Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Colombiana de Ciencias Pecuarias
Print version ISSN 0120-0690
Rev Colom Cienc Pecua vol.28 no.4 Medellín Sep./Dec. 2015
https://doi.org/10.17533/udea.rccp.v28n4a01
ORIGINAL ARTICLE
doi: 10.17533/udea.rccp.v28n4a01
Edible mushroom powder (Agaricus bisporus) and flavophospholipol improve performance and blood parameters of broilers¤
Champiñón en polvo (Agaricus bisporus) y flavofosfolipol mejoran el desarrollo y parámetros sanguíneos en pollos de engorde
O uso de champignon em pó (Agaricus bisporus) e flavofosfolipol melhoram o desenvolvimento e parâmetros sanguíneos em frangos de corte
Sepideh Shamsi1, BSc (Ag), MSc; Alireza Seidavi1*, BSc (Ag), PhD; Maliheh Rahati1, BSc (Ag), PhD; José Ángel G Nieto2, MV, PhD.
1Department of Animal Science, Rasht Branch, Islamic Azad University, Rasht, Iran.
2Consejería Agricultura Castilla-La Mancha. OCA La Roda, 02630, Albacete, Spain.
*Corresponding author: Alireza Seidavi. Department of Animal Science, Rasht Branch, Islamic Azad University, Rasht, Iran. Tel: +989113313073. Email: alirezaseidavi@iaurasht.ac.ir
Received: January 13, 2015; accepted: June 12, 2015
Summary
Background: flavophospholipol is an antibiotic growth promoter (AGP). The current ban of AGP in some countries is controversial because their benefits on the environment and economy by saving feed and reducing nitrogen excretion have been overlooked. White button mushrooms have important nutritional properties and the industry discards large quantities of waste that could be fed to animals. Objective: to evaluate the effect of dietary inclusion of five levels of edible mushroom powder (EMP) and flavophospholipol on the performance and blood serum metabolites of broilers. Methods: a total of 300 one-day-old male broiler chicks were randomly distributed into 10 treatments with three replicates of 10 chicks per pen. The experiment consisted of a factorial arrangement (2x5 treatments) with five inclusion levels of EMP supplementation (0, 0.5, 1.0, 1.5, and 2.0 g/kg of diet) and the addition of 0 or 5 mg/kg of flavophospholipol. Results: supplementation with EMP and flavophospholipol, as individual factors, had a negative effect on feed intake, but positively affected broiler weight gains and feed conversion ratio. Antibiotic supplementation increased uric acid concentration and, as an interaction with mushroom powder, reduced serum triglycerides, and very low density lipoprotein cholesterol (VLDL). The EMP also affected serum concentration of total cholesterol. Conclusion: the two substances studied, but not their combination, had a positive effect on growth performance of chickens that could be translated into economic benefits.
Keywords: antibiotic, blood chemical analysis, fungi, poultry.
Resumen
Antecedentes: el flavofosfolipol es un antibiótico promotor del crecimiento (AGP). La actual prohibición de los AGP en muchos países es polémica porque sus beneficios económicos y medioambientales a través del ahorro en pienso y la disminución en la producción de heces y nitrógeno son pasados por alto. El champiñón común tiene importantes propiedades nutricionales y la industria desecha grandes cantidades de subproducto que podrían ser aprovechables en la alimentación de animales. Objetivo: evaluar el efecto de cinco niveles de champiñón en polvo (EMP) y flavofosfolipol sobre el desarrollo y metabolitos sanguíneos en suero de pollos de engorde. Métodos: trescientos pollos de engorde machos de un día de vida fueron distribuidos aleatoriamente en 10 grupos en función del tratamiento con tres réplicas de 10 animales. El diseño experimental consistió en un arreglo factorial (2x5 tratamientos), incluyendo cinco concentraciones de champiñón en polvo (0, 0,5, 1,0, 1,5 2,0 g/kg de alimento) y la adición de 0 o 5 mg/kg de flavofosfolipol. Resultados: la suplementación con EMP y flavofosfolipol, individualmente, tiene un efecto negativo en la ingestión diaria de alimento, pero positivo sobre la ganancia de peso vivo y el índice de conversión. La suplementación con antibióticos aumenta la concentración de ácido úrico en sangre e, interaccionando con el champiñón en polvo, disminuye la concentración sérica de triglicéridos y de colesterol de muy baja densidad (VLDL). El EMP también afectó la concentración sérica de colesterol total. Conclusiones: las dos sustancias estudiadas, pero no su combinación, tuvieron un efecto positivo sobre el desarrollo de los pollos de engorde que se puede traducir en un beneficio económico.
Palabras clave: análisis químico sanguíneo, antibiótico, aves de corral, hongo.
