Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Colombiana de Ciencias Pecuarias
Print version ISSN 0120-0690
Rev Colom Cienc Pecua vol.29 no.1 Medellín Jan./Mar. 2016
https://doi.org/10.17533/udea.rccp.v29n1a06
ORIGINAL ARTICLE
doi: 10.17533/udea.rccp.v29n1a06
Ivermectin resistance of three Rhipicephalus microplus populations using the larval immersion test¤
Resistencia de tres poblaciones de Rhipicephalus microplus a la ivermectina por el ensayo de inmersión de larvas
Resistência de três populações de Rhipicephalus microplus à ivermectina pelo teste de imersão de larvas
David Villar1*, MV, MSc, PhD; Jonathan Puerta1, Est MV; Anderson López1, MV; Jenny J Chaparro1, MV, MSc, DrSc.
1Grupo de Investigación VERICEL, Laboratorio de Parasitología, Escuela de Medicina Veterinaria, Facultad de Ciencias Agrarias, Universidad de Antioquia (UdeA), Medellín, Colombia.
*Corresponding author: David Villar. Facultad de Ciencias Agrarias, Universidad de Antioquia, Calle 70 No. 52-21, Medellín, Colombia. E-mail: davidvillar2003@yahoo.com
Received: March 19, 2015; accepted: July 07, 2015
Summary
Background: in Colombia, the control of tick infestation in cattle is almost exclusively performed with chemical acaricides. It is important to determine the degree of resistance of Riphicephalus microplus field populations to ivermectins (IVM) as the first step to design strategies to increase the useful life of acaricides and decrease the reliance on chemical controls. Objective: to test the degree of resistance to IVM. Methods: three cattle farms in Antioquia (Colombia) where IVM had previously failed to control infestations of R. microplus ticks were studied. Ticks were collected several months apart in 2013, and the larval immersion test (LIT) was performed on the progeny of the adult females. Concentration-mortality data were subjected to probit analysis. Results: the three populations showed lethal concentrations (LC) 50 and 99 of ≥ 30 ppm and ≥ 400 ppm, respectively. There was no difference observed in the LC at different times of collection for each population studied. Such high values for LC, together with very low regression slopes (≤ 2), indicated a very heterogeneous response to increasing concentrations of IVM, which is common for resistant populations. At two farms, subcutaneous injection with a long-acting formulation of IVM at a dosage of 630 μg/Kg was ineffective at eliminating existing infestations and protecting against reinfestations, confirming farmer suspicions of lost efficacy. Conclusion: epidemiological studies are necessary to assess the current status of resistance to IVM in this region of Colombia, and it is likely that the intensive use of IVM will aggravate this situation in the future.
Keywords: low efficacy, macrocyclic lactones, ticks.
Resumen
Antecedentes: en Colombia, el control de la infestación de garrapatas en el ganado se ha realizado casi exclusivamente con acaricidas químicos y es importante determinar el grado de resistencia de las poblaciones de campo de Riphicephalus microplus a ivermectinas (IVM) como el primer paso para diseñar tácticas que aumenten su vida útil y disminuyan la dependencia de los controles químicos. Objetivo: probar el grado de resistencia a IVM. Métodos: se evaluó la resistencia de R. microplus a IVM en tres fincas ganaderas en Antioquia (Colombia) donde se sospechaba que productos con IVM estaban fracasando para controlar infestaciones por garrapatas. Se recolectaron garrapatas con varios meses de diferencia durante el año 2013 y se empleó la prueba de inmersión de larvas (LIT) con la descendencia de las hembras adultas. Los datos de concentraciónmortalidad fueron sometidos a análisis probit. Resultados: los resultados para las tres poblaciones estudiadas mostraron concentraciones letales (LC) 50 y 99 de ≥ 30 ppm y ≥ 400 ppm, respectivamente. No se observó diferencia en las LC obtenidas en diferentes momentos de recogida para cada población estudiada. Los altos valores de LC, junto con pendientes muy bajas en las rectas de regresión (≤ 2), indicaron una respuesta muy heterogénea a concentraciones crecientes de IVM, propio de poblaciones resistentes. En dos de las granjas, la administración de inyecciones subcutáneas con una formulación de acción prolongada de la IVM a dosis de 630 μg/Kg fue incapaz de eliminar las infestaciones y proteger frente a reinfestaciones, confirmando las sospechas de los ganaderos de pérdida de eficacia. Conclusión: estudios epidemiológicos son necesarios para evaluar el estado actual de la resistencia a IVM en esta región de Colombia, y es probable que el uso intensivo de IVM agrave esta situación en el futuro.
