Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista Colombiana de Ciencias Pecuarias
versión impresa ISSN 0120-0690
Rev Colom Cienc Pecua vol.28 no.4 Medellín sep./dic. 2015
https://doi.org/10.17533/udea.rccp.v28n4a07
ORIGINAL ARTICLE
doi: 10.17533/udea.rccp.v28n4a07
Insemination of bocachico fish (Prochilodus magdalenae) with fresh or cryopreserved semen: effect of spermatozoa/oocyte ratio¤
Inseminacion de bocachico (Prochilodus magdalenae) con semen fresco o crioconservado: efecto de la proporción espermatozoide/oocito
Inseminação do bocachico (Prochilodus magdalenae) usando sêmen fresco o criopreservado: efeito da razão espermatozoide/oócito
Víctor J Atencio García1*, MSc; José A Espinosa1, MSc; José G Martínez2, MSc; Sandra C Pardo Carrasco3, PhD.
1Centro de Investigación Piscícola, Facultad de Medicina Veterinaria y Zootecnia, Universidad de Córdoba.
2Laboratory of Animal Genetics and Evolution - LEGAL, Institute of Biological Sciences, Federal University of Amazonas, Manaus, Brasil.
3Facultad de Ciencias Agrarias, Departamento de Producción Animal, BIOGEM, Universidad Nacional de Colombia Sede Medellín, Colombia.
*Corresponding author: Víctor J Atencio García. Universidad de Córdoba. Carrera 6 N° 76-103. Phone: (57)4 7860151. Email: vatencio@hotmail.com
Received: September 4, 2014; accepted: January 11, 2015
Summary
Background: cryopreservation is an important biotechnological tool in the conservation of biodiversity, particularly for endangered species. Objective: to evaluate six different spermatozoa/oocyte ratios using fresh and cryopreserved semen in bocachico fish (Prochilodus magdalenae). Methods: fresh semen was collected and its quality determined to verify cryopreservation feasibility. The semen was put in 5 mL straws and mixed with a solution (5.5% glucose, 12% egg yolk, and 10% dimethyl sulfoxide -DMSO-) in a 1:4 dilution (semen:solution). The semen was frozen in nitrogen vapor dry shipper for 30 min, rapidly transferred to storage thermos, and submerged directly into liquid nitrogen (LN; -196 °C). Straws were thawed at 60 ºC for 45 seconds. Motility, velocity, and sperm progressivity of fresh and cryopreserved semen were assessed using Sperm Class Analyzer (SCA®) software. Each proportion of spermatozoa/oocyte was assessed with 2 g of eggs (1,630 ± 87 eggs/g) to evaluate fertility (F), hatching (H), and larval survival (LS) rates. Results: the best reproductive performance for fresh semen was obtained inseminating with 160,000 spermatozoa/oocyte (F = 75.0%, H = 67.7%, LS = 32.7%). Similarly, the best reproductive performance for cryopreserved semen was achieved with 320,000 spermatozoa/oocyte (F = 70.0%, H = 48.6%, LS = 19.5%). Conclusion: it is possible to achieve adequate reproductive performance in bocachico fish using cryopreserved sperm (10% DMSO, 5.5% glucose, and 12% egg yolk) at twice the spermatozoa/oocyte ratio used with fresh semen.
Keywords: artificial reproduction, cryobiology, fertility, Prochilontidae.
Resumen
Antecedentes: la crioconservación es una herramienta biotecnológica importante para la conservación de la biodiversidad, particularmente de especies en peligro. Objetivo: evaluar seis proporciones diferentes entre espermatozoides/ovocito en la fertilización de bocachico (Prochilodus magdalenae), usando semen fresco o crioconservado. Métodos: se colectó semen y se determinó su calidad para verificar su viabilidad de crioconservación. El semen fue colocado en pajillas de 5 mL y mezclado con una solución crioconservante (5,5% glucosa, 12% yema de huevo y 10% dimethyl sulfoxido -DMSO-) en una dilución 1:4 (semen:solución). El semen fue congelado en un termo de vapores de nitrógeno por 30 min y rápidamente se transfirió a termos de almacenamiento sumergiéndolo directamente en nitrógeno líquido (LN; -196 °C). Las pajillas fueron descongeladas a 60 ºC por 45 segundos. La motilidad, velocidad y progresividad de los espermatozoides, tanto de semen fresco como del congelado, fueron evaluadas usando el software Sperm Class Analyzer (SCA®). Cada proporción de espermatozoides/ovocito fue evaluada en 2 g de huevos (1.630 ± 87 huevos/g) para evaluar fertilidad (F), eclosión (H) y sobrevivencia larval (LS). Resultados: el mejor desempeño reproductivo con semen fresco fue obtenido inseminando con la proporción de 160.000 espermatozoides/ovocito (F = 75,0%, H = 67,7%, LS = 32,7%). De manera similar, el mejor desempeño reproductivo con semen crioconservado fue logrado con la proporción de 320.000 espermatozoide/ovocito (F = 70,0%, H = 48,6%, LS = 19,5%). Conclusión: es posible lograr un adecuado desempeño reproductivo en bocachico usando semen crioconservado (10% DMSO, 5,5% glucosa y 12% yema de huevo) cuando la relación espermatozoide/ovocito usada es del doble de la proporción aplicada para semen fresco.
