Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Revista Colombiana de Ciencias Pecuarias
versão impressa ISSN 0120-0690
Rev Colom Cienc Pecua vol.29 no.1 Medellín jan./mar. 2016
https://doi.org/10.17533/udea.rccp.v29n1a05
ORIGINAL ARTICLE
doi: 10.17533/udea.rccp.v29n1a05
Nitric oxide and malondialdehyde in gastric contents and blood in an equine model of gastric ulcer induced by phenylbutazone¤
Concentración de óxido nítrico y malonaldehído en contenido gástrico y en sangre en un modelo de úlcera gástrica equina inducida por fenilbutazona
Concentração de óxido nítrico e malondialdeído no conteúdo gástrico e no sangue em modelo equino de úlcera gástrica
Angélica M Zuluaga1, MV; Geraldo E Silveira A2, MV, MS, PhD; José R Martínez A1*, MVZ, MS, PhD.
1Grupo de Investigación CENTAURO, Línea de Investigación LIMCE, Escuela de Medicina Veterinaria, Universidad de Antioquia UdeA, Calle 70 No. 52 – 21, AA 1226, Medellín, Colombia.
2Departamento de Clinica e Cirurgias Veterinárias, Escola de Veterinária, Universidade Federal de Minas Gerais (UFMG), Belo Horizonte, MG, Brazil.
*Corresponding author: José R Martínez A. Facultad de Ciencias Agrarias, Universidad de Antioquia UdeA, Calle 70 No. 52 – 21, AA 1226, Medellín, Colombia. Tel: 57-0542199155. E-mail: jose.martinez@udea.edu.co; jrramonmvz@yahoo.com
Received: January 22, 2015; accepted: July 23, 2015
Summary
Background: mechanisms of gastric mucosal injury include cellular damage by oxygen free radicals, which can be indirectly measured through malondialdehyde (MDA). Production of nitric oxide (NO) maintains gastric tissue perfusion through vasodilatation. Objective: to demonstrate oxidative stress and impaired gastric perfusion measuring NO and MDA in gastric contents and blood of equines subjected to a gastrointestinal ulceration induction protocol. Methods: a gastrointestinal ulceration induction protocol involving fasting and oral administration of phenylbutazone was performed on five horses. NO and MDA were measured before and after protocol induction and presence of fecal occult blood (FOB) was evaluated. Animals underwent gastroscopy at the beginning and end of the protocol. Results: horses presented variability in hematological and FOB exams. Azotemia, hyperphosphatemia, and hypocalcemia were found in all animals. No significant changes were found in enzymatic activity. At the end of the protocol, 40% of the horses showed varying degrees of gastric ulceration. NO production in the stomach decreased by 60%, whereas MDA production increased by 55% after the protocol. Plasma concentration of MDA average increased 96 hours after starting the protocol. There were no significant differences in mean plasma NO during the protocol. Conclusion: the protocol used to induce gastric ulcers produces diminished cytoprotection (by NO) and induces oxidative stress in the gastric mucosa.
Keywords: anti-inflammatory, antioxidant, NSAID, oxidative stress, stomach.
Resumen
Antecedentes: los mecanismos de lesión de la mucosa gástrica incluyen daño celular por radicales libres de oxígeno que puede ser medido a través del malonaldehído (MDA). La producción de óxido nítrico (NO) sostiene la perfusión del tejido gástrico mediante vasodilatación. Objetivo: demostrar el estrés oxidativo y alteración de la perfusión gástrica mediante la medición de NO y MDA en contenido gástrico y sangre de equinos sometidos a un protocolo de inducción de ulceración gastrointestinal. Métodos: el protocolo de ulceración se hizo en 5 caballos con fenilbutazona y periodos de ayuno. Antes y después de aplicar el protocolo fueron medidos NO y MDA y se evaluó la presencia de sangre oculta en heces (FOB). Los animales fueron sometidos a gastroscopia al inicio y final del protocolo. Resultados: los equinos presentaron variabilidad en los exámenes hematológicos y FOB. Se encontró Azotemia, hiperfosfatemia e hipocalcemia en todos los animales. No fueron encontradas alteraciones significativas en la actividad enzimática. Al final del protocolo, el 40% de los equinos presentó ulceración gástrica en grado variable. La producción de NO en estómago disminuyó 60%, mientras que la producción de MDA aumentó 55% después del protocolo. El promedio de la concentración plasmática de MDA aumentó después de 96 horas de iniciar el protocolo. No hubo diferencias significativas en el promedio de NO plasmático durante el protocolo. Conclusión: el protocolo utilizado para inducir úlceras gástricas produce disminución en la citoprotección derivada del NO e induce estrés oxidativo en la mucosa gástrica.
