Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Colombiana de Ciencias Pecuarias
Print version ISSN 0120-0690
Rev Colom Cienc Pecua vol.28 no.3 Medellín July/Aug. 2015
https://doi.org/10.17533/udea.rccp.v28n3a01
ORIGINAL ARTICLE
doi: 10.17533/udea.rccp.v28n3a01
Escherichia coli lipopolysaccharide affects intestinal mucin secretion in weaned pigs¤
El lipopolisacárido de Escherichia coli afecta la secreción de mucinas intestinales en cerdos destetados
O lipopolissacárido de Escherichia coli afecta a secreção de mucina intestinal em porcos desmamados
Deny J Zapata1*, MV, MSc; Berardo de J Rodríguez1, MV, Esp Patol, PhD; María C Ramírez2 MV, MSc; Albeiro López3, Zoot MV, MSc, DrSci; Jaime Parra3, Zoot MSc, PhD.
1CENTAURO research group, Facultad de Ciencias Agrarias, Universidad de Antioquia, Calle 70 No. 52-21, Medellín, Colombia.
2QUIRON pathobiology research group, Facultad de Ciencias Agrarias, Universidad de Antioquia, Calle 70 No. 52-21, Medellín, Colombia.
3BIOGEM research group, Universidad Nacional de Colombia, AA 1779, Colombia.
*Corresponding author: Deny J Zapata, MV, MSc. Grupo de Investigación CENTAURO, Facultad de Ciencias Agrarias, Universidad de Antioquia, Calle 70 No. 52-21, Medellín, Colombia. Email: deny.zapata@udea.edu.co; juli2341@yahoo.es
Received: December 3, 2013; accepted: July 21, 2014
Summary
Background: to the best of our knowledge, the effects of lipopolysaccharide (LPS) from Escherichia coli on goblet cells and intestinal mucin secretion of weaned pigs has not been reported, and it is unknown whether these effects could trigger enteritis. Objective: to determine the effect of E. coli LPS on intestinal mucin secretion in weaning piglets. Methods: fifty-two piglets weaned at 21 days of age were fed a basal diet supplemented with four LPS levels (0.0, 0.3, 0.5, and 1.0 μg/mg) during 10 days. Piglets were slaughtered on days 1, 5, 7, and 10 post-weaning and samples of small and large intestine were taken for histochemical staining to determine goblet cell population and type of mucins produced (acidic, sulphated, non-sulphated, or neutral). Results: acidic mucin was reduced on day 5 post-weaning independently of the dietary LPS level supplied to piglets. Recovery of acidic mucins was observed during days 7 and 10 post-weaning. Neutral mucins increased on day 5 and decreased on days 7 and 10 post-weaning. High LPS levels decreased goblet cells population and secretion of all types of mucins. This effect was remarkably high for diet two (D2: 0.5 mg LPS/mg food). Conclusions: early weaning (21 d) and LPS addition to the diet affect mucin secretion and intestinal epithelium integrity by modifying goblet cell populations and their balance between acidic and neutral mucin secretion. These findings explain some abnormalities related with post-weaning diarrhea syndrome and help to explain its pathophysiology.
Keywords: goblet cells, histochemistry, histopathology, intestine, pigs.