Resumo
Antecedentes: o flavofosfolipol é um antibiótico promotor de crescimento (AGP). A proibição de AGP em muitos países é controversa pois os seus benefícios económicos e ambientais a través da poupança de subministro de alimento e diminuição da produção de fezes e nitrogênio tendem a ser esquecidos. A respeito disso, o cogumelo tem propriedades nutricionais interessantes e a indústria alimentícia rejeita grandes quantidades deste produto que poderiam ser de utilidade para ser aproveitadas na alimentação animal. Objetivo: avaliar o efeito de cinco níveis diferentes do cogumelo em pó (EMP) e da presença ou não de flavofosfolipol sobre o desenvolvimento e a concentração sérica de vários metabolitos em frangos de corte. Métodos: trezentos frangos de um dia foram aleatoriamente divididos em dez grupos de acordo com o tratamento, com três repetições de dez animais. Usou-se um delineamento experimental fatorial (2x5 tratamentos), em que os tratamentos realizados incluíram cinco concentrações de champignon em pó (0, 0,5, 1,0, 1,5 e 2,0 g/kg de ração) e da adição da 0 ou 5 mg/kg flavofosfolipol. Resultados: adicionar flavofosfolipol e cogumelo em pó às dietas exerce, independentemente, uma diminuição sobre a ingestão diária da ração, mas com resultados positivos no ganho de peso e na taxa de conversão alimentar. O antibiótico aumenta a concentração de ácido úrico no sangue; quando está associado com o pó de cogumelo, diminui a concentração sérica de triglicerídeos e colesterol de baixa densidade (VLDL). O consumo do pó de cogumelo afetou a concentração sérica do colesterol total. Conclusões: as duas substâncias estudadas, mas consumidas independentemente, têm um efeito positivo sobre o desenvolvimento com o intuito de ter além, um benefício econômico na produção de frangos de corte.
Palavras chave: antibiótico, composição química sanguínea, frangos de corte, fungo.
Introduction
The increase in bacterial antibiotic resistance could be directly linked to the abuse of antibiotic growth promoters (AGP) in livestock production (De Barros et al., 2012). This has resulted in banning their use in many countries, mainly within the European Union. Others, including Iran, following the recommendations of the international office of epizootics (OIE) have relied on prudent use guidelines and programs that reduce total microbial loads, rather than banning AGP. According to the available data, the use of antibiotics for livestock and poultry in Iran is higher than that in developed countries with the exception of South Korea (Aalipour et al., 2014).
Until today, a direct and clear link between AGP use and increased antibiotic resistance has not been established (Bywater, 2005). Moreover, for some researches, the strategy of banning or restricting the use of antibiotics in animals has had limited success: it has been followed in many cases by deterioration of animal health and increase in human illnesses and resistance rates (Cox Jr and Ricci, 2008). Therefore, the use of antimicrobial feed additives of different class from the antibiotics used in humans, and hence not putatively cause of resistance, seems reasonable. Flavophospholipol is a phosphoglycolipid antimicrobial produced by various strains of Streptomyces (Pfaller, 2006), has no therapeutic use in human or veterinary medicine, and can still be legally added to poultry diets in many countries (Butaye et al., 2003).
Additionally, several medicinal mushrooms have demonstrated potent antioxidant activities and, as a consequence, have potential application as natural antioxidants (Barros et al., 2006; Minareci et al., 2011; Liu et al., 2013). Agaricus bisporus, commonly known as white button mushroom or champignon, is one of the most commonly and widely consumed mushrooms in the world. It is considered a valuable healthy food with high content of polyphenols, ergothioneine, vitamins, minerals, and polysaccharides (Tian et al., 2012). Moreover, A. bisporus has demonstrated various valuable biological properties including antitumor, anti-aromatase, antimicrobial, immune-modulatory, anti-inflammatory, and antioxidant activities (Chen et al., 2006; Tsai et al., 2009; Moro et al., 2012; Liu et al., 2013). During the mushroom production process, high quantities of this mushroom are wasted, which could be recycled as a feed additive for broiler production.
Therefore, the aim of this study was to determine the effects of edible mushroom powder (EMP) on performance and haematology parameters of broiler chickens. Simultaneously, the use of flavophospholipol as an antimicrobial feed additive and its interaction with EMP was also evaluated.
Material and methods
Ethical considerations
The experiments were approved by the Scientific Board of the Islamic Azad University and were conducted according to the International Guidelines for research involving animals (Directive 2010/63/EU).
Location
This study was conducted at the Poultry Farm Facilities in the city of Ramsar (latitude 50°40' N and longitude 36°54' E) and the Agriculture Faculty of the Islamic Azad University, Rasht Branch, Iran, between July-September, 2013 (summer, warm season), during a total of 42 days.