Palabras clave: AINE, anti-inflamatório, antioxidante, estómago, estrés oxidativo.
Resumo
Antecedentes: mecanismos de lesão da mucosa gástrica incluem o dano celular pela ação dos radicais livres de oxigênio que pode ser medido a través do malonaldehido (MDA). A produção de óxido nítrico (NO) mante a perfusão do tecido gástrico a través da vasodilatação. Objetivo: demostrar estrese oxidativo e alteração da perfusão gástrica pela quantificação de NO e MDA a partir do conteúdo gástrico e sangue de equinos que foram submetidos a um protocolo de indução de ulceração gastrointestinal. Métodos: realizou-se um protocolo a base de fenilbutazona e períodos de jejum em 5 cavalos. Antes e depois do protocolo foram dosados NO e MDA e se avaliou a presencia de sangue oculta em fezes (FOB). No início e no final do protocolo, os animais foram avaliados pela gastroscopia. Resultados: os equinos apresentaram variabilidade nos exames hematológicos e FOB. Encontrou-se azotemia, hiperfosfatemia e hipocalcemia em todos os animais. Não foram achadas alterações significativas na atividade enzimática. Ao final do protocolo, o 40% dos equinos apresentou grado variável de ulceração gástrica. A produção de NO no estômago diminuiu 60%, entretanto a produção de MDA aumentou 55% após do protocolo. A concentração média plasmática de MDA aumentou depois de 96 horas de começar o protocolo. Não houve diferencias significativas na média de NO plasmático durante o protocolo. Conclusão: o protocolo utilizado para induzir úlceras gástricas produz diminuição na citoproteção derivada do NO e induz estresse oxidativo na mucosa gástrica.
Palavras chave: AINE, anti-inflamatório, antioxidante, estômago, estresse oxidativo.
Introduction
Gastrointestinal mucosa can be damaged by factors that cause the expression of complex defense mechanisms in the gastrointestinal system, including nitric oxide production to maintain tissue perfusion through vasodilatation (Brzozowski et al., 1999) and prostaglandin E2 to stimulate mucus production.
Nitric oxide (NO) results from nitric oxide synthase (NOS) reaction on an l-arginine substrate. There are three NOS isoforms, two of which are constitutively expressed (neuronal and endothelial), and one isoform induced by inflammatory and immune processes (Squadrito and Pryor, 1998). The NO has multiple functions: it participates in vascular reactivity, generates vasodilatation, inhibits platelet aggregation and leukocyte adhesion, and participates in phagocytosis and capture of free radicals.
Multiple risk factors have been associated with the frequency and severity of gastrointestinal ulcerative lesions in horses, including the use of non-steroidal anti-inflammatory drugs (NSAIDs), which affect protective mechanisms of gastrointestinal mucosa and kidney (Blikslager and Jones, 2005; Martinez and Silveira, 2014). Phenylbutazone (PBZ) and prolonged fasting periods (PFP) have been associated with gastric ulcers due to the systemic and local effects caused by inhibition of cytoprotective prostaglandins and the caustic action of gastric acid, respectively (Dowling, 2002; Chapman, 2009).
Other mechanisms of gastrointestinal mucosal injury include the release of oxygen free radicals (oxidative stress). Malondialdehyde (MDA) is an indicator of oxidative stress and can be measured in ulcerative and inflammatory conditions of the gastrointestinal tract. The enzymatic pathway is not the major route of MDA production, but it is the best studied (Onyango and Baba, 2010). The enzymatic pathway involves the action of H2 (PGH2) on prostaglandin thromboxane synthase. MDA generation by the non-enzymatic pathway is based on the production of hydroperoxides from fatty acids (Del Rio et al., 2005). MDA causes potent biological effects on protein, DNA, lipoproteins, and collagen (Del Rio et al., 2005).
