Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Colombiana de Ciencias Pecuarias
Print version ISSN 0120-0690
Rev Colom Cienc Pecua vol.28 no.3 Medellín July/Aug. 2015
https://doi.org/10.17533/udea.rccp.v28n3a7
SHORT COMMUNICATION
doi: 10.17533/udea.rccp.v28n3a7
Surgical placement and management of jugular vascular access ports in dogs and cats: description of technique¤
Implantación quirúrgica y gestión de catéter venoso central en la vena yugular de perros y gatos: descripción de la técnica
Implante cirúrgico e manejo de cateter venoso central na veia jugular de cães e gatos: descrição da técnica
Simone D Guérios1*, MV, MSc PhD; Daniella M Silva1, MV; Carlos HM Souza2, MV, MSc; Nicholas J Bacon2, MA, VetMB, DECVS, DACVS, MRCVS.
1Hospital Veterinário, Serviço de Oncologia Veterinária, Departmento de Medicina Veterinária, Universidade Federal do Paraná, Curitiba, Brasil.
2Department of Small Animal Clinical Sciences, College of Veterinary Medicine, University of Florida, Gainesville, USA.
*Corresponding author: Simone Guérios. Department of Veterinary Medicine, Federal University of Parana. Rua dos Funcionários 1540, Curitiba, Parana, Brazil. Email: sdguerios@gmail.com
Received: July 23, 2014; accepted: October 24, 2014
Summary
Background: vascular access ports (VAPs) are designed to allow repeated access to the vascular system with minimum patient distress. Objective: to describe the surgical technique, care and complications of jugular VAPs currently used at the Veterinary Oncology Service of the University of Florida Small Animal Hospital. Conclusion: the VAPs can remain in site for long terms (months) with minimum complications, and its placement is a reliable technique that should be considered by veterinarians, particularly for a longterm treatment.
Keywords: chemotheraphy, intravenous access, medical oncology, vascular surgical procedures.
Resumen
Antecedentes: los catéteres venosos centrales (VAPs) están diseñados para permitir el acceso repetido al sistema vascular con las mínimas molestias para el paciente. Objetivo: describir la técnica quirúrgica, atención de mantenimiento y las complicaciones de los VAPs en vena yugular, que se utiliza actualmente en el Veterinary Oncology Service en la University of Florida Small Animal Hospital. Conclusión: el VAP puede permanecer en el lugar durante períodos largos (meses) con pocas complicaciones, y su implantación quirúrgica debe ser considerada por los veterinarios, en particular para el tratamiento a largo plazo.Palabras clave: acceso intravenoso, oncología médica, procedimientos quirúrgicos vasculares, quimioterapia.
Resumo
Antecedentes: os cateteres venosos centrais (VAPs) permitem acessos repetidos ao sistema vascular com mínimo desconforto ao paciente. Objetivo: descrever a técnica cirúrgica, cuidados de manutenção e complicações dos VAPs de veia jugular utilizados atualmente no Serviço de Oncologia Veterinária do Hospital Veterinário de Pequenos Animais da Universidade da Florida. Conclusão: o VAP pode permanecer no local por longos períodos (meses) com poucas complicações. O implante cirúrgico descrito no presente trabalho deve ser considerado pelos médicos veterinários, particularmente para tratamentos de longa duração.
Palavras chave: acceso intravenoso, oncología, procedimentos cirúrgicos vasculares, quimioterapia.
Introduction
Totally implantable subcutaneous vascular access ports (VAPs) are devises used to access the circulatory system with less discomfort for the patient compared to a standard venipuncture. The port consists of a small titanium or plastic chamber with an injection membrane of compressed silicone septum. Attached to the base of the port there is the catheter that is placed in large veins. VAPs provide repeated access to the vascular system for long-term blood sampling and blood donation, analgesia, chemotherapy, parenteral nutrition, antibiotics, fluids and drugs. It is indicated especially for small patients or fractious animals. (Bagley and Flandres, 1990; Webb et al., 1995; Albarellos et al., 2003; Swindle et al., 2005; Cahalane et al., 2007). The major benefit of a VAP implant is reduced pain and stress associated with repeated venipuncture. This is especially true in cancer patients due to repeated blood sampling for cell counts and administration of intravenous chemotherapy, plus anesthetic drugs for radiotherapy for patients undergoing up to 18-20 doses of radiation (Niederhuber et al., 1982; Newman et al., 1989; Schwarz et al., 1997; Ignatov et al., 2008).