Resumen
Antecedentes: actualmente se desconoce el efecto del lipopolisacárido (LPS) de Escherichia coli sobre la cantidad de células caliciformes y la secreción de mucinas en diferentes regiones del intestino en cerdos durante el período pos-destete. Tampoco se ha descrito si cambios en la distribución de las mucinas en el intestino están relacionados con el desarrollo de enteritis. Objetivo: determinar el efecto del LPS de E. coli sobre la secreción de mucinas en el intestino de lechones recién destetados. Métodos: cincuenta y dos lechones destetados a los 21 días fueron alimentados con una dieta basal adicionada con cuatro niveles de LPS (0,0, 0,3, 0,5 y 1,0 μg/mg) durante 10 días. Los cerdos se sacrificaron los días 1, 5, 7 y 10 pos-destete y se tomaron muestras de intestino delgado y colon para realizar coloraciones histoquímicas, que permitieran calcular la cantidad de células caliciformes y el tipo de mucinas ácidas sulfatadas, no sulfatadas o neutras por ellas producidas. Resultados: la producción de mucinas ácidas en las células caliciformes se redujo el día 5 del período pos-destete, con posterior restauración de los parámetros a los días 7 y 10 e independientemente de la dosis de LPS suministrada en la dieta. En contraste, la producción de mucinas neutras aumentó en el día 5 y disminuyó en los días 7 y 10 del período pos-destete. Al comparar las dietas experimentales, se observó que dosis mayores de LPS, disminuyen el número de células caliciformes y la secreción de los diferentes tipos de mucinas. Este efecto fue más marcado con la dieta dos (D2: 0,5 mg de LPS/mg de alimento). Conclusiones: el destete a los 21 días y la adición LPS de E. coli a la dieta generan cambios en la secreción de mucinas, afectan la integridad del epitelio y el balance entre la secreción de mucinas ácidas y neutras por las células caliciformes. Estos hallazgos sugieren explicaciones de algunas alteraciones que se producen en el síndrome de diarrea pos-destete y contribuyen a explicar su fisiopatología.
Palabras clave: células caliciformes, histopatología, histoquímica, intestino, porcinos.
Resumo
Antecedentes: actualmente é desconhecido o efeito do lipopolissacarídeo (LPS) de Escherichia coli sobre a quantidade de células caliciformes, sobre a secreção de mucinas em diferentes regiões do intestino em porcos durante o período de pós-desmame e se o padrão de distribuição das mucinas está relacionado com o desenvolvimento de enterite. Objetivo: determinar o efeito da LPS de E. coli sobre a secreção de mucinas no intestino de leitões recém desmamados. Métodos: foi realizado um estudo experimental com 52 leitões desmamados aos 21 dias, que foram alimentados com uma dieta basal adicionada com quatro níveis de LPS (0,0, 0,3, 0,5 y 1,0 μg/mg) durante 10 dias. Os porcos foram sacrificados os dias 1, 5, 7 e 10 pós-desmame para tirar amostras de intestino delgado e grosso, realizar colorações histoquímicas, calcular a quantidade de células caliciformes e o tipo de mucinas ácidas sulfatadas, não sulfatadas e neutras por elas produzidas. Resultados: observou-se que ao número de células caliciformes que expressaram mucinas ácidas nos diferentes tempos do período pós-desmame, apresentaram uma diminuição da secreção de mucinas ácidas no quinto dia, com posterior recuperação dos parâmetros os dias 7 e 10 Independentemente da dose de LPS disso. Ao contrário, as mucinas neutras incrementaram o dia 5 com uma posterior diminuição os dias 7 e 10 pós-desmame. Ao comparar as dietas experimentais, observou-se uma diminuição do número de células caliciformes secretando os diferentes tipos de mucinas a doses maiores de LPS, principalmente com a dieta dois (D2: 0,5 mg LPS/mg comida). Conclusões: o desmame aos 21 dias e a adição de LPS de E. coli na dieta, gera mudanças na secreção de mucinas, afetam a integridade do epitélio e o balanço entre a secreção de mucinas ácidas e neutras pelas células caliciformes; isto modela algumas alterações que se produzem no síndrome de diarreia pós-desmame e contribui a explicar a sua fisiopatologia.
Palavras chave: células caliciformes, histopatología, histoquímica, intestino, porcinos.
Introduction
Alterations of intestinal epithelium of piglets have been reported, including changes in absorptive activity and predisposition to enteritis, with subsequent weight loss and mortality during post-weaning (Reis de souza et al., 2012).
Early weaning causes bacterial imbalances in the intestinal populations, favoring Escherichia coli growth and subsequent release of lipopolysaccharide (LPS) from the cell wall of these bacteria (Amador et al., 2007). The LPS is considered an important pathogenic agent and a potent stimulator of innate immunity and inflammation (Zhenfeng et al., 2008).