Animals and housing
Three hundred one-day-old male Ross 308 broilers (Aviagen, 2007) were randomly assigned to 10 treatments with three replicates per treatment. Each replicate consisted of 10 chicks housed in pens of 1.2 m2. The pens were equipped with electrical heaters. The room temperature was adjusted to approximately 33 ºC on day 1, and was then gradually reduced to 24 ºC. Lighting was provided with a 23L:1D (from 19:00 to 20:00) program. Humidity was maintained between 55 and 65% in the early growing period by spraying water on the floor. The chicks were vaccinated against Newcastle and Gumboro. Within 24 h after vaccination, a multiple-vitamin and electrolyte solution (1:1000) was offered via drinking water to reduce stress.
Diets and experimental design
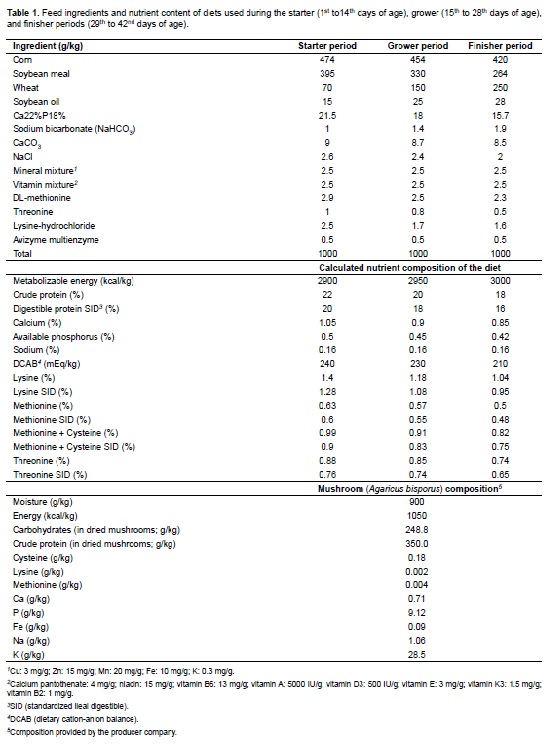
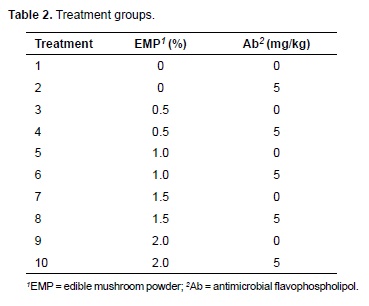
Feed and water were supplied ad libitum during the entire experimental period. The nutritional requirements were met according with the Ross rearing catalogue (Aviagen, 2007). Feeds were formulated to be iso-proteic and iso-energetic for all treatments. The composition of diets and their nutrient content are presented in Table 1. The feed remaining in the feeders (orts) was weighed and weekly recorded. Mushrooms (A. bispours) were obtained from a national mushroom producer (Pars Company, Ramsar, Iran). Mushroom composition was provided by the same company and is presented in Table 1. Whole mushrooms were dried at 60 ºC and added to the experimental diets after grinding. The treatments consisted of five levels of EMP (0, 0.5, 1.0, 1.5, and 2.0 g/kg) and 0 or 5.0 mg/kg flavophospholipol (Teif Azmoon Pars, Co., Tehran, Iran). According to De Barros et al. (2012), the best performance is obtained with 10 mg/kg flavophospholipol. Regarding mushroom powder, Giannenas et al. (2011) reported positive results on turkey poults when it was added up to 20 g/kg feed. Dietary treatments used in the present study are detailed in Table 2.
Body weight at birth was 43.5 ± 1.2 g. Weight gain was weekly recorded by pen. At the end of the study (42 days of age) the mean body weight of birds (commercial weight) was 2,715.3 ± 31.7 g. At this day one bird per group, totalling three birds per treatment, were selected for blood collection. Care was taken to choose the most representative birds with respect to body weight compared to the mean body weight of the group. Blood samples (1 mL/bird) were collected into EDTA tubes from the wing veins. Samples were transferred to the laboratory for analysis within 2 hours of collection. After centrifugation (3,000 g x 10 min at room temperature) serum was harvested and stored in Eppendorf tubes at -20 ºC until analysis.
Chemical analysis
Serum cholesterol and triglyceride levels were determined using enzymatic methods (Teif Azmoon Pars Co., Tehran, Iran). High-density lipoprotein (HDL) and low-density lipoprotein (LDL) cholesterol were measured directly with HDL-C and LDL-C diagnostic kits (Teif Azmoon Pars Co., Tehran, Iran). Colorimetric determination of cholesterol in blood serum samples was done by the cholesterol oxidase procedure by Barham and Trinder (1972), which is based on the formation of a red-purple quinoneimine produced by oxidative condensation of a phenolic compound with 4-aminoantipyrine in the presence of hydrogen peroxide. Quinoneimine absorbance, measured spectrophotometrically, has a direct relationship with the amount of cholesterol in the sample.