In dogs and cats, VAPs with open-ended catheters are usually placed into the jugular vein in animals. The rate of complications for jugular use is less than 5%, with most being minor, and not necessitating VAP removal (Hai, 1982; Henry et al., 2002; Morrison et al., 2007).
The purpose of this article is to describe the surgical technique for jugular VAP placement, removal, maintenance and complications in dogs and cats, routinely used at the Veterinary Oncology Service of the University of Florida Small Animal Hospital.
VAP device
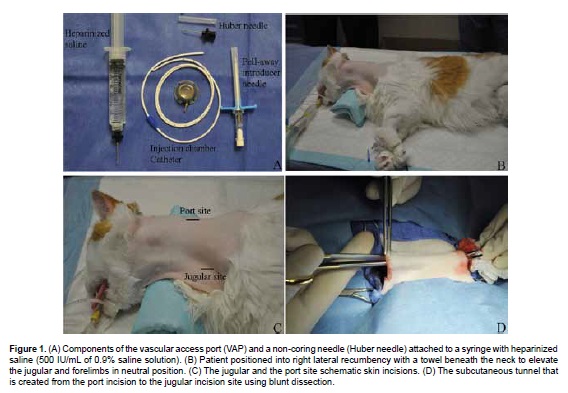
The Veterinary Oncology Service at the University of Florida Small Animal Hospital uses a VAP device (Vascular Access Port, Norfolk Vet products, 7350 N. Ridgeway, Skokie, IL 60076) that consists of a titanium injection chamber with a compressed silicone septum and a attachable radiopaque silicon rubber catheter with depth markings, boot connector and rounded tip (Figure 1A). The catheter used for cats and small dogs (<15 kg bodyweight) is a 5 French/16 G, and for larger dogs (>15 kg bodyweight) a 7 French/13 G silicone catheter is preferred.
VAP implantation technique
Animals should be fasted for 6 to 12 hours and prepared for a minor surgical procedure. VAPs are placed under general anesthesia, using a modified technique described by Seldinger (1953). The left ventrolateral area of the neck is clipped and aseptically prepared. Patients are positioned into right lateral recumbency with a sandbag or towel beneath the neck to elevate the jugular and create a slightly convex surgical field (Figure 1B). The VAP is inspected for defects and tested for pressure with heparinized saline (500 IU/mL of 0.9 saline solution). The distance from the mid neck jugular puncture to approximately the second rib is recorded and marked to predetermine the catheter length. At this location the catheter will lie in the cranial vena cava, just cranial to the heart.
The left external jugular vein is found in mid neck and a 2 cm skin incision is made parallel and lateral to the vein in the mid-cervical region. A second incision 3 cm long is made in a dorso-ventral direction at the level of the cranial end of the jugular incision, in the dorsal third of the neck (Figure 1C). A subcutaneous pocket caudal to this second incision is created using blunt dissection to accommodate the VAP injection chamber.
A subcutaneous tunnel is created from the port incision to the jugular incision site using blunt dissection (Figure 1D) and the unmarked end of the catheter is passed through the tunnel.
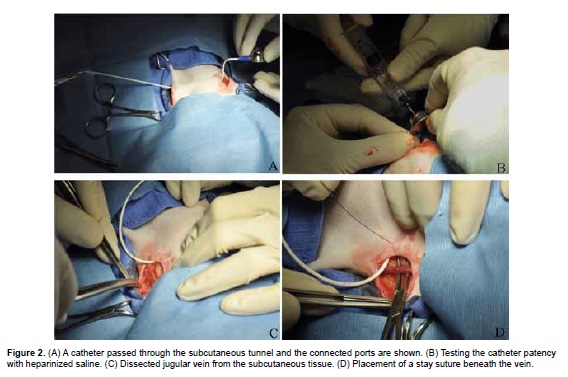
At this point the excess catheter is cut and it is connected to the port (Figure 2A). The VAP is flushed with a non-coring needle (Huber needle) with 1 ml heparinized saline (500 IU/mL of 0.9% saline solution; Figure 2B). The left external jugular vein is gently dissected and it is isolated by placing a stay suture beneath the vein at both the cranial and caudal ends of the dissection with 3-0 polydioxanone suture (Figure 2C and 2D).