Recent studies show that E. coli LPS induces morphological and histological changes in the intestine of weaned piglets, such as: atrophy of villi, increase in crypt depth, and injury to the epithelial barrier (Ospina et al., 2011; Parra et al., 2011; Montoya et al., 2012; McLamb et al., 2013). LPS also induces morphological changes in goblet cells, which cause variations in their proliferative activity in different organs (Shimizu et al., 2011).
Goblet cells synthesize glycoproteins called mucins. A protective mucus gel composed predominantly of these proteins covers the gastrointestinal epithelium. Mucins constitute an important element of natural immunity and its secretion is associated with different physiological and pathological conditions (Voynow et al., 2009; Nakamura et al., 2012). Mucins are classified as neutral, acidic, sulphated, and non-sulphated.
In this study we evaluated the effect of E. coli LPS on goblet cells and the type of mucins secreted during post-weaning.
Material and methods
Ethical considerations
All experimental procedures followed guidelines set forth in the International Guiding Principles for Biomedical Research Involving Animals (CIOMS, 1985) approved by the Ethics Committee for Animal Experimentation of Universidad Nacional de Colombia, Medellin (CEMED 001, January 26, 2009).
Location
The animal work was conducted at the San Pablo Experimental Center of the Universidad Nacional de Colombia. The farm is located in Rionegro municipality, at 2,100 m.a.s.l. with an average environmental temperature between 12 to 18 ºC.
Experimental design
Durocs x Landrace pigs weaned at 21 days of age with 6.5 ± 0.5 kg body weight were used. The experiment followed a randomized block design (two blocks) in a 4 x 4 factorial arrangement (Steel and Torrie, 1985) with four diets and four sampling days for histochemical analyses. The experimental diets contained LPS from E. coli, serotype 0111: B4 (Sigma- Aldrich, St. Louis, MO, USA), as follows: a control basal diet (BD) without LPS addition, Diet 1 (D1): BD plus 0.3 mg LPS/mg food, Diet 2 (D2): BD plus 0.5 mg LPS/mg food, and Diet 3 (D3): BD plus 1 mg LPS/mg food. A total of 52 piglets were slaughtered. Four piglets (n = 1 per diet) were slaughtered for samplings on day 1 post weaning and were used as the reference group for each diet. Then, four piglets per treatment were slaughtered on days 5, 7, and 10 post weaning (n = 16 on each slaughtering day). Only one piglet per diet instead of 4 was slaughtered on day 1 following recommendations by the Ethics Committee.
Pigs were housed at a density of eight pigs per pen (64 pigs total) in environmentally controlled rooms (26 ± 3 °C) with ad libitum water. The basal diet included milk and milk derivatives, vitamins, minerals and lysine-HCl. Diets were formulated according to minimal nutritional requirements proposed by the National Research Council (NRC, 2012; Table 1). The amount of food offered per animal was 3,000 g/day. Experimental diets were offered from days 1 to 10 post weaning. No solid food was offered to piglets during lactation.
Intestinal sampling
The animals were sedated by inhalation of carbon dioxide during 3 minutes, and then slaughtered by ex-sanguination through section of the jugular vein. Afterwards, they were put in supine position and the intestine was extracted trough abdominal incision. The intestine was aligned on a table, measured without any tension, divided into three sections of equal size (corresponding to the duodenum, jejunum, and ileum), and 20 cm sections were taken from the center of each segment. Once the portions were cut, intestinal content was washed with cold saline solution as described by Reis de souza et al. (2005). Then, 1 cm long sub-samples were obtained from each segment. All samples were kept in 10% neutral buffered formalin solution until analysis.