The aim of this study was to confirm oxidative stress and impaired gastric perfusion caused by gastrointestinal ulcerative lesions, by evaluating the dynamics of NO and MDA from the gastric contents and blood of horses subjected to an induction protocol for gastrointestinal ulceration.
Material and methods
Ethical considerations
This assay was evaluated and approved by the Federal University of Minas Gerais (UFMG) Ethics Committee for Animal Experimentation (CETEA) through protocol 234/09.
Location
This uncontrolled assay was conducted at the School of Veterinary Medicine of the Federal University of Minas Gerais (EV-UFMG), Brazil. Five clinically healthy adult mongrel horses (two males and three females) between 10 and 22 years of age and 487 Kg body weight (BW) were used. During the experiment, animals were individually housed and fed 1.5% BW Tifton hay (Cynodon dactylon), water, mineral salt ad libitum, and commercial concentrate at 1% BW supplied twice daily.
Gastroscopy was performed after 10 days of adaptation, clinical and laboratory examinations. Gastroscopies were also performed after administering a gastric ulcer induction protocol.
The protocol to induce gastric ulcers involved oral administration of PBZ associated with PFP. Animals were subjected to PFP 24 hours every other day for six days, and were not fed grass during that period. PBZ was administered at a dose of 10.5 mg/Kg BW/BID twice a day on the first two days, and four days after the dose decreased to 5.25 mg/Kg BW/BID twice a day.
Clinical examinations were performed daily during the protocol. Blood samples were collected every 48 hours (T0, T1, T2, T3, T4) for hematological and biochemical analyses. Plasma NO and MDA were measured before and after applying the protocol. Gastric content was collected by nasogastric tube before and after the protocol to measure NO and MDA through the Griess reaction and high-performance liquid chromatography (HPLC; Nielsen et al., 1997), respectively. Presence of fecal occult blood (FOB) was also evaluated in the same period using a commercial kit (Hemoplus®, Newprov Produtos para Laboratório Ltda., Pinhais, PR, Brazil),.
Animals underwent gastroscopy at the beginning and the end of the protocol. Animals were not offered solid food for 12 to 14 hours before gastroscopy, and were sedated by intravenous administration (IV) of 10 μg/Kg BW detomidine hydrochloride (Dormosedan®, Zoetis, New York, NY, USA). Presence of gastric ulcers was diagnosed with a flexible endoscope video (PortaScope®, 1800 PVS, Bradenton, FL, USA) 12 mm in diameter and 300 cm in length. The number and severity of injuries before and after the protocol were analyzed through the score suggested by MacAllister et al. (1997).
Data analysis
For statistical analysis, descriptive statistics were performed, and Tukey's test was used to determine significant differences (p<0.05) between hematological and metabolite values. Linear regression analysis and calculation of the correlation coefficient between time to progression of PBZ administration combined with fasting periods and NO concentration were conducted.
Results
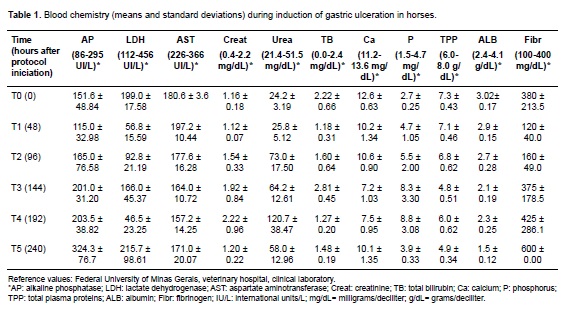
Horses showed different degrees of dehydration according to the following hematological parameters: hematocrit, erythrocyte count, hemoglobin, and total plasma protein (TPP). Progressive leukopenia and lymphopenia with neutropenia were evident in all animals during the trial. Platelet and leukocyte counts showed similar results. Albumin and TPP concentrations decreased at the end of the treatment, except for equine 4 (Table 1).