Palabras clave: garrapatas, lactonas macrocíclicas, pérdida de eficacia.
Resumo
Antecedentes: na Colômbia, o controle de infestação com carrapatos em bovinos tem sido realizado quase exclusivamente com acaricidas químicos pelo qual se faz importante para determinar o grau de resistência de populações de campo de Rhipicephalus microplus a uma das drogas antiparasitárias de maior uso no manejo de gado, a ivermectina (IVM), como o primeiro passo para projetar táticas para aumentar a sua vida útil e diminuir a dependência de controles químicos. Objetivo: testar o grau de resistência à ivermectina. Método: foi avaliada a resistência de R. microplus à ivermectina em três fazendas de gado leiteiro localizadas no departamento de Antioquia (Colômbia) com suspeita de que o controle dos carrapatos com a utilização deste produto não estava sendo efetivo. Coletaram-se carrapatos em diferentes meses durante o ano 2013, e foi utilizado o teste de imersão de larvas (LIT) na descendência das fêmeas adultas, como método para detecção da resistência. Os dados de concentração-mortalidade foram submetidos à análise Probit. Resultados: todas as três populações avaliadas mostraram concentrações letais (LC) 50 de ≥ 30 ppm, e LC 99 de ≥ 400 ppm. Não foram observadas diferenças na LC entre as coletas feitas nos diferentes meses, em cada população amostrada. Os altos valores de LC encontrados junto com uma baixa regressão linear (≤ 2), indicam uma resposta muito heterogênea as concentrações crescentes de ivermectina, próprio de populações resistentes. Em duas das fazendas, a administração de injeções subcutâneas de ivermectina com uma formulação de longa ação, em doses de 630 μg/Kg, não foram capazes de eliminar as infestações e proteger contra a reinfestação, confirmando as reclamações dos fazendeiros para a perda de eficácia da droga antiparasitária. Conclusões: os estudos epidemiológicos são necessários para avaliar o estado atual da resistência à ivermectina nesta região da Colômbia, e é provável que o uso intensivo da ivermectina agrave ainda mais esta situação no futuro.
Palavras chave: carrapatos, lactonas macrocíclicas, perda de eficácia.
Introduction
The cattle tick Rhipicephalus (Boophilus) microplus is considered the main external parasite affecting cattle productivity in the tropical countries of America (Grise et al., 2014). In Colombia, control of this parasite is almost exclusively performed with chemical acaricides (García, 2011). However, resistance to most acaricides is a growing phenomenon worldwide; therefore, it is necessary to identify strategies to increase their useful life and to decrease reliance on chemical controls. The first cases of ivermectin resistance were reported in Brazil 14 years ago (Martins and Furlong, 2001), but, as the compound was introduced in most countries in the early 1980s, it is likely that resistance developed earlier. There are many types of generic ivermectin (IVM) in the Colombian market, with at least 29 different names registered. The farms analyzed here use IVM routinely, regardless of real or perceived needs.
Confirmation of resistance is routinely determined using dose-response bioassays and/or assessment of the field efficacy of acaricides according to methods approved by the FAO (FAO, 2004) or the World Association for the Advancement of Veterinary Parasitology (WAAVP; Holdsworth et al., 2006). The standard in vitro bioassays recommended in the FAO guidelines are the larval packet test (LPT) and the adult immersion test (AIT). Although it remains difficult to predict field acaricide efficacy based on these resistance bioassays, they can diagnose resistance before control failures are obvious. The LPT is laborious and modifications such as the larval immersion test (LIT), initially developed in Brazil (Klafke et al., 2006) is more sensitive in discriminating between resistant and susceptible strains against macrocyclic lactones (Sabatini et al., 2001; Klafke et al., 2012). Numerous reports using the LIT in the last decade show R. microplus resistance to ivermectin in Latin American countries, including Mexico (Perez-Cogollo et al., 2010a; 2010), Uruguay (Castro-Janer et al., 2011), and Brazil (Klafke et al., 2010; 2012). To complement the above in vitro assays, field studies using the WAAVP guidelines should be used simultaneously to confirm a reduction in therapeutic efficacy and protective period (Holdsworth et al., 2006).
The objective of this study was to determine the degree of resistance of R. microplus field populations to IVM at three cattle farms in the province of Antioquia (Colombia). This study complements a field study conducted at two farms in Antioquia and reported elsewhere (López et al., 2015).
Material and methods
The authors considered that this study did not require approval of an Ethics Committee for Animal Experimentation.
Type of study
A descriptive study was performed.