Palabras clave: criobiología, fertilidad, Prochilontidae, reproducción artificial.
Resumo
Antecedentes: a criopreservação é uma ferramenta biotecnológica importante na conservação da biodiversidade, particularmente de espécies ameaçadas. Objetivo: foram avaliadas seis proporções de espermatozoides/ovócito na fertilização usando sêmen fresco e crioconservado em fertilização de bocachico (Prochilodus magdalenae), Métodos: o sêmen fresco foi coletado e determinada sua qualidade para verificar a viabilidade de crioconservação. O sêmen foi colocado em palhetas de 5 mL e misturado com a solução crioconservante (5,5% glicose, 12% gema de ovo e 10% dimetilsulfóxido -DMSO-) em numa diluição 1:4 (sêmen:solução). O sêmen foi congelado em botijão de vapores de nitrogênio por 30 min e rapidamente transferido a botijão de armazenagem submergindo-os diretamente em nitrogênio líquido (LN; -196 °C). As palhetas foram descongeladas a 60 ºC por 45 segundos. A motilidade, velocidade e progressividade dos espermatozoides, tanto de sêmen fresco quanto de congelado, foram avaliadas usando o software Sperm Class Analyzer (SCA®). Para avaliar fertilidade (F), eclosão (H) e sobrevivência larval (LS), cada relação de espermatozóide/oócito foi avaliada em 2 g de oócitos (1.630 ± 87 ovos/g). Resultados: o melhor desempenho reprodutivo com sêmen fresco foi obtido inseminando com proporção 160.000 espermatozoides/oócito (F = 75,0%, H = 67,7%, LS = 32,7%). O melhor desempenho reprodutivo com sêmen crioconservado foi verificado na proporção de 320.000 espermatozoides/oócito (F = 70,0%, H = 48,6%, LS = 19,5%). Conclusão: é possível alcançar um adequado desempenho reprodutivo em bocachico usando sêmen crioconservado (10% DMSO, 5,5% glicose e 12% gema de ovo) quando a proporção espermatozoide/oócito usada é o dobro da utilizada para sêmen fresco.
Palavras chave: criobiología, fertilização, Prochilontidae, reprodução artificial.
Introduction
Cryopreservation is an important biotechnological tool for the conservation of biodiversity (Wildt and Wemmer, 1999). Additional advantages include broadening artificial fertilization processes in aquaculture (Watson and Holt, 2001) as well as increasing the availability of semen during periods of low sperm production observed in captive fish. Reports on advances in the development of protocols for bocachico semen cryopreservation include: a study on the effect of glucose concentration on sperm motility (Martínez et al., 2011), another study on the use of dimethylacetamide as cryoprotectant (Atencio-García et al., 2013), a further study into the use of different concentrations of dimethyl sulfoxide (DMSO 5, 10, 15%) and glucose (5.5, 6.0, 6.5%) as cryoprotectants (Martínez et al., 2012a), an assessment of membrane damage and DNA fragmentation of bocachico spermatozoa caused by cryopreservation with DMSO and glucose (Martínez et al., 2012b), and a report on the effects of freezing and thawing rates on sperm motility (Martínez et al., 2013). In spite of this, there is little information concerning effective spermatozoa/oocyte ratios for bocachico using fresh and cryopreserved semen to improve the efficiency of semen usage in artificial reproduction processes.