Serum triglycerides were measured using a series of coupled reactions in which triglycerides are hydrolyzed to produce glycerol. Glycerol is then converted to pyruvate and then to lactate. Decreased absorbance, measured spectrophotometrically, is proportional to the triglyceride concentration in the sample (Schmid and von Forstner, 1986).
A glucose oxidase kit (Teif Azmoon Pars Co., Tehran, Iran), based on an oxidase-peroxidase procedure, was used to measure serum glucose. In this assay, glucose is oxidized in the presence of the glucose oxidase catalyst into H2O2 and gluconic acid. The reactions involving gluconic acid, hydrogen peroxide, a phenolic compound and 4-aminoantipyrine form a red-violet quinoneimine, and the absorbance of the quinoneimine chromagen, measured by spectrophotometry, is directly related to the amount of glucose in the sample.
A uric acid-uricase enzyme kit (Teif Azmoon Pars Co., Tehran, Iran), based on an oxidase-peroxidase procedure (Trinder, 1969), was used to measure serum uric acid. In this procedure uric acid is oxidized with uricase and, in the presence of the generated hydrogen peroxide, a phenolic compound and 4-aminoantipyrine forms a red-colored quinoneimine. The absorbance of quinoneimine chromagen, measured by spectrophotometry, is directly associated with the amount of uric acid in the sample (Thomas, 1998).
Serum alkaline phosphatase (ALP) was determined enzymatically using a commercial kit (Teif Azmoon Pars Co., Tehran, Iran). In this procedure, ALP activity was determined colorimetrically by a modification of the Bessey et al. (1964) method, using p-nitrophenol phosphate as the enzyme substrate, which is converted to phosphate and p-nitrophenol by the ALP. The released p-nitrophenol is proportional to the ALP activity.
Albumin (Alb) was determined based on the bromocresol green method (Doumas et al., 1971) using a commercial kit (Teif Azmoon Pars Co., Tehran, Iran), while total protein (TP) was assayed by the Biuret method (Gornall et al., 1949; Teif Azmoon Pars Co., Tehran, Iran). Total protein determination was based on the principle that protein forms an intense violet-blue complex with copper salts in alkaline media. Iodine was included as an antioxidant, the intensively color formed is proportional to the total protein concentration in the sample. The globulin values were calculated by subtracting albumin values from the corresponding total protein.
Statistical analysis
General lineal models (GLM, SPSS 15.0, Chicago, IL, USA) were used to analyse the effects of EMP and flavophospholipol on feed intake (FI), weight gain (WG), feed conversion ratio (FCR), body weight at 42 d, and blood metabolites in a 2x5 factorial arrangement. Tukey's test was used to determine disparities among the groups.
Results
Growth performance
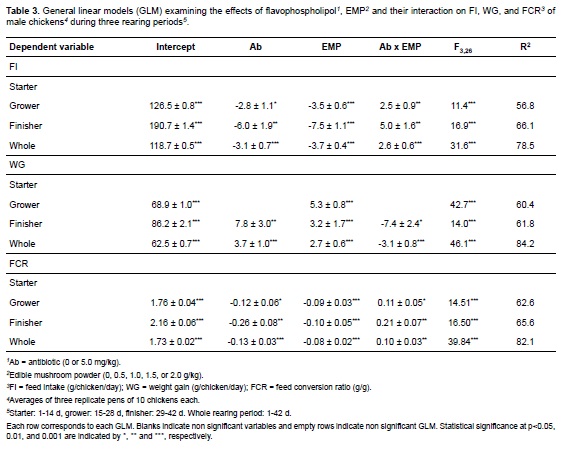
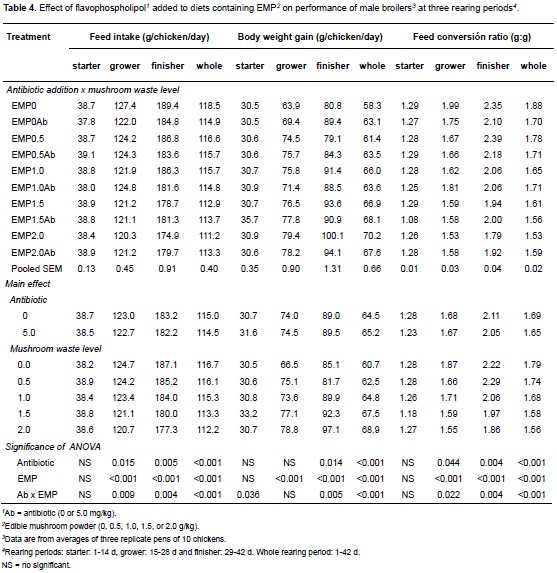
The effects of dietary EMP and antibiotic supplementation on bird performance are detailed in Tables 3 and 4, respectively. Results from the current work show that dietary EMP as well as the addition of flavophospholipol had a negative effect on FI. These effects were absente in the starter period, but during the grower period both variables reached significance (see GLM on Table 3). Similarly, both EMP and flavophospholipol affected FI during the finisher period. Finally, the GLM showed a negative effect of these variables on FI for the whole growth period (from birth to 42 days; Table 3). Regarding WG, results showed a positive effect of EMP and antibiotic supplementation, but showed a negative effect when combined. This effect was not found in the starter period, while only EMP effects reached significance during the grower period. In the finisher period, as well as in the GLM analysing the whole rearing period, both variables were significant. Results similar to those for FI were observed for FCR, i.e., EMP and antibiotic supplementation decreased FCR for all rearing periods except for the first one. Finally, results showed that both flavophospholipol and EMP addition positively affected final body weight (model F3,26 = 23.1, p<0.001, R2= 72.7%), coefficients were: EMP = 99.3 ± 34.9, p<0.001; antibiotic = 185.7 ± 60.5, p<0.01; interaction = -173.6 ± 49.4, p<0.01.