Histochemical analysis
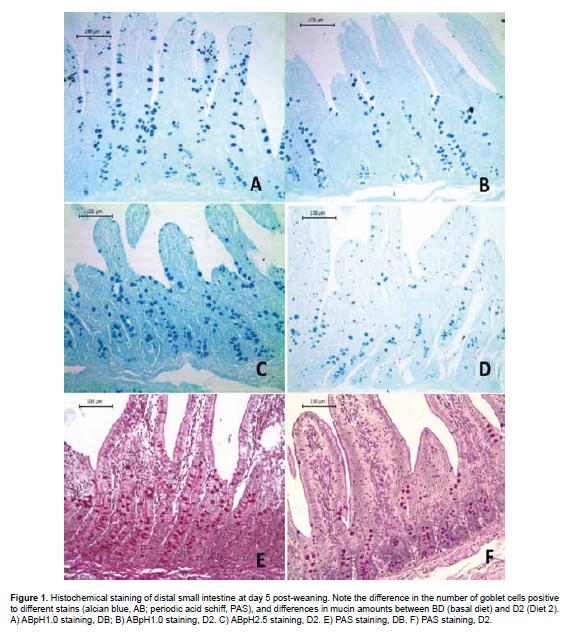
The tissues were embedded in paraffin and sliced in 4 μm thick cuts according to conventional techniques. Histochemical staining was done in three intestinal samples to identify mucin types (AFIP, 1992). Alcian blue stain pH 1.0 was used for identifying significantly sulphated acid mucins, alcian blue pH 2.5 for non-sulphated acid mucins, and periodic acid schiff (PAS) for neutral mucins.
Microscopic evaluation and morphometric analysis of images
Quantitative evaluation of tissue slides was carried out according to the following procedure: tissue identification was performed with a Leica DMLB optical microscope (Meyer Instruments, Houston, TX, USA). Subsequently, images from the tunica epithelial were captured using a Leica EC3 microscope camera (Leica microsystems, Heerbrugg, Switzerland) and magnified 200X. Images were analyzed with ZEN software (blue edition, Carl Zeiss, 2011).
Assessment of dietary effects on mucin secretion was conducted as follows: a circular fold of mucosa was selected and ten circular sections (diameter of 200 μm) of villi and intestinal glands were drawn, for a total evaluated area of 0.3 mm2 per tissue slide. Afterwards, a semiautomatic count of goblet cells positive to each stain was conducted. Each villi region was equally represented in the assessment. This process was repeated on each section of the small intestine (duodenum, jejunum, and ileum). Statistical data analysis was conducted using a multivariable lineal GLM model with SPSS software (version 19, 2010, IBM). Duncan test was used to compare treatment means (p<0.05).
Results
Piglets fed the basal diet were in good health whereas those receiving LPS showed a sporadic increase in rectal temperature (above 38 ºC). However, none of the animals had symptoms of illness, therefore isolation or sacrifice was not required.
Initially, the effect of early weaning on goblet cells population and mucin secretion was determined by comparing data of the piglets fed the basal diet (control diet) at different days post-weaning. The levels of acid mucins decreased on day 5 post-weaning and increased later on days 7 and 10 (Table 2). The goblet cells that secrete neutral mucins increased on day 5 and decreased at days 7 and 10 post-weaning, showing significant differences in the evaluated days (p<0.05).
Piglets fed different LPS levels exhibited a similar trend to that described for the basal diet on days 1, 5, 7, and 10 post-weaning. This is, acid mucins decreased on day 5 and increased at days 7 and 10. On the contrary, neutral mucins increased on day 5 and subsequently decreased (Table 3). No statistical interaction was observed between LPS concentrations and post-weaning periods for any of the variables studied. Therefore, it was not necessary to analyze or break down factors independently.
Analyzing the effect of different LPS doses (experimental diets) on the population of goblet cells and their mucins secretion, a deleterious effect was observed, especially on acid mucins over all the intestinal regions. This effect was dependent on the LPS concentration and was associated to the highest doses (Table 4). In this way, significant differences (p<0.05) were observed in all the intestinal regions for diet D2 and in duodenum for diet D3 with respect to diets BD and diet D1 (Figure 1).