The FOB test was positive for equine 2 on the third day of the protocol; the remaining animals showed FOB since day six. Urea and creatinine concentration increased in all horses during the protocol. Hyperphosphatemia and hypocalcemia were observed in all animals (Table 1). No significant alterations were found in enzyme activity or bilirubin concentrations.
Initial gastroscopic examination revealed no ulcers, whereas 40% of the horses presented gastric ulcers at the end of the protocol. Equine 5 exhibited non-glandular mucosal ulcerations (4/4 and 3/5 grades in number and intensity, respectively). Meanwhile, equine 2 had ulcers in glandular and non-glandular mucosa (4/4 grade for both number and intensity). Horses 3 and 4 did not develop ulcers. Equine 1 did not undergo the second gastroscopy due to sudden death at the end of the protocol.
The average NO concentration in gastric content was 0.321 ± 0.151 mmol/L and 0.199 ± 0.085 mmol/L before and after induction of gastric ulceration, respectively. MDA average concentration in gastric juice was 1.257 ± 0.667 mmol/L and 2.089 ± 1.369 mmol/L before and after the protocol, respectively. NO production in the stomach decreased by 60%, whereas MDA production increased by 55% after the protocol.
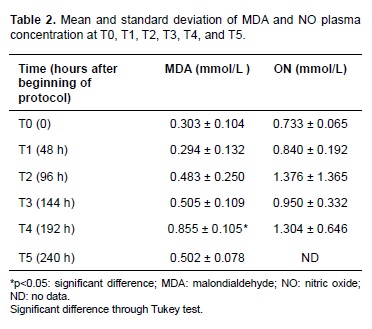
Average MDA (mmol/L) plasma concentration increased 96 hours after starting the protocol. T4 (192 hours after protocol initiation) showed a significant difference (p = 0.02) in MDA average and coincided with the last dose of PBZ. MDA concentration decreased during T5 (240 hours after protocol initiation; Table 2).
Changes in plasma NO were not significant despite increases in time and PBZ dose, compared to plasma NO at T0. This observation was confirmed by linear regression analysis and correlation between PBZ progression and NO concentration. Plasma NO increased with time in a constant ratio (r = 0.999).
Discussion
Prolonged fasting and/or PBZ affect the gastrointestinal tract of equines, acting synergistically in terms of side effects, and both are frequently used in medical routine. The combination of both factors is usually underestimated. The clinical and laboratory effects found in this study were similar to those reported under experimental conditions and case reports of colitis associated to PBZ administration (El-Ashker et al., 2012). However, variations of these results were not analyzed for statistical significance; additionally, horse 1 died and its data were not included in the analysis.
In addition to fasting, anorexia -derived from the low palatability of the drug- contributed to dehydration and alterations in TPP and calcium levels. Increased concentrations of urea and creatinine is a response to poor renal perfusion and a nephropathy caused by inhibition of cytoprotective prostaglandins resulting from PBZ administration (Blikslager and Jones, 2005). Hyperphosphatemia may be compatible with kidney damage and/or associated with hypocalcemia.
Hypoproteinemia and hypoalbuminemia are also considered sensitive biochemical indicators of PBZ-induced enteropathy in the horse (El-Ashker et al., 2012). Loss of plasma proteins through the gastrointestinal tract is the result of ulcerations and bleeding, evidenced by the presence of FOB and initial thrombocytopenia, as shown in this assay.
Leukogram changes were detected during PBZ administration, indicating an inflammatory process with tissue infiltration by leukocytes in organs potentially affected by the ulceration protocol. This finding is characteristic in horses treated with PBZ or horses with severe endotoxemia (McConnico et al., 2008; El-Ashker et al., 2012). The role of infiltrating neutrophils in damaged intestine is well known (Gayle et al., 2000; Tomlinson and Wilder, 2004); however, there is evidence of response variability in segments of the digestive tract of horses in accordance with the nonsteroidal anti-inflammatory drug (NSAID) type (Marshall and Blikslager, 2011).
