphonomy over space and time: discussion of mechanisms and implications for vertebrate paleobi-ology. In: Allison, PA, Bottjer, DJ, editors. Taphonomy: process and bias through time. Dordrecht: Springer; 2011. p. 287-336. [ Links ]
Ogawa M. Moody MW, Portier RJ, Bell J, Schexnayder MA, Losso JN. Biochemical properties of black drum and sheepshead seabream skin collagen. J Agri food Chem 2003; 51:8088-8092. [ Links ]
Ogawa M, Portie RJ, Moody MW, Bell J, Schexnayder MA, Losso JN. Biochemical properties of bone and scale collagens isolated from the subtropical fish black drum (Pogonia cromis) and sheepshead seabream (Archosargus probatocephalus). Food Chem 2004; 88:495-501. [ Links ]
Olszta MJ, Cheng X, Jee SS, Kumar R, Kim YY, Kaufman MJ, Douglas EP, Gower LB. Bone structure and formation: a new perspective. Mater Sci Eng 2007; 58:77-116. [ Links ]
Pasteris JD, Wopenka B, Valsami-Jones E. Bone and tooth mineralization: why apatite? Elements 2008; 4:97-104. [ Links ]
Pati F, Adhikari B, Dhara, S. Isolation and characterization of fish scale collagen of higher thermal stability. Bioresour Technol 2010; 101:3737-3742. [ Links ]
Regenstein JM, Zhou P, Shahidi F. Collagen and gelatin from marine by-products. Maximis Val Marine Prod 2007;1:279-303. [ Links ]
Sadowska M, Kotodziejska I, Niecikowska C. Isolation of collagen from the skins of Baltic cod (Gadus morhua). Food Chem; 2003; 81:257-262. [ Links ]
Sasaki N, Tagami A, Goto T, Taniguchi M, Nakata M, Hikichi K. Atomic force microscopic studies on the structure of bovine femoral cortical bone at the collagen fibril-mineral level. J Mater Sci: Materials in Medicine 2002; 13:333-337. [ Links ]
Smith RE. Structural Bone Density of Pacific Cod (Gadus macrocephalus) and Halibut (Hippoglossus stenolepis): Taphonomic and archaeological implications. Portland State University. Department of Anthropology 2008; p. 289. [ Links ]
Suárez H, De francisco A, Beirão LH, Block JM, Saccol A, Pardo-Carrasco S. Importância de ácidos graxos poliinsaturados presentes em peixes de cultivo e de ambiente natural para a nutrição humana. Bol Inst Pesca; 2002; 28:101-110. [ Links ]
Suarez H, Pardo S, Rodríguez MC. Calidad físico-química y atributos sensoriales de filetes sajados biopreservados de cachama, empacados al vacío bajo refrigeración. Rev Col Cien Pec 2008; 21:330-339. [ Links ]
Suárez Mahecha H, Pardo SC, Cortés RM, Ricaurte S, Rojano B. Evaluation of new technology to mitigate intramuscular thorns in cachama fillets. Rev Fac Nal Agro 2009; 62:4989-4997. [ Links ]
Walsh WR, Ohno M, Guzelsu N. Bone composite behaviour: effects of mineral-organic bonding. J Mater Sci-Mater M 1994; 5:72-79. [ Links ]
Wang X, Bank RA, TeKoppele JM, Hubbard GB, Athanasiou KA, Agrawal CM. Effect of collagen denaturation on the toughness of bone. Clin Orthop Relat Res 2000; 371:228-239. [ Links ]
Weiner S, Traub W, Wagner HD. Lamellar bone: structurefunction relations. J struct biol 1999; 126:241-255. [ Links ]
Zioupos P, Currey JD, Hamer AJ. The role of collagen in the declining mechanical properties of aging human cortical bone. J Biomed Mater Res1999; 45:108-116. [ Links ]