Discussion
In this study, E. coli LPS caused a dose-dependent decrease in acid and neutral mucin secretion in all intestinal regions. This effect was more evident on day 5 post-weaning for acid mucins. Additionally, an inverse relationship was observed between the number of goblet cells secreting acid and neutral mucins on day 5 post-weaning.
Quantification of the type of mucin secreted by goblet cells in piglets fed the BD showed that sulphated and non-sulphated acid mucins decrease on day 5 post-weaning with a subsequent increase on days 7 and 10; these results show the base effect of early weaning, and are similar to what has been described in pigs and other species (Hedemann et al., 2003; Vente-Spreeuwenberg et al., 2004; Brown et al., 2006). These authors found that goblet cell population in the intestinal mucous decreases at the beginning of the weaning period followed by a recovery of these cells during days 6 and 8. Finally, goblet cell population reaches normal levels at days 9 and 14.
The decrease in goblet cell secretion of acid mucins observed at the beginning of weaning— not only in piglets fed BD, but also in those fed with diets containing LPS (D1, D2, D3)—is due to changes in cell architecture during post-weaning, as has been reported by Parra et al. (2011). These intestinal changes are associated with a reduction of villi height and area (Parra et al., 2011) and epithelial injury, necrosis principally, which causes loss of goblet cells secreting acid mucins (Liu et al., 2013).
It has been suggested that sulphated and nonsulphated acid mucins protect against enteric illness in piglets (Strous et al., 1992; Betscher et al., 2010); therefore, a detriment in intestinal mucous and decrease in goblet cells secreting acid mucins could increase susceptibility to enteric disturbances (Looft, 2013). Nevertheless, the intestine can react to a harmful environment by increasing the rate of epithelium regeneration (Moeser et al., 2012). This is in agreement with results of this study, since recovery in the number of goblet cells secreting acid mucins was evidenced at days 7 and 10 post-weaning.
Neutral mucins increased on day 5, as opposite to acid mucins. This could be related with less maturity since this mucin type is mostly found in the fetal stage, and are widely distributed in stomach and intestine after birth. Besides, it is also known that neutral mucin population is lower than acid mucins under normal conditions (Deplancke et al., 2012).
Goblet cells differentiate from endoderm stem cells in that they are found deeply in the crypts, and when mature they migrate to the villous surface (Dunsford et al., 1991). Therefore, it can be suggested that increase in neutral mucins during post-weaning is related to variation in villi and crypt depth, affecting goblet cell differentiation and normal maturation (Ghaleb et al., 2011).
E. coli LPS caused a deleterious effect in goblet cells and secretion of acid and neutral mucins that was dependent on LPS concentration, and it was more evident for D2 diet (0.5 μm LPS/mg of food). This shows that LPS addition potentiates the effect of early weaning on goblet cell population.
The highest LPS level did not have the strongest effect on goblet cells and mucin secretion, as evidenced in the results of diet D3. This could be due to a reduction of food consumption caused by higher secretion of pro-inflammatory cytokines (Bauer et al., 2011; Parra et al., 2013). It may also suggest that D3 results are related to a saturation of the recognition receptors that mediate cellular signaling induced by LPS (Gomes et al., 2010).
It can be concluded that dietary LPS addition increase the negative effect of early weaning on pig intestine. LPS generates morphological alterations reflected in changes of goblet cell population and distribution in the intestine. Dietary addition of LPS also causes alterations of the intestinal physiology of piglets, evidenced in secretion changes of mucins during post-weaning (McGuckin et al., 2011). LPS effects can occur during natural infections after weaning, causing post-weaning diarrhea (Blanco et al. 2011). It is known that there is a strong interaction between epithelium and intestinal microbiota. Changes associated with mucin secretion can lead to variations in the protecting function of intestinal mucous and bacteria-host interactions (Deplancke et al., 2012) facilitating the occurrence of enteritis and septicemia during post-weaning.