For bocachico, Martínez et al. (2013) used a ratio of 100,000 spermatozoa/oocyte and performed the insemination using cryopreserved semen (5% and 10% DMSO, and 5.5 and 6% glucose) and fresh semen. The fertility rates obtained with cryopreserved semen (between 23.4% and 27.6%) were lower than those using fresh semen (69.3%). This was attributed to the low spermatozoa/oocyte ratio used in the study. According to Martínez and Pardo-Carrasco, (2010), the cellular membrane is damaged during the cryopreservation process, ATP degradation takes place, nuclear and mitochondrial DNA is fragmented, and enzymes, kinases and cytosolic proteins are degraded. This reduces the fertilizing capacity and motility of fish spermatozoa, suggesting that increased spermatozoa/oocyte ratios when using cryopreserved semen could compensate the effects of cryopreservation on fertility rates. Lahnsteiner et al. (2004) found that despite the acceptable motility and velocity observed after sperm cryopreservation of Starlet (Acipenser ruthenus L.) fertility rates were low for cryopreserved (10% DMSO + NaCl) compared to fresh semen (6.9% and 33.9%, respectively). They attributed this to ignorance of the correct sperm:egg proportion, which they, in suitable amounts, compensated for the low motility observed with cryopreservation.
The present study aimed to determine the spermatozoa/oocyte ratio, for both fresh and cryopreserved semen, that improves the efficiency of semen used in artificial reproduction.
Material and methods
This study was approved by the Animal Experimentation Ethics Committee of the Fish Research Center (CINPIC), Universidad de Córdoba, Colombia (CINPIC 005 - June 23, 2012).
Layout
This study was conducted at the Fish Research Center of Universidad de Córdoba (CINPIC; Montería, Colombia), located at latitude 8º 48´ N and longitude 75º 52´ W. Two-year-old bocachico males and females were kept in earthen ponds (density: 0.5 kg/m2). Weight and total length (TL) of males was 178 ± 20.1 g and 26.2 ± 2.0 cm, respectively. Males were selected during the spermiation phase, i.e. when gentle abdominal pressure in cephalocaudal direction causes releasing of seminal fluid. Weight and TL of females (n = 7) was 238.8 ± 51.8 g and 30.7 ± 1.5 cm, respectively. Females were selected during the final maturity phase, determined by the germinal vesicle position of the oocyte in an ovarian biopsy sample. Ovulation was induced by administering 5 mg carp pituitary extract (CPE)/kg body weight in two doses: 20% of the extract was first administered and the remaining 80% twelve hours later. Males were subsequently treated with a single dose of 4 mg CPE/ Kg. This dose was administered two hours before that for females, facilitating to extract and analyze the semen before female ovulation. Semen was obtained after drying and cleaning the genital papilla region with a lab wipe and expulsion of urine and fecal matter. Milt was expressed with gentle abdominal pressure and collected in 2 mL Eppendorf vials.
Evaluation of sperm quality
Color was recorded and the samples contaminated with feces, blood, or urine, were discarded. Total motility, curvilinear velocity (CLV), straight line velocity (SLV), spermatozoa rate with rapid motility (type a, greater than 100 μm/s), medium motility (type b, 46 to 100 μm/s), slow motility (type c, 10 to 45 μm/s), and static spermatozoa (type d) were assessed. A 0.25 μL semen aliquot was taken from each male (n = 7), put in a Makler counting chamber (Sefi, Medical Instruments Ltd, Haifa, Israel) and activated with 75 μL bi-distilled water (1:300 dilution). Semen was analyzed with a contrast optical phase microscope (Nikon, Tokio, Japan) adapted to the Sperm Class Analyzer SCA® (Microptic SL, Barcelona, Spain) computer assisted system for seminal analysis. This allowed to obtain the average data (from two analyses, approximately 450 spermatozoa per field) for each sample analyzed. Semen was considered suitable for cryopreservation only when total motility was above 90%. The activation time elapsed from the moment in which the activating solution (bi-distilled water) was added to the sample until the moment when approximately 90% spermatozoa ceased to move. It was measured with a contrast optical phase microscope (Japan) adapted with a Basler camera (A100 FireWire, 50 frame/s; Germany). A mixture of 1 μL of semen and 699 μL 6% glucose in a 2 mL Eppendorf vial (1:699 dilution) was used to estimate sperm concentration. This mixture was then homogenized for five seconds in a vortex mixer at 1200 rpm (Usmate, Milano, Italy). Subsequently, 10 μL was placed in the Makler chamber and concentration was estimated through an SCA®.