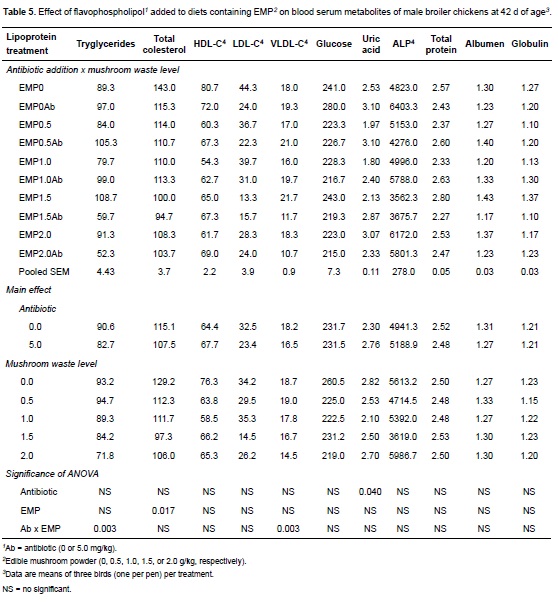
Blood serum metabolites
Values for selected serum metabolites in birds fed the 10 experimental diets are given in Table 4. The antibiotic supplementation did not affect serum concentration of total cholesterol, HDL, LDL, glucose, or ALP. However, dietary flavophospholipol linearly increased uric acid concentration (p = 0.040) and, as an interaction with EMP, decreased triglycerides serum concentration (F1,28 = 5.04, p<0.01, R2 = 27.2%) and VLDL (F1,28 = 5.29, p<0.05, R2 = 28.2%). EMP affected, individually or combined with antibiotic, the serum concentration of triglycerides, total cholesterol (F1,28 = 6.38, p<0.05, R2 = 18.6%; coefficient = -12.27 ± 4.86, p<0.05), and VLDL.
No statistically significant differences were found for total protein concentration at day 42 between treatment groups and control (Table 5). Similarly, no significant differences were observed for total albumin or globulin concentrations in treatment groups in comparison to controls.
Discussion
In general, adding flavophospholipol and EMP to diets caused positive effects on broiler performance. The results for flavophospholipol addition are similar to those reported by De Barros et al. (2012), who also found improvements in body weight gains and feed conversion ratio. However, there are two main differences between their study and ours. First, they found these benefits throughout the whole rearing period while we found improvements only from the third week of rearing onward. This difference can be explained by the different doses of antibiotic employed in both studies; in our work we used 5 mg/kg of the antibiotic while De Barros et al. (2012) used 10 mg/kg of flavophospholipol.
The second difference is that they did not find a decrease in feed intake like the one found in the present study. A possible explanation for this negative effect of flavophospholipol and EMP on feed intake could be that supplementation of both products reduces feed palatability. To our knowledge, there are no studies concluding that EMP or flavophospholipol addition could affect feed intake by changing its palatability.
However, it has been demonstrated that mushrooms have a quite intense taste (Tsai et al., 2009) so it can affect feed flavour (Perdok et al., 2003; Isabel and Santos, 2009).
The positive impact of growth promoting antibiotics is well known in the poultry industry. Antibiotics suppress harmful microorganisms and the intestinal bacterial population comes into balance as a more beneficial microbiota. In this sense, a well-balanced gut microbe population is considered an effective barrier against pathogenic bacteria, which positively influences animal growth through increased nutrient absorption (Pfaller, 2006). Inhibition of inflammatory effects has also been proposed as a means of action for this type of compound (Niewold, 2007).