The results of this study help to understand the role of mucins in keeping intestinal integrity during post-weaning. These findings underline the need of further studies to understand the mechanisms involved in the regulation of intestinal mucin secretion. That knowledge would help to design strategies for preventing and controlling diseases associated with this critical phase of swine production.
Acknowledgements
The authors acknowledge the support from bacteriologist Maria Idalba Morales and staff at the animal pathology laboratory, Universidad de Antioquia. This study obtained financial support from Colciencias, ''Convocatoria Jóvenes Investigadores 2011'' and ''Estrategia de Sostenibilidad 2013-2014 of Universidad de Antioquia''.Conflicts of interest
The authors declare they have no conflicts of interest with regard to the work presented in this report.
Notes
¤To cite this article: Zapata DJ, Rodríguez BJ, Ramírez MC, López A, Parra J. Escherichia coli lipopolysaccharide affects intestinal mucin secretion in weaned pigs. Rev Colomb Cienc Pecu 2015; 28:209-217.
References
Amador P, García-Herrera J, Marca MC, de la Osada J, Acin S, Navarro MA, Salvador MT, Lostao MP, Rodríguez-Yoldi MJ. Intestinal D-galactose transport in an endotoxemia model in the rabbit. J Membr Biol 2007; 2153:125-133. [ Links ]
Bauer E, Metzler-Zebeli BU, Verstegen, MW, Mosenthin R. Intestinal gene expression in pigs: effects of reduced feed intake during weaning and potential impact of dietary components. Nutr Res Rev 2011; 24.2:155-175. [ Links ]
Betschera S, Beinekeb A, Schönfelda L, Kamphues J. Effects of diet's physical form (grinding intensity; meal/pellets) on morphological and histological parameters (e.g. ratio of neutral to acid mucins) of the gastrointestinal tract in weaned piglets. Livest Sci 2010; 134:149-151. [ Links ]
Blanco M, Lazo L, Blanco JE, Dahbi G, Mora A, López C, Blanco J. Serotypes, virulence genes, and PFGE patterns of enteropathogenic Escherichia coli isolated from Cuban pigs with diarrhea. Int Microbiol 2010; 9:53-60. [ Links ]
Brown DC, Maxwell CV, Erf GF, Davis ME, Singh S, Johnson ZB. The influence of different management systems and age on intestinal morphology, immune cell numbers and mucin production from goblet cells in post-weaning pigs. Vet Immunol Immunopathol 2006; 111:187-198. [ Links ]
Deplancke B, Gaskins, HR. Microbial modulation of innate defense: goblet cells and the intestinal mucus layer. Am J Clin Nutr 2012; 73(6):1131S-1141S. [ Links ]
Dunsford B, Haensly W, Knabe D. Effects of diet on acidic and neutral goblet cell populations in the small intestine of early weaned pigs. Am J Vet Res 1991; 52:1743-1746. [ Links ]
Ghaleb AM, McConnell BB, Kaestner KH, Yang VW. Altered intestinal epithelial homeostasis in mice with intestine-specific deletion of the Krüppel-like factor 4 gene. Dev Biol 2011; 349: 310-320. [ Links ]
Gomes N, Brunialti M, Mendes M, Freudenberg M, Galanos C, Salomão R. Lipopolysaccharide-induced expression of cell surface receptors and cell activation of neutrophils and monocytes in whole human blood. Braz J Med Biol Res 2010; 43:853-858. [ Links ]
Hedemann MS, Hojsgaard S, Jensen BB. Small intestinal morphology and activity of intestinal peptidases in piglets around weaning. J Anim Physiol Anim Nutr 2003; 87:32-41. [ Links ]