Experimental design
Six spermatozoa/oocyte ratios were evaluated for both fresh and cryopreserved semen: 20.000, 40.000, 80.000, 160.000, and 320.000 spermatozoa/ oocyte, with three replicates per treatment. Semen was cryopreserved in a 6% glucose solution (weight/ volume), 12% chicken egg yolk (volume/volume), and 10% DMSO (volume/volume; Panreac, Barcelona, Spain). Semen was diluted at 1:3 ratio, packed in 5 mL straws (Minitüb, Tiefenbach, Germany) and placed into a 4 L nitrogen vapor dry shipper (MVE, Braunfels, Germany) for 30 minutes. This provided a 27.3 ºC/min freezing rate from 28 to -20 ºC, 29.9 ºC/min from -20 to -100 ºC, 5.5 ºC/min from -100 to -196 ºC, as described by Cruz-Casallas et al. (2006). Straws were quickly transferred to storage thermos (MVE, USA) and plunged directly into liquid nitrogen. After two weeks, straws were thawed by submersion into a 60 ºC water bath for 45 s using a programmable serological bath (Memmert, Schabach, Germany).
Assessment of fertility, hatching and larval survival rates
Fertility and hatching rates were assessed using two g of oocytes (analytical balance: Ohaus, Parsippany, Denmark) treated with several spermatozoa/oocyte ratios. The number of oocytes per gram (1.630 ± 87 oocytes/g) was previously determined. A micropipette (Transferpette®, Wertheim, Germany) was used to inseminate the two g of oocytes with spermatozoa. Activation was performed with 20 mL bi-distilled water in 50 mL containers. Incubation was performed in 2.5 L experimental conical incubators with 120 mL/min flow rate. Fertility rate was assessed five hours after fertilization (HAF) by collecting a sample of 50 embryos in final gastrula stage (90% epiboly). Embryos were randomly selected using a glass pipette with 0.5 cm diameter. Fertility rate was estimated by dividing viable embryos by the total number of analyzed embryos. Viable embryos were translucent, while non-viable embryos were opaque and off-white under a light stereoscope (Leica, Wetzlar, Germany). Hatching was evaluated 12 hours after fertilization (HAF) using the procedure previously described. Live larvae were counted 48 hours after hatching when bocachico began to feed exogenously (Atencio-García et al., 2013), in order to determine larval survival at that moment over the total number of hatched eggs.
Statistical analysis
A completely randomized design was used. Normality of variables and homogeneity of variances were verified with the Kolmogorov-Smirnov test and Levene's test, respectively. After such verifications, analysis of variance (ANOVA) was conducted and the Tukey multiple range test was used whenever statistical differences were noted. Additionally, regression analysis was performed between fertility percentage and sperm/oocyte ratio. All statistical analyses were conducted using SAS software version 9.0 (SAS Inst. Inc, Cary, NC, USA). A 5% significance level was considered. All data are expressed as mean ± standard deviation (SD).
Results
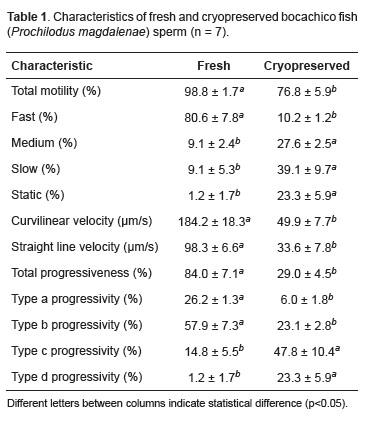
Cryopreservation promoted reduction (p<0.05) of sperm quality in all traits (Table 1).
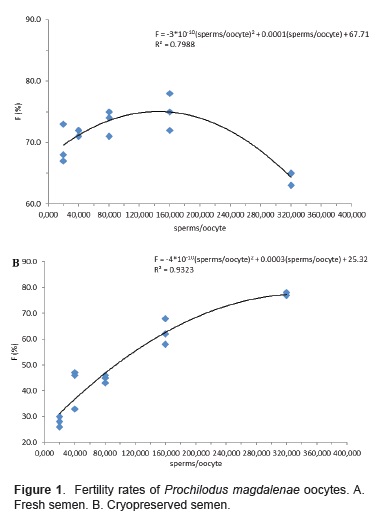
The best fit curve for fresh semen was polynomial (Figure 1A). By deriving regression equation between fresh semen and fertility 166,667 sperm/oocyte was the best theoretical ratio for the highest fertility using fresh semen (81.6%).