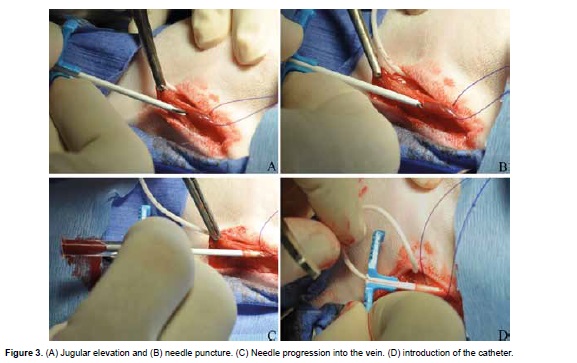
The jugular vein is gently elevated by the stay sutures and the inner introducer needle is used to puncture the vein in a caudal direction (Figure 3A, 3B, and 3C). The needle is removed, leaving the peelaway sheet in place and the catheter is introduced and advanced into the vessel up the previously recorded mark (Figure 3D).
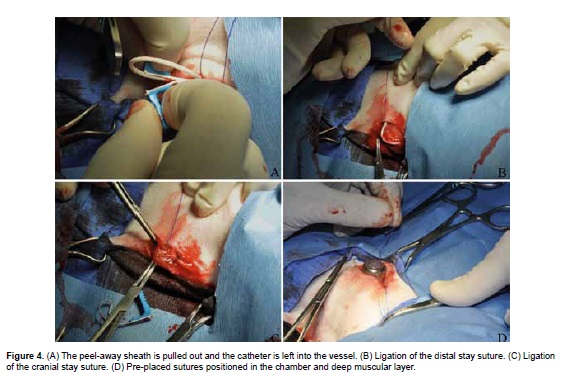
The two handles of the peel-away sheath are grasped and pulled out until the sheath is removed leaving the catheter into the vessel (Figure 4A). The distal stay suture is used to gently ligate the vein and catheter, distal to the venotomy puncture site (Figure 4B). The cranial stay suture is tightened to occlude the jugular vein proximal to the venotomy site in order to reduce hemorrhage (Figure 4C). Sutures of 2-0 polypropylene are pre-placed through the anchor points into the underlying fascia. Deep fascial bites are taken and tension is applied to ensure they do not tear through prior to performing the knot (Figure 4D). The chamber is positioned inside the subcutaneous pocket, away from the skin incision with the catheter exiting cranio-ventrally, and sutures are locked in order to avoid the chamber from becoming dislodged and flipping over.
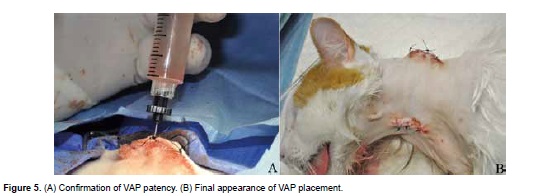
VAP patency is confirmed by blood aspiration with a non-coring needle (Huber needle) prior to flushing the system with 3 ml heparinized saline (500 IU/mL of 0.9% saline solution; Figure 5A). The two incised sites are closed in a routine pattern with an appositional continuous 3-0 polyglecaprone suture in the subcutaneous tissue and simple interrupted 3-0 nylon for skin (Figure 5B).
VAP maintenance
Patients are evaluated one day after surgery, and after that rechecks are determined by the treatment protocol for each patient. In cases where the VAP is not being used it is recommended to flush it with heparinized saline once a month. At each VAP use, the skin above the port is aseptically prepared, the chamber is hold in place and a Huber needle (22G x ¾ inch needle with a bent tip) is inserted through the skin into the chamber. Blood aspiration is performed and the system is flushed with 1-2 mL of 0.9% saline solution containing 500 IU heparin/mL in order to clear the VAP and catheter. The VAP is then flushed after the blood draw with 3 mL heparinized saline solution and the needle is removed. Repeated vascular access through the VAP can be obtained without sedation or anesthesia and with minimal physical restraint.
VAP removal
VAPs are normally removed when long term treatment is over or if there are complications. Patients are anesthetized and the skin is clipped and prepared as for VAP placement. A 2 cm skin incision is made directly over the chamber and subcutaneous and fibrotic tissues are incised. The sutures anchoring the port to the fascia are located and cut and all suture remnants are removed. External pressure is applied to the jugular vein and port and catheter are withdrawn with traction. Mild pressure is applied over the jugular vein for five minutes to avoid blood extravasation. The incision is closed using a two layer suture.