It is not surprising that mushroom powder had a positive effect on broiler performance; it is acknowledged that mushrooms have a good effect on human and animal health. Giannenas et al. (2010) reported that A. bisporus has a prebiotic-like effect on turkeys since fermentable polysaccharide content in mushrooms may improve growth of Lactobacilli and Bifidobacteria populations and inhibit E. coli leading to a more balanced biota population in the gastrointestinal tract of poultry, as a consequence, to a greater efficiency in digestibility and feed utilization. Furthermore, Lactobacilli may produce organic acids such as lactic acid (Rehman et al., 2007) and bactericidal substances (Neal-McKinney et al., 2012) that may improve gastrointestinal function and feed digestibility, resulting in enhanced growth and improved FCR (Ferket, 2004).
An important result of the present work is the combined negative effect of the antibiotic and EMP on weight gain, FCR and body weight at 42-d. Since flavophospholipol promotes proliferation of a bacterial population capable of profiting in a more effective way the mushroom nutritional properties, including sugar composition as polysaccharide fractions, we expected to find the opposite results. In contrast, the present study showed that the combination of the antibiotic and EMP decreases the positive effect on growth produced by the two substances added separately.
Limited information is available on changes in blood metabolites associated with the addition of flavophospholipol or EMP. In the present study, the addition of EMP showed an overall decrease on blood lipid metabolite profiles. In this sense, results show that EMP added individually or with flavophospholipol decreases serum triglycerides, total cholesterol and VLDL concentration but no LDL or HDL. So, the decrease in serum total cholesterol may be a consequence of the decrease in VLDL.
Abdel-Fatah et al. (2008) showed that total blood lipids and cholesterol decreased significantly with dietary acidifiers. Panda et al. (2006) discovered that the addition of probiotics in diets reduced total serum cholesterol and triglycerides. They attributed this to a reduced absorption and/or synthesis of cholesterol in the gastro-intestinal tract of probioticsupplemented chickens (Mohan et al., 1995; Mohan et al., 1996). Similarly, Lactobacillus acidophilus and other acidophilic bacteria lower the pH of the environment it occupies. Bile acids are less soluble at low pH, are absorbed less in the intestine, and are more likely to be excreted in the faeces (Klaver and van der Meer, 1993).
Since fermentable polysaccharides in mushrooms may improve Lactobacilli growth, this could explain part of the results in the present study. Furthermore, flavophospholipol inhibits transglycosylase, an enzyme necessary for the formation of bacterial cell wall, and its consequent intestinal microbiota modulation promotes the development of Lactobacilli and Bifidobacteria, which are generally considered beneficial bacteria (Bolder et al., 1999). Another possible explanation, or at least partially, is that the observed lower feed consumption and consequently lower fat intake may also contribute to reducing blood lipid content. This increment in beneficial bacterial populations would lead to a more efficient assimilation of the feed throughout a better intestinal absorption that could explain the observed reduction in FI and FCR increment.
Flavophospholipol treatment showed an increment in serum concentration of uric acid. Uric acid is the major end product of protein metabolism in poultry (Griminger and Scames, 1986) so increased serum concentration of uric acid could indicate increased growth potential in broilers treated with the antibiotic, which would be reflected in the increased weight gain observed in our results. Supporting this idea, Darsi et al. (2012) observed that uric acid plasma levels were reduced in parallel with dietary crude protein. On the other hand, increased plasma uric acid has anti-oxidant activity in chickens (Klandorf et al., 2001; Carro et al., 2010), which suggest an anti-oxidative effect of flavophospholipol.
Finally, treatments failed to induce any significant effect on total protein, albumin or globulin serum concentrations. Higher serum globulin is an indicator of better immune response and source of antibody production, and low albumin to globulin ratio signifies better disease resistance and immune response (Griminger, 1986), so the good health status of birds in the present study can explain why there was no evident influence of mushrooms on these blood compounds. Similarly, Guo et al. (2003) suggested that the mushrooms effect was more pronounced under infectious conditions than under normal ones.
In conclusion, the use of flavophospholipol and EMP has a positive effect on growth performance and these benefits can be translated into important amounts of feed saved in animal production. Moreover, results showed an overall decrease in blood lipid metabolite profiles and uric acid content of chicks.
Acknowledgements
This manuscript has been prepared from the MSc thesis of the first author at Islamic Azad University, Rasht Branch, Rasht, Iran. The authors wish to thank the Islamic Azad University for their support and the two anonymous referees for their comments.
Conflicts of interest
The authors declare they have no conflicts of interest with regard to the work presented in this report.
Notes
¤To cite this article: Shamsi S, Seidavi A, Rahati M, Nieto JAG. Edible mushroom powder (Agaricus bisporus) and flavophospholipol improve performance and blood parameters of broilers. Rev Colomb Cienc Pecu 2015; 28:291-302.