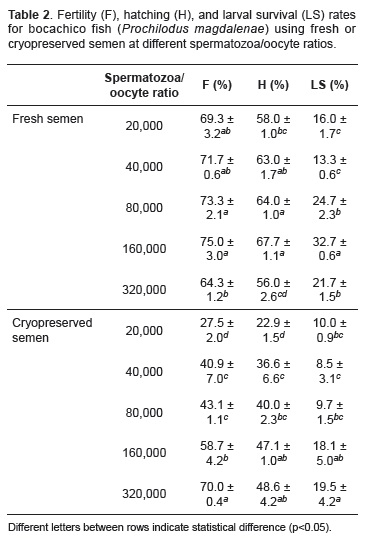
The highest fertility (F) rate was obtained with 320,000 spermatozoa/oocyte (70.0 ± 0.4%) ratios while the lowest rate was obtained with 20,000 spermatozoa/oocyte (27.5 ± 2.0%). A significant difference was observed between these values (p<0.05; Table 2). The highest hatching (H) rate for cryopreserved semen was obtained with 320,000 spermatozoa/oocyte (48.6 ± 4.2%) ratio and was no different compared with 160,000 (47.1 ± 1.0%) spermatozoa/oocyte ratio (p>0.05; Table 2). Conversely, the lowest hatching rate was obtained with 20,000 spermatozoa/oocyte (22.9 ± 1.5%) ratio and was different to the other treatments (p<0.05). The highest larval survival (LS) for cryopreserved semen was observed with 320,000 (19.5 ± 4.2%) and 160,000 spermatozoa/oocyte (18.1 ± 5.0%) ratios, and no difference was found between these values (p>0.05). The lowest LS rate was for 40,000 spermatozoa/oocyte (8.5 ± 3.1%) ratio and was not different (p>0.05) to 80,000 spermatozoa/ oocyte (9.7 ± 1.5%) or 20,000 spermatozoa/oocyte (10.0 ± 0.9%) ratios.
The best-fit curve for cryopreserved semen was also polynomial (Figure 1B). Deriving the regression equation between fresh semen and fertility, 375,000 sperm/oocyte gave the best theoretical ratio for the highest fertility rate with fresh semen (76.0%).
Discussion
Cryopreserved sperm presented low motility, velocity and sperm progressivity, which explains its low fertility, hatching and larval survival. Low quality of cryopreserved semen is suggested as the cause for its reduced fertility, hatching and larval survival. Cryopreservation damages the sperm deteriorating its quality (Lahnsteiner et al., 1992; Morisawa, 1994). Cruz-Casallas et al. (2006) reported 92% total motility for fresh semen and 76% for cryopreserved semen of Brycon amazonicus. Ramírez et al. (2005) reported 84% total motility for fresh semen and 33.3% for cryopreserved semen of Piaractus brachypomus. Cryopreservation generally decreases motility and stimulates increased circular motion (Lahnsteiner et al., 2000; Wamecke and Pluta, 2003).
In this study, motility of fresh semen had the highest percentage of fast spermatozoa, whereas cryopreserved semen had the highest percentage of slow spermatozoa. This proves that fast, medium, and slow spermatozoa in cryopreserved semen had fertilizing capacity. Similarly, straight line and curvilinear velocities observed in fresh semen were higher compared to cryopreserved semen because spermatozoa were damaged by cryopreservation and thawing. Ogier de Baulny et al. (1997) reported losses in seminal properties during cryopreservation, with only a small amount of viable sperm cells, thus reducing the fertilizing capacity of cryopreserved semen. Martinez and Pardo (2010) stated that, even when effectiveness is known for a given cryoprotectant in a fish species, the variation in its concentration could have toxic effects that can affect velocity.
Insemination with fresh semen resulted in the highest fertility rates for ratios ranging from 80,000 to 160,000 spermatozoa/oocyte. The regression equation (fertility vs ratio, R2 = 0.7988) with fresh semen suggests that 166,667 sperm/oocyte ratio would yield the best fertility. The highest fertility rate for cryopreserved semen was obtained with 320,000 spermatozoa/oocyte ratio. The regression equation (fertility vs ratio, R2 = 0.9323) for cryopreserved semen suggests that a 375,000 sperm/ oocyte ratio would yield the best fertility rate. Results also suggest that doubling the best ratio for fresh semen, i.e. increasing it from 160,000 to 320,000 spermatozoa/oocyte, would reduce fertility rate. Velasco-Santamaría et al. (2006) observed the same change in fertility for fresh and cryopreserved semen of B. amazonicus. One possible cause for fertility decrease upon doubling the best spermatozoa/ oocyte ratio using fresh semen could be the limit that gamete-quality impose on fertility. It is thus conceivable that an increase beyond the best ratio does not imply an increase in fertility. Another possible cause for the decrease could be that spermatozoa/ oocyte ratios above the best ratio could cause obstructions and thus hinder spermatozoa movement due to excessive concentration in the activation volume used. In this study, the same volume of activation (20 mL) was used for the various ratios of spermatozoa/oocyte (two g of oocytes are equivalent to 1.5 mL).