VAP complications
In human patients, the two most significant VAPrelated complications are thrombosis and infection. Catheter-related thrombosis may be linked to preexisting hypercoagulability, venous irritation due to chemotherapeutic agents, and venous stasis caused by tumor compression (Denny, 1993; Sehirali et al., 2005). In dogs and cats, VAP-related thrombosis has only been reported in a small numbers of patients, even with the placement of small diameter catheters (Culp et al., 2010). Infection is considered a major catheter complication. When it occurs between 3 to 5 days post operatively, it is assumed to be due to contamination introduced at the time of surgery (Cowart et al., 1999; Henderson et al., 2003). The use of prophylactic antibiotics for VAP placements is controversial, though a single dose of a broad-spectrum antibiotic prior the incision is prudent as a potentially permanent implant is being introduced. Delayed infection can occur at the catheter exit, in the pocket, or via hematogenous seeding from a distant site (Denny, 1993). In veterinary medicine, the systemic infection rate is reportedly lower than 10% and the infection is resolved after VAP removal and antibiotic therapy based on a sensitivity test (Valentini et al., 2013).
Local seroma formation is a common complication in veterinary patients, particularly when the jugular vein is used, since VAP placement at this location requires more subcutaneous dissection (Aubert et al., 2011). To avoid seroma formation it is recommended to eliminate dead space by using subcutaneous sutures and to place the port within the pocket, not directly beneath the skin incision (Mayer et al., 2008).
Mechanical complications are usually related to poor port anchoring and suture breakage which may result in VAP mal positioning and difficulty accessing the port. Anchoring the port in a stable position might be challenging (Cahalane et al., 2007). Pre-placing sutures to the anchor points into the underlying muscle are recommended to avoid port malposition (Farrow et al., 2013). Furthermore, getting familiar with the surgical technique reduces positioning and catheter length problems.
The optimal length of the catheter can be determined by anatomic landmarks or with intraoperative fluoroscopy to assess the position of the catheter tip. The use of fluoroscopy is more accurate to access catheter tip placement before cutting the desired length, and the tip location should be reassessed by fluoroscopy after anchoring the port to the fascia layers (Mayer et al., 2008). When fluoroscopy is not available, the final position of the catheter can be assessed with radiographs that should be taken immediately after surgery. Recommended location for the catheter tip for a VAP in a jugular vein is the cranial vena cava (Bagley and Flanders, 1990). Placement of the tip within the heart is not recommended because of the risk of endocardial irritation and arrhythmia (Evans et al., 1994).
Conclusion
The VAPs allows continuous vascular access in patients that need repeated intravenous blood draws or injections, and are well tolerated by dogs and cats. VAPs are implanted to aid treatment in veterinary cancer patients with chronic illness and immune-compromise. The VAP system should be considered by the veterinary oncologist to improve the welfare of animals receiving multiple injections as part of long-term treatments.
Acknowledgements
To CAPES foundation, Ministry of Education of Brazil fellowship to Simone Guérios process number 3293-1 3-0.
Conflicts of interest
The authors declare they have no conflicts of interest with regard to the work presented in this report.
Notes
¤To cite this article: Guérios SD, Silva DM, Souza CHM, Bacon NJ. Surgical placement and management of jugular vascular access ports in dogs and cats: description of technique. Rev Colomb Cienc Pecu 2015; 28:265-271.