References
Aalipour F, Mirlohi M, Jalali M. Determination of antibiotic consumption index for animal originated foods produced in animal husbandry in Iran, 2010. J Environ Health Sci Eng 2014; 12:42-48. [ Links ]
Abdel-Fattah SA, El-Sanhoury MH, El-Mednay NM, Abdel- Azeem F. Thyroid activity, some blood constituents, organs morphology and performance of broiler chicks feed supplemental organic acids. Int J Poult Sci 2008; 7(3):215-222. [ Links ]
Aviagen. Ross 308 broiler: Nutrition Specification 2007; [Access date; November 24, 2014]. URL: http://www.natchix.co.za/pdf/nutrition_specifications.pdf [ Links ]
Barham D, Trinder P. An improved colour reagent for the determination of blood glucose by the oxidase system. Analyst (Lond) 1972; 97:142-145. [ Links ]
Barros L, Ferreira MJ, Queirós B, Ferreira ICFR, Baptista P. Total phenols, ascorbic acid, ß-carotene, and lycopene in Portuguese wild edible mushrooms and their antioxidant activities. Food Chem 2006; 103:413-419. [ Links ]
Bessey OA, Lowry OH, Brock MJ. A method for the rapid determination of alkaline phosphatase with five cubic millimeters of serum J Biol Chem 1964; 164:321-329. [ Links ]
Bolder NM, Wagenaar JA, Putirulan FF, Veldman KT, Sommer M. The effect of flavophospholipol (Flavomycin®) and salinomycin sodium (Sacox®) on the secretion of Clostridium perfringens, Salmonella enteritidis, and Campylobacter jejuni in broilers after experimental infection. Poult Sci 1999; 78:1681-1689. [ Links ]
Butaye P, Devriese LA, Haesebrouck F. Antimicrobial growth promoters used in animal feed: effects of less well known antibiotics on gram-positive bacteria. Clin Microbiol Rev 2003; 16(2):175-188. [ Links ]
Bywater RJ. Identification and surveillance of antimicrobial resistance dissemination in animal production. Poult Sci 2005; 84:644-648. [ Links ]
Carro MD, Falkenstein E, Radke WJ, Klandorf H. Effects of allopurinol on uric acid concentrations, xanthine oxidoreductase activity, and oxidative stress in broiler chickens. Comp Biochem Physiol C 2010; 151:12-17. [ Links ]
Chen S, Oh SR, Phung S, Hur G, Ye JJ, Kwok SL, Shrode GE, Belury M, Adams LS, Williams D. Anti-aromatase activity of phytochemicals in white button mushrooms (Agaricus bisporus). Cancer Res 2006; 66(24):12026-12034. [ Links ]
Cox Jr LA, Ricci PF. Casual regulations vs. political will: Why human zoonotic infections increase despite precautionary bans on animal antibiocics. Environ Int 2008; 34(8):459-475. [ Links ]
Darsi E, Shivazad M, Zaghari M, Namroud NF, Mohammadi R. Effect of reduced dietary crude protein levels on growth performance, plasma uric acid and electrolyte concentration of male broiler chicks. J Agric Sci Technol 2012; 14:789-797. [ Links ]
De Barros RD, Vieira SL, Favero A, Taschetto D, Mascarello NC, Cemin HS. Reassessing flavophospholipol effects on broiler performance. Rev Bras Zootec 2012; 41(12):2458-2462. [ Links ]
Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 of the protection of animals used for scientific purposes. Official Journal of the European Union of 20th October 2010. L276/33. [ Links ]
Doumas BT, Watson WA, Biggs HG. Albumin standards and the measurement of serum albumin with bromcresol green. Clin Chim Acta 1971; 31:87-96. [ Links ]
Ferket PR. Alternatives to antibiotics in poultry production. In: Lyons TP, Jacques KA, editors. Nutritional Biotechnology in the Feed and Food Industries. Nottingham: E-publishing Inc; 2004. p.57-67. [ Links ]
Giannenas I, Pappas IS, Mavridis S, Kontopidis G, Skoufos J, Kyriazakis I. Performance and antioxidant status of broiler chickens supplemented with dried mushrooms (Agaricus bisporus) in their diet. Poult Sci 2010; 89:303-311. [ Links ]
Giannenas I, Tsalie E, Chronis EF, Mavridis S, Tontis D, Kyriazakis I. Consumption of Agaricus bisporus mushroom affects the performance, intestinal microbiota composition, and morphology, and antioxidant status of turkey poults. Anim Feed Sci Techol 2011; 165:218-229. [ Links ]
Gornall AG, Bardawill CJ, David MM. Determination of serum proteins by means of the biuret reaction. J Biol Chem 1949; 177:751-766. [ Links ]