De Souza et al. (2007) found that the volume required to activate two mL of Prochilodus lineatus oocytes ranged from 60 to 100 mL. Sanches et al. (2009) reported that appropriate water volume for sperm activation guarantees significant increase in fertility rates. According to Chereguini et al. (1999), the dilution volume of the fertilizing material has two important implications on fertility. Using small water volumes for activation tends to compromise sperm activation itself and results in an inadequate medium for gathering the gametes. On the other hand, excessive amounts of water in the fertilization process may excessively dilute the medium, thus preventing spermatozoa from reaching the micropyle during the short time in which sperm activation takes place. It has also been observed that regardless of dilution volume for activation, high and low sperm/ egg ratios negatively affect oocyte fertilization (Rurangwa et al., 1998).
On analyzing the hatching results obtained for fresh semen, a trend similar to that obtained for fertility was noted. The highest rates were obtained with ratios between 80,000 and 160,000 spermatozoa/oocyte. For cryopreserved semen, the highest hatching rate was achieved with 320,000 spermatozoa/oocyte. However, the ratio at which the best fertility rate was obtained was not the same observed during the hatching rate evaluation. This may be explained by the fact that hatching rate is evaluated after a longer time and, therefore, the longer time between insemination and variable measurement (i.e. fertility, hatching, larval survival), it is likely that the result is affected by other variables. Such variables include handling, incubation conditions, and embryo and larva handling (incubation flows, water quality, etc). These could affect the results and prevent them from indicating the quality of the semen. For that reason, fertility is considered closely related to seminal quality, and therefore it is thought to best measure the effects of cryopreservation. For this reason, few authors report cryopreservation results based on hatching rate, and even fewer based on spermatozoa/oocyte. Despite this, there are studies which used hatching rate as a criterion for assessing the quality of cryopreserved semen. Ramírez et al. (2005) reported 36.6% hatching rate using cryopreserved P. brachypomus semen and 85.3% for fresh semen.
In a similar manner, studies evaluating cryopreserved semen based on larval survival are also uncommon for the reasons stated above. In this study, larval survival using fresh semen yielded the highest rates with 160,000 spermatozoa/oocyte ratio. For cryopreserved semen, the highest rates were obtained with 320,000 spermatozoa/oocyte ratio. Few studies have assessed the effects of cryopreservation on larval survival. The work by Ramirez et al. (2005) is worth mentioning. That study, conducted on P. brachypomus, reported 72% larval survival rates for fresh semen and 62% for cryopreserved semen. It is worth mentioning that, in these studies, calculation for determining larval survival rate was based on the amount of hatched larvae, whereas the present study estimated it based on the number of incubated oocytes.
Based on the results presented here, it may be suggested that 20,000 to 40,000 spermatozoa/ oocyte ratio could be considered as the minimum for inseminating bocachico with fresh semen. Likewise, between 80,000 and 160,000 spermatozoa/oocyte ratio might be considered as the maximum proportion to achieve efficient reproductive performance. Furthermore, the minimum ratio for cryopreserved semen could be 160,000 spermatozoa/oocyte and the maximum 320,000 spermatozoa/oocyte. The study by Rogamosa et al. (2010) on P. lineatus, a conspecific of bocachico, suggested a very wide range of spermatozoa/oocyte ratio for fresh semen -between 320,000 and 3,200,000- to obtain good fertility rates. This study, however, achieved 75% fertility rate with 160,000 spermatozoa/oocyte ratio. Optimal reported ratios for other species include 314,428 spermatozoa/ oocyte for Brycon insignis (Shimoda et al., 2007). Recently, Viveiros et al. (2009) studied P. lineatus, conspecific with bocachico, suggesting 500,000 spermatozoa/oocyte ratio for cryopreserved semen to ensure proper fertility rates (47 to 82%). For the present study, 70% fertility rate could be obtained with only 320,000 spermatozoa/oocyte ratio.