Side effects are mainly attributed to the inhibition of cytoprotective PGS prostaglandins because of cyclooxygenase pathway blockage by PBZ. However, recent studies have shown that NSAID does not affect the expression of COX 1 and COX-2 genes (Nieto et al., 2012). Another study also showed increased PGE2 concentration in gastric contents after oral administration of PBZ (Martínez- Aranzales et al., 2014). The findings support the hypothesis that oxidative stress is another pathway in gastrointestinal mucosal injury; for instance, NO and MDA concentrations have been experimentally observed in gastric tissue after a single administration of PBZ (Martínez-Aranzales et al., 2014), and plasma concentration of those metabolites has been reported in clinical cases (El-Ashker et al., 2012).
NO is involved in maintaining the integrity of gastric mucosa and is considered gastroprotective, as it regulates the production of hydrochloric acid, modulates perfusion, captures free radicals (Wink et al., 2001; Abdallah, 2010), and accelerates mucosal re-epithelialization (Li et al., 2000). Imbalance in the concentration of this metabolite in rat gastric tissue has been described after indomethacin treatment (Słomiany et al., 1999; Polat et al., 2010).
This study showed decreased NO concentration in gastric contents due to PBZ. However, no significant change was observed in plasma indicating that oral PBZ administration and fasting affect gastric mucosa integrity in a localized mode, and that gastroprotection assessment is more representative in the gastric juice. Similar findings were reported in gastric tissue of rats treated with indomethacin (Motawi et al., 2008; Abdallah, 2010). However, El-Ashker et al. (2012) found high prognostic value of NO in colitis associated with PBZ administration, significantly increasing in non-surviving patients.
Because of its nature, NO concentration is likely to be variable based on the complexity of the biological matrices (e.g., interaction with hemoglobin). Similarly, the technique used explains the different results in this study compared with those previously reported (Beall et al., 2012; Sun et al., 2003).
MDA is an end product of cell membrane lipid peroxidation by free radicals; therefore, MDA is considered as a primary biomarker of oxidative stress. Naito et al. (1998) considered oxidative stress as an injury mechanism of NSAIDs, independent of PG prostaglandin inhibition. Rat studies have attributed MDA increase in gastric mucosa to oxidative damage after diclofenac administration (Sánchez et al., 2002).
In this study, MDA showed an upward dynamic in both gastric juice and blood, suggesting local and systemic effects of free radicals derived from PBZ administration during fasting. Similarly, a study showed that MDA increased in equine gastric mucosa after PBZ administration, and a single administration of antioxidants decreased oxidative stress parameters related to PBZ (superoxide dismutase, catalase, and NO; Martínez-Aranzales et al., 2014) indicating a direct involvement of free radicals in cell damage.
Previous horse studies have measured MDA in PBZ-associated colitis. Plasma concentration of MDA is considered a prognostic biomarker in this condition (El-Ashker et al., 2012). Increased blood MDA followed by flatulent and impaction colic have suggested oxidative stress in a previous report (Ibrahim, 2014). However, McConnico et al. (2008) did not find significant differences for MDA in samples of right dorsal colon of horses treated with PBZ for 21 days. In this study, plasma MDA at T4 showed a significant increase, possibly indicating that systemic expression of gastric oxidative stress requires the course of at least 8 days; however, this behavior must be re-evaluated with a greater number of animals, and the presence of systemic inflammatory response syndrome (SIRS) must be considered in such protocols as well.
The increase of free radicals due to alterations of endogenous antioxidant mechanisms and increased leukocyte infiltration in the gastrointestinal mucosa can be measured indirectly through MDA, and both effects were observed in this study after PBZ administration. An increase of this biomarker has also been observed with other NSAIDs, such as indomethacin and diclofenac (Devi et al., 2007; Abdallah, 2010; Polat et al., 2010). However, it must be considered that MDA increase can also occur in the absence of NSAIDs, such as during ischemiareperfusion injury in equine jejunum (Kooreman et al., 1998) and other enzyme and non-enzyme sources (Hecker and Ullrich, 1989; Onyango and Baba, 2010).