References
Albarellos GA, Bonafine RR, Kreil VE, Ambros LA, Montoya L, Hallu RF. A non-surgical jugular catheterization technique for multiple blood sampling in cats. Lab Anim 2003; 37:188-192. [ Links ]
Aubert I, Abrams-Ogg, ACG, Sylvestre AM, Dyson DH, Allen DG, Johnstone IB. The use of vascular access ports for blood collection in feline blood donors. Can J Vet Res 2011; 75:25-34. [ Links ]
Bagley RS, Flanders JA. The use of totally implantable vascular access systems. Comp Cont Educ Pract 1990; 12:22-27. [ Links ]
Cahalane AK, Rassnick KM, Flanders JA. Use of vascular access ports in femoral veins of dogs and cats with cancer. J Am Vet Med Assoc 2007; 231:1354-1360. [ Links ]
Cowart RP, Payne JT, Turk JR, Tyler JW, Casteel AW. Factors optimizing the use of subcutaneous vascular access ports in weaned pigs. Contemp Top Lab Anim Sci 1999; 38:67-70. [ Links ]
Culp WTN, Mayhew PD, Reese MS, Duda L, Glassman MM, Brown DC. Complications associated with use of subcutaneous vascular access ports in cats and dogs undergoing fractionated radiotherapy: 172 cases (1996-2007). J Am Vet Med Assoc 2010; 236:1322-1327. [ Links ]
Denny DF Jr. Placement and management of long-term central venous access catheters and ports. AJR 1993; 161:385-393. [ Links ]
Evans KL, Smeak DD, Couto CG, Hammer AS, Gaynor JS. Comparison of two indwelling central venous access catheters in dogs undergoing fractionated radiotherapy. Vet Surg 1994; 23:135-142. [ Links ]
Farrow HA, Rand JS, Burgess DM, Coradini M, Vankan DM. Jugular vascular access port implantation for frequent, long-term blood sampling in cats: methodology, assessment, and comparison with jugular catheters. Res Vet Sci 2013; 95:681-686. [ Links ]
Hai NP. Technical notes on long-term vascular access for more than 12 months in conscious dogs. J Pharmacol Method 1982; 7:5-64. [ Links ]
Henderson KK, Mokelke EA, Turk JR, Rector RS, Laughlin MH, Sturek M. Maintaining patency and asepsis of vascular access ports in Yucatan miniature swine. Contemp Top Lab Anim Sci 2003; 42:28-32. [ Links ]
Henry CJ, Russell LE, Tyler JW, Buss MS, Seguin B, Cambridge AJ, Moore ME. Comparison of hematologic and biochemical values for blood samples obtained via jugular venipuncture and via vascular access ports in cats. J Am Vet Med Assoc 2002; 220:482-485. [ Links ]
Ignatov A, Hoffman O, Smith B, Fahlke J, Peters B, Bischoff J, Costa SD. An 11-year retrospective study of totally implanted central venous access ports: complications and patient satisfaction. Eur J Surg Oncol 2008; 35:241-246. [ Links ]
Mayer MN, Grier CK, Yoshikawa H, Ringwood B. Complications associated with the use of vascular access ports in dogs receiving external beam radiation therapy. J Am Vet Med Assoc 2008; 233:96-103. [ Links ]
Morrison JA, Lauer SK, Baldwin CJ, Evans RB, Andreasen CB, Kinyon JM, Swanson E. Evaluation of the use of subcutaneous implantable vascular access ports in feline blood donors. J Am Vet Med Assoc 2007; 230:855-861. [ Links ]
Newman KA, Reed WP, Bustamante CI, Schimpff SC, Wade JC. Venous access devices utilized in association with intensive cancer chemotherapy. Eur J Cancer Clin On 1989: 1375-1378. [ Links ]
Niederhuber JE, Ensminger W, Gyves JW, Liepman M, Doan K, Cozzi E. Totally implanted venous and arterial access system to replace external catheters in cancer treatment. Surgery 1982; 92:706-712. [ Links ]
Schwarz RE, Groeger JS, Coit DG. Subcutaneously implanted central venous access devices in cancer patients. Cancer 1997; 79:1635-1640. [ Links ]
Sehirali S, Inal MM, ÖzsezgIn S, Sanci M, Atli Ö, Nayki C, Yildirim Y, Tinar S. A randomized prospective study of comparison of reservoir ports versus conventional vascular access in advanced-stage ovarian carcinoma cases treated with chemotherapy. Int J Gynecol Cancer 2005; 15:228-232. [ Links ]
Seldinger SI. Catheter replacement of the needle in percutaneous arteriography: a new technique. Acta Radiol 1953; 39:368-376. [ Links ] Swindle MM, Nolan T, Jacobson A, Wolf P, Dalton MJ, Alison C. Vascular access port (VAP) usage in large animal species. Contemp Top Lab Anim Sci 2005; 44:7-17. [ Links ]
Valentini F, Fassone F, Pozzebon A, Gavazza A, Lubas G. Use of totally implantable vascular access port with mini-invasive Seldiger technique in 12 dogs undergoing chemotherapy. Res Vet Sci 2013; 94:152-157. [ Links ]
Webb AI, Bliss JM, Herbst LH. Use of vascular access ports in the cat. Lab Anim Sci 1995; 45:110-114. [ Links ]