Liu Y, Ipharraguerre IR, Pettigrew JE. Digestive physiology of the pig symposium: Potential applications of knowledge of gut chemosensing in pig production. J Anim Sci 2013; 91:1982-1990. [ Links ]
Looft TP. The swine intestinal microbiota: localized adaptations and responses to in-feed antibiotics. Graduate Theses and Dissertations 2012; [Access date: September 13, 2014] URL: http://lib.dr.iastate.edu/etd/12390 [ Links ]
McGuckin MA, Lindén SK, Sutton P, Florin TH. Mucin dynamics and enteric pathogens. Nat Rev Microbiol 2011; 9:265-27. [ Links ]
McLamb BL, Gibson AJ, Overman EL, Stahl C, Moeser AJ. Early weaning stress in pigs impairs innate mucosal immune responses to enterotoxigenic E. coli challenge and exacerbates intestinal injury and clinical disease. Plos One 2013; 8(4):e59838. doi:10.1371/journal.pone.0059838 [ Links ]
Moeser AJ, Borst LB, Overman BL, Pittman JS. Defects in small intestinal epithelial barrier function and morphology associated with peri-weaning failure to thrive syndrome (PFTS) in swine. Res Vet Sci 2012; 93:975-982. [ Links ]
Montoya R, Carolina M, López A, Parra S. Alteraciones en la producción mRNA de enzimas intestinales de cerdos durante varios períodos pos-destete. Rev Bio Agro 2012; 10:126-134. [ Links ]
Nakamura Y, Hamajima Y, Komori M. The role of Atoh1 in mucous cell metaplasia. International Journal of Otolaryngology 2012; [Access date: September 13, 2014] URL: doi: 10.1155/2012/438609 [ Links ]
Ospina D, Ciro J, Román Y, Peláez C, López A, Parra J. Cambios en la actividad enzimática en duodeno y yeyuno de cerdos durante varios periodos posdestete. Rev Med Vet Zoot 2011; 58:156-165. [ Links ]
Parra J, Agudelo J, Ortiz L, Ramírez MC, Rodríguez B, López A. Lipopolysaccharide (LPS) from Escherichia coli has detrimental effects on the intestinal morphology of weaned pigs. Rev Colomb Cienc Pecu 2011; 24:598-608. [ Links ]
Parra J, Agudelo J, Sanín P, Forero J, Muskus C, López A. Intestinal expression of pro-infammatory cytokines induced by oral intake of lipopolysaccharide (LPS) from Escherichia coli in weaned pigs. Rev Colomb Cienc Pecu 2013; 26:108-118. [ Links ]
Reis de souza T, Guerrero C, Aguilera B, Mariscal L. Efecto de diferentes cereales sobre la morfología intestinal de lechones recién destetados. Téc Pecu Méx 2005; 43:309-321. [ Links ]
Reis de souza T, Guerrero C, Aguilera B, Mariscal L. Cambios nutrimentales en el lechón y desarrollo morfofisiológico de su aparato digestivo. Vet Méx 2012; 43:155-173. [ Links ]
Shimizu T, Shimizu S. Differential properties of mucous glycoproteins produced by allergic inflammation and lipopolysaccharide stimulation in rat nasal epithelium. Adv Otorhinolaryngol 2011; 72:107-109. [ Links ]
Steel RG, Torrie JH. Principles and procedures of statistics: a biometrical approach. 2nd ed. New York: McGraw-Hill; 1985. [ Links ] Strous GJ, Dekker J. Mucin-type glycoproteins. Crit Rev Biochem Mol Biol 1992; 27:57-92. [ Links ]
Vente-Spreeuwenberg MA, Verdonk JM, Bakker GC, Beynen AC, Verstegen MW. Effect of dietary protein source on feed intake and small intestine morphology in newly weaned piglets. Livest Prod Sci 2004; 86:169-177. [ Links ]
Voynow JA, Bruce KR. Mucins, mucus and sputum. Chest 2009; 135:505-512. [ Links ]
Zhenfeng Z, Deyuan O, Xiangshu P, Sung WK, Yanhong L, Junjun W. Dietary arginine supplementation affects microvascular development in the small intestine of early-weaned pigs. J Nutr 2008; 138:1304-1309. [ Links ]