The fact that spermatozoa/oocyte ratio doubled for cryopreserved semen, when compared with fresh semen, is associated with spermatozoa damage during cryopreservation, affecting the insemination capacity (María et al., 2006). Martínez et al. (2009) considered that sperm cryopreservation in fish causes damage that reduces motility and velocity after thawing. According to them, this has negative effects on the inseminating capacity of Prochilodus magdalenae. Additionally, this damage may be linked to several stages of the cryopreservation process, the period of time in which sperms are exposed to the cryoprotectant, and to the freezing and thawing curves. Recently Morris et al. (2012) indicated that the damage in sperm cells is related to the osmotic imbalance resulting from the thawing process, and not to the formation of intracellular ice. Damage compromises the cell at the morphological level (membrane), affecting mitochondria, which are critical for energy production and post-thawing cell movement. Additionally, damage also occurs at the DNA level, causing the alteration of the genetic material despite the fact that cells can still move (Martínez and Pardo, 2013).
The results of this study allow concluding that it is possible to obtain adequate bocachico reproductive performance with cryopreserved semen (10% DMSO, 5.5% glucose, and 12% egg yolk) when using 320,000 spermatozoa/oocyte ratio. This is twice the best ratio used for insemination with fresh semen (160,000 spermatozoa/oocyte).
Acknowledgements
The authors would like to thank the Colombian Ministry of Agriculture and Rural Development for sponsoring this study, which is part of the project entitled Establishment of an Experimental Semen Bank of Native Fish with Farming Potential in the Colombian Humid Caribbean Zone (Code: MADR 2007U7723-401).
Conflicts of interest
The authors declare they have no conflicts of interest with regard to the work presented in this report.
Notes
¤To cite this article: Atencio VJ, Espinosa JA, Martínez JG, Pardo SC. Insemination of bocachico fish (Prochilodus magdalenae) with fresh or cryopreserved semen: effect of spermatozoa/oocyte ratio. Rev Colomb Cienc Pecu 2015; 28:347-355.
References
Atencio García VJ, Pérez EJ, Espinosa JA, Pardo SC. Evaluación de dimetilacetamida como crioprotector para la crioconservación de semen de bocachico Prochilodus magdalenae. Arch Med Vet 2013; 45(2):151-158. [ Links ]
Chereguini O, De la Banda IG, Rasines I, Fernández A. Artificial fertilization in turbot, (Scopothalmus maximus L.): different methods and determination of the optimal sperm-egg ratio. Aquac Res 1999; 30:319-324. [ Links ]
Cruz Casallas P, Medina Robles V, Velasco Santamaría Y. Protocolo para la crioconservación de semen de yamú (Brycon amazonicus Spix & Agassiz 1829). Rev Colomb Cienc Pecu 2006; 19(2):146-151. [ Links ]
De Souza BES, Sanches EA, Baggio DM. Interação entre a relação de espermatozoide/ovócito e o volume de água empregados na fertilização artificial de ovócitos de curimbatá Prochilodus lineatus. In: Proceeding 1° Congresso Brasileiro de Produção de Peixes Nativos de Água Doce, Dourados 2007, Embrapa [CD-ROM] [ Links ].
Lahnsteiner F, Weismann T, Patzner R. Fine structure changes in spermatozoa of the grayling, Thymallus thymallus (Pisces: Teleostei), during routine cryopreservation. Aquaculture 1992; 103: 73-84. [ Links ]
Lahnsteiner F, Berger B, Horvath A, Urbányi B, Weismann T. Cryopresevation of spermatozoa in cyprindid fishes. Theriogenology 2000; 54: 1477-1496. [ Links ]
Lahnsteiner F, Berger B, Horváth A, Urbányi B. Studies on the semen biology and sperm cryopreservation in the sterlet, (Acipenser ruthenus L.) Aquac Res 2004; 35:519-528. [ Links ]
Maria AN, Viveiros ATM, Orfão LH, Oliveira AV, Morães GF. Effects of cooling and freezing on sperm motility of the endangered fish piracanjuba Brycon orbignyanus (Characiformes, Characidae). Anim Reprod 2006; 3:55-60. [ Links ]