Differences between MDA concentrations in this study and those reported in other studies of the same nature are possibly due to the diversity of samples (urine, plasma, serum, and tissue) and method variability due to chemical interferences. This study used HPLC, which has great sensitivity and specificity by separating contaminant complexes in specific columns (Karatas et al., 2002). Other studies used spectrophotometry.
Furthermore, oxidative stress observed in animals that did not develop ulcers was probably due to housing stress and damage from PBZ in other segments of the gastrointestinal tract (El-Ashker et al., 2012). FOB was observed as a result of severe ulcerative lesions developed in intestinal segments (data not shown). It is known that continued PBZ administration generates ulcerations in the colon and ileocecal valve (El-Ashker et al., 2012). However, the absence of ulcers does not preclude the presence of non-ulcerative inflammatory lesions.
It can be concluded that the protocol used in this study to induce gastric ulcers decreases cytoprotection and generates oxidative stress in the gastric mucosa, as evidenced by increased MDA in the gastric contents. Oxidative stress by PBZ is directly proportional to administration time, and fasting can potentiate this effect.
Acknowledgements
The authors thank EV-UFMG, Belo Horizonte, Brazil, and the Sustainability Strategy 2013–2014 from CODI-Universidad de Antioquia, Medellin, Colombia.
Conflicts of interest
The authors declare they have no conflicts of interest with regard to the work presented in this report.
Notes
¤To cite this article: Zuluaga AM, Silveira GE, Martínez JR. Nitric oxide and malondialdehyde in gastric contents and blood in an equine model of gastric ulcer induced by phenylbutazone. Rev Colomb Cienc Pecu 2016; 29:43-50.
References
Abdallah DM. Nicotinamide alleviates indomethacin-induced gastric ulcers: a novel antiulcer agent. Eur J Pharmacol 2010; 627: 276-80. [ Links ]
Beall CM, Laskowski D, Erzurum SC. Nitric oxide in adaptation to altitude. Free Radical Biol Med 2012; 52:1123-34. [ Links ]
Blikslager A, Jones S. NSAIDs. J Equine Vet Sci 2005; 25:98-102. [ Links ]
Brzozowski T, Konturek PC, Konturek SJ, Pajdo R, Duda A, Pierzchalski P, Bielanski W, Hahn EG. Leptin in gastroprotection induced by cholecystokinin or by a meal: role of vagal and sensory nerves and nitric oxide. Eur J Pharmacol 1999; 374:263-76. [ Links ]
Chapman AM. Acute diarrhea in hospitalized horses. Vet Clin N Am Equine 2009; 25: 363-80. [ Links ]
Del Rio D, Stewart AJ, Pellegrini N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr Metab Cardiovas 2005; 15: 316-28. [ Links ]
Devi RS, Narayan S, Vani G, Shyamala Devi CS. Gastroprotective effect of Terminalia arjuna bark on diclofenac sodium induced gastric ulcer. Chem Biol Interact 2007; 167:71-83. [ Links ]
Dowling PM. Adverse drug reactions in horses. Clin Tech Equine Pract 2002; 1:58-67. [ Links ]
El-Ashker M, El-Khodery S, Metwally N, Hussein H, El-Boshy M. Prognostic significance of oxidative stress markers in colitis associated with phenylbutazone administration in draft horses. J Equine Vet Sci 2012; 32:146-52. [ Links ]
Gayle J, Blikslager A, Jones S. Role of neutrophils in intestinal mucosal injury. J Am Vet Med Assoc 2000; 217: 498-500. [ Links ]
Hecker M, Ullrich V. On the mechanism of prostacyclin and thromboxane A2 biosynthesis. J Biol Chem 1989; 264:141-50. [ Links ]
Ibrahim HMM. Oxidative stress associated with spasmodic, flatulent, and impaction colic in draft horses. J Equine Vet Sci 2014; 34:1205-10. [ Links ]