Griminger P. Lipid metabolism. In: Sturkie PD, editor. Avian Physiology 4th ed. New York: Springer; 1986. p.345-358. [ Links ]
Griminger P, Scames CG. Protein metabolism. In: Sturkie PD, editor. Avian Physiology 4th ed. New York: Springer; 1986. p.326-344. [ Links ]
Guo FC, Savelkoul HFJ, Kwakkel RP, Williams BA, Verstegena MWA. Immunoactive, medicinal properties of mushroom and herb polysaccharides and their potential use in chicken diets. World's Poult Sci J 2003; 59(4):427-440. [ Links ]
Isabel B, Santos Y. Effects of dietary organic acids and essential oils on growth performance and carcass characteristics of broiler chickens. J App Poult Res 2009; 18:472-476. [ Links ]
Klandorf H, Rathore D, Iqbal M, Shi X, Simoyi M, Van Dyke KV. Accelerated tissue aging and increased oxidative stress in broiler chickens fed allopurinol. Comp Biochem Physiol C 2001; 129:93-104. [ Links ]
Klaver FAM, van der Meer R. The assumed assimilation of cholesterol by Lactobacilli and Bifidobacterium bifidum is due to their bile salt deconjugating activity. App Environ Microbiol 1993; 59:1120-1124. [ Links ]
Liu J, Jia L, Kan J, Jin CH. In vitro and in vivo antioxidant activity of ethanolic extract of white button mushroom (Agaricus bisporus). Food Chem Toxicol 2013; 51:310-316. [ Links ]
Minareci E, Ergonul B, Kayalar H, Kalyoncu F. Chemical mompositions and antioxidant activities of five endemic asperula TAXA. Arch Biol Sci Belgrade 2011; 63(3):537-543. [ Links ]
Mohan B, Kadirvel R, Bhaskaran M, Natarajan M. Effect of probiotic supplementation on serum/yolk cholesterol and on egg shell thickness in layers. Br Poult Sci 1995; 36:799-803. [ Links ]
Mohan B, Kadirvel R, Natarajan M, Bhaskaran M. Effect of probiotic supplementation on growth, nitrogen utilization and serum cholesterol in broilers. Br Poult Sci 1996; 37:395-401. [ Links ]
Moro C, Palacios I, Lozano M, D'Arrigo M, Guillamón E, Villares A, García-Lafuente A. Anti-inflammatory activity of methanolic extracts from edible mushrooms in LPS activated RAW 264.7 macrophages. Food Chem 2012; 130(2):350-355. [ Links ]
Neal-McKinney JM, Lu X, Duong T, Larson CL, Call DR, Shah DH, Konkel ME. Production of organic acids by Probiotic Lactobacilli can be used to reduce pathogen load in poultry. PLoS ONE 2012; 7:1-11. [ Links ]
Niewold TA. The nonantibiotic anti-inflammatory effect of antimicrobial growth promoters, the real mode of action? A hypothesis. Poult Sci 2007; 86:605-609. [ Links ]
Panda AK, Rama Rao SV, Raju MVLN, Sharma SR. Dietary supplementation of Lactobacillus Sporogenes on performance and serum biochemico-lipid profile of broiler chickens. J Poult Sci 2006; 43:235-240. [ Links ]
Perdok H, Langhout P, Van Vugt P. Stimulating appetite. Feed Mix 2003; 11:10-13. [ Links ]
Pfaller MA. Flavophospholipol use in animals: Positive implications for antimicrobial resistance based on its microbiologic properties. Diagn Microbiol Infec Dis 2006; 56(2):115-121. [ Links ]
Rehman H, Vahjen W, Awad WA, Zentek J. Indigenous bacteria and bacterial metabolic products in the gastrointestinal tract of broilers. Arch Anim Nutr 2007; 61:319-335. [ Links ]
Schmid M, von Forstner D. Laboratory testing in veterinary medicine diagnosis and clinical monitoring. Mannheim: Boehringer Mannheim GmbH; 1986. [ Links ]
Thomas L. Clinical laboratory diagnostics: Use and assessment of clinical laboratory results. Verlagsgeselschaft: TH-Books; 1998. [ Links ]
Tian Y, Zeng H, Xu Z, Zheng B, Lin Y, Gan C, Lo YM. Ultrasonicassisted extraction and antioxidant activity of polysaccharides recovered from white button mushroom (Agaricus bisporus). Carbohydr Polym 2012; 88(2):522-529. [ Links ]
Tsai SY, Huang SJ, Lo SH, Wu TP, Lian PY, Mau JL. Flavour components and antioxidant properties of several cultivated mushrooms. Food Chem 2009; 113(2): 578-584. [ Links ]
Trinder P. Determination of blood glucose using an oxidaseperoxidase system with a non-carcinogenic chromogen. J Clin Pathol 1969; 22:158-16. [ Links ]