Martínez JG, Atencio García VJ, Tarazona AM, Pardo Carrasco SC. Estandarización de protocolos para la crioconservación de semen de bocachico y el análisis de la movilidad mediante el software sperm class analyzer. Rev Fac Nal Agr Medellín 2009; 62 suppl 3-19. [ Links ]
Martínez JG, Pardo Carrasco SC. Crioconservación de semen en peces: efectos sobre la movilidad espermática y la fertilidad. Acta Biol Colomb 2010; 15(2):3-24. [ Links ]
Martínez JG, Atencio Garcia VJ, Pardo Carrasco SC. Efectos de la concentración de glucosa sobre la activación de la movilidad espermática en bocachico Prochilodus magdalenae (Pisces, Characiformes). Rev Mvz Cordoba 2011; 16:2554-2563. [ Links ]
Martínez JG, Tarazona Morales AM, Pardo Carrasco SC. Sperm cryopreservation of freshwater fish bocachico Prochilodus magdalenae in DMSO and glucose and its effects on fertilization and hatching efficiency. Anim Reprod 2012a; 9(1):19-26. [ Links ]
Martínez JG, Atencio García VJ, Pardo Carrasco SC. DNA fragmentation and membrane damage of bocachico Prochilodus magdalenae (Ostariophysi, Prochilodontidae) sperm following cryopreservation with dimethylsulfoxide and glucose. Neotrop Ichthyol 2012b; 10(3):577-586. [ Links ]
Martínez JG, Pardo Carrasco SC. Effect of freezing and thawing rates on sperm motility in Bocachico Prochilodus magdalenae (Pisces, Characiformes). Rev Mvz Cordoba 2013; 18(1):3295-3303. [ Links ]
Morris GJ, Acton E, Murray JB, Fonseca F. Freezing injury: The special case of the sperm cell. Cryobiology 2012; 64:71-80. [ Links ]
Morisawa M. Cell signaling mechanism for sperm motility. Zoo Sci 1994; 11: 647-662p. [ Links ]
Ogier de Baulny B, Le Vern Y, Kerboeuf D, Maisse G. Flow cytometric evaluation of mitochondrial activity and membrane integrity in fresh and Cryopreserved Rainbow trout (Oncorhynchus mykiss) spermatozoa. Cryobiology 1997; 34:141-149. [ Links ]
Ramírez MJA, Velasco Santamaría YM, Medina Robles VM, Cruz Casallas PE. Crioconservación de semen de Cachama blanca (Piaractus brachypomus Cuvier, 1818): efectos del volumen de empaque y de la sustancia crioprotectora sobre la calidad seminal. Rev Colomb Cienc Pec 2005; 18:331. [ Links ]
Romagosa E, Souza BE, Sanches EA, Baggio M, Bombardelli RA. Sperm motility of Prochilodus lineatus in relation to dilution rate and temperature of the activating medium. J Appl Ichthyol 2010; 26:678-681. [ Links ]
Rurangwa E, Roclants I, Huyskens G, Ebrahimi M, Kime DM, Ollevier F. The minimum effective spermatozoa:egg ratio for artificial insemination and the effects of mercury on sperm motility and fertilization ability in (Clarias gariepinus). J Fish Biol 1998; 53:402-413. [ Links ]
SAS Institute Inc. 2004. SAS/STAT® 9.1 User's Guide. Cary, NC, USA: SAS Institute Inc. [ Links ]
Sanches EA, Bombardelli RA, Baggio DM, Souza BE. Dose inseminante para fertilização artificial de ovócitos de dourado. R Bras Zootec 2009; 38:2091-2098. [ Links ]
Shimoda E, Andrade DR, Vidal Júnior MV, Godinho HP, Yasui GY. Determinação da razão ótima de espermatozóides por ovócitos de piabanha Brycon insignis (Pisces-Characidae). Arq Bras Med Vet Zootec 2007; 59:877-882. [ Links ]
Velasco Santamaría YM, Medina Robles VM, Cruz Casallas PE. Cryopreservation of yamú (Brycon amazonicus) sperm for large scale fertilization. Aquaculture 2006; 256(1-4):264-271. [ Links ]
Viveiros ATM, Orfão LH, Maria AN, Allaman IB. A simple, inexpensive and successful freezing method for curimba Prochilodus lineatus (Characiformes) semen. Anim Reprod Sci 2009; 112:293-300. [ Links ]
Wamecke D, Pluta H. Motility and fertilizing capacity of frezen/ thawed common carp (Cyprinus carpio L.) sperm using dimetilacetamida as the mam cryoprotectant. Aquaculture 2003; 187: 361-375. [ Links ]
Watson PF, Holt WV. Cryobanking the genetic resource: Wildlife conservation for the future. London, UK: Taylor & Francis; 2001. [ Links ]
Wildt DE, Wemmer C. Sex and wildlife: the role of reproductive science in conservation. Biodivers Conserv 1999; 8:965-976. [ Links ]