Karatas F, Karatepe M, Baysar A. Determination of free malondialdehyde in human serum by high-performance liquid chromatography. Anal Biochem 2002; 311:76-9. [ Links ]
Kooreman K, Babbs C, Fessler J. Effect of ischemia and reperfusion on oxidative processes in the large colon and jejunum of horses. Am J Vet Res 1998; 59:340-6. [ Links ]
Li Y, Wang WP, Wang HY, Cho CH. Intragastric administration of heparin enhances gastric ulcer healing through a nitric oxidedependent mechanism in rats. Eur J Pharmacol 2000; 399:205-14. [ Links ]
MacAllister CG, Andrews FM, Deegan E, Ruoff W, Olovson SG. A scoring system for gastric ulcers in the horse. Equine Vet J 1997; 29:430-3. [ Links ]
Marshall J, Blikslager A. The effect of non-steroidal antiinflammatory drugs on the equine intestine. Equine Vet J 2011; 43:140-4. [ Links ]
Martínez Aranzales JR, Cândido de Andrade BS, Silveira Alves GE. Orally administered phenylbutazone causes oxidative stress in the equine gastric mucosa. J Vet Pharmacol Ther 2014; 2015; 38: 257-64 [ Links ]
Martinez Aranzalez JR, Silveira Alves GE. Equine gastric ulcer syndrome: risk factors and therapeutic aspects. Rev Colomb Cienc Pecu 2014; 27:157-70. [ Links ]
McConnico RS, Morgan TW, Williams CC, Hubert JD, Moore RM. Pathophysiologic effects of phenylbutazone on the right dorsal colon in horses. Am J Vet Res 2008; 69:1496-505. [ Links ]
Motawi T, Elgawad H, Shahin N. Gastroprotective effect of leptin in indomethacin-induced gastric injury. J Biomed Sci 2008; 15:405-12. [ Links ]
Naito Y, Yoshikawa T, Yoshida N, Kondo M. Role of oxygen radical and lipid peroxidation in indomethacin-induced gastric mucosal injury. Digest Dis Sci 1998; 43 Suppl 9:30S-34S. [ Links ]
Nielsen F, Mikkelsen BB, Nielsen JB, Andersen HR. Plasma malondialdehyde as biomarker for oxidative stress : reference interval and effects of life-style factors. Clin Chem 1997; 43: 1209-14. [ Links ]
Nieto JE, Aleman M, Anderson J D, Fiack C, Snyder JR. Effects of phenylbutazone on gene expression of cyclooxygenase-1 and -2 in the oral, glandular gastric, and bladder mucosae of healthy horses. Am J Vet Res 2012; 73:98-104. [ Links ]
Onyango AN, Baba N. New hypotheses on the pathways of formation of malondialdehyde and isofurans. Free Radical Bio Med 2010; 49:1594-1600. [ Links ]
Polat B, Suleyman H, Alp HH. Adaptation of rat gastric tissue against indomethacin toxicity. Chem Biol Interact 2010; 186:82-9. [ Links ]
Sánchez S, Martín M, Ortiz P. Effects of dipyrone on inflammatory infiltration and oxidative metabolism in gastric mucosa: comparison with acetaminophen and diclofenac. Digest Dis Sci 2002; 47: 1389-98. [ Links ]
Slomiany BL, Piotrowski J, Slomiany A. Role of caspase-3 and nitric oxide synthase-2 in gastric mucosal injury induced by indomethacin: effect of sucralfate. J Physiol Pharmacol 1999; 50:3-16. [ Links ]
Squadrito GL, Pryor WA. Oxidative chemistry of nitric oxide: the roles of superoxide, peroxynitrite, and carbon dioxide. Free Radical Biol Med 1998; 25:392-403. [ Links ]
Sun J, Zhang X, Broderick M, Fein H. Measurement of nitric oxide production in biological systems by using Griess Reaction Assay. Sensors 2003; 3:276-84. [ Links ]
Tomlinson J, Wilder B. Effects of flunixin meglumine or etodolac treatment on mucosal recovery of equine jejunum after ischemia. J Am Vet Med Assoc 2004; 65:761-9. [ Links ]
Wink DA, Miranda KM, Espey MG, Pluta RM, Hewett SJ, Colton C, Vitek M, Feelisch M, Grisham MB. Mechanisms of the antioxidant effects of nitric oxide. Antioxid Redox Signal 2001; 3:203-13. [ Links ]