Selection of farms
Three Antioquian farms with failures to control tick infestation, that previously claimed a lack of satisfactory control despite of the use of one or more commercial trade versions of IVM, were visited on three separate occasions for the collection of ticks at approximately two-month intervals between March and October of 2013. On each occasion, the samples were taxonomically identified as R. microplus. Except for the Tarso farm, herds were typical of the predominant production system and management practices of their area. The tick from San Jerónimo was obtained at a family-run mixed dairy farm with approximately 30 Simmental, Normandy and Holstein crosses. The Yarumal ticks originated from a small dairy farm of approximately 32 cattle of Jersey and Holstein crosses. The Tarso ticks were collected from a beef farm of highly selected Brangus imported from Texas, and comprising approximately 250 cattle. IVM was used for an uncertain number of years at the three locations, and producers had failed to clear the initial invasion of engorging ticks after use of one or several trade versions of IVM.
Larval immersion test (LIT)
A control solution containing 1% ethanol and 0.02% Triton X-100 in distilled water was used to prepare all IVM immersion dilutions and to test control larvae. A commercial 1% IVM (Ivomec – Merial Saúde Animal, Brazil. Batch number BE314/11, expiration date 11/2016) was used to prepare all serial dilutions with the control solution. The analysis of this particular product reported a concentration of 0.85% ivermectin (López et al., 2015). For the LIT, ten different concentrations of IVM dilutions were prepared, ranging from 10 to 300 ppm, in a final immersion volume of 4 mL; larvae arising from eggs of at least 25 ticks were first mixed in distilled water to avoid exposing only those originating from a few ticks. Approximately 300 - 400 larvae were then immersed in different concentration solutions for 10 minutes under gentle agitation. They were immediately placed in Petri dishes and were transferred with a paintbrush to filter papers (850 x 750 mm) that were folded and sealed with sticky tape, forming a packet.
Three packets containing approximately 100 larvae each were used for each IVM dilution. The packets were placed in the incubator for 24 h, after which they were opened under a desk lamp and the larvae were gently transferred with a paintbrush to a white sheet of paper. Mortality was immediately determined using a magnifying lens because the larvae are incapable of locomotion. To assist with counting, those that moved spontaneously were removed with sticky tape and considered alive. Bioassays for each farm were replicated at least three times.
Data analysis
The number of dead versus exposed larvae was introduced into IBM SPSS Statistics 21 software (version 2003) and submitted to probit analysis to calculate the LC50 and LC99% with their respective confidence intervals (CI95%), chi-square tests for homogeneity, and slopes of the regression line.
Results
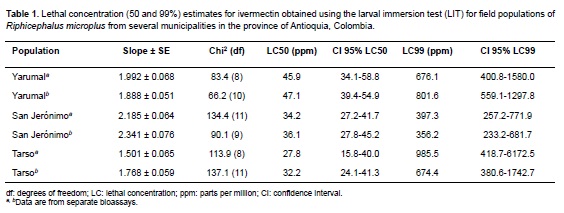
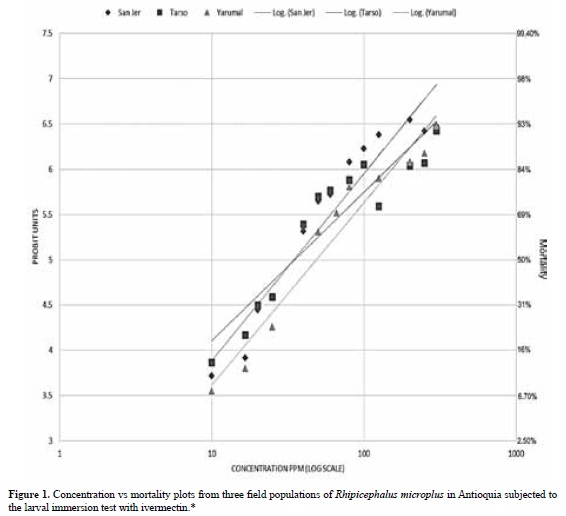
Concentration-mortality regressions, lethal concentrations LC50 and LC99, their 95% confidence intervals and slopes for each population are shown in Table 1. There was no difference in the LC50 and LC99 estimates for each of the three populations studied when bioassays were performed at different times of collection. Mortality in the control groups exposed to the ethanol-Triton X solution was zero; therefore, no correction was necessary for the ivermectin-exposed groups. Figure 1 represents the probit mortality plots with the regression lines for each of the populations studied. The small slopes of the regression (≤ 2.5) and the distant values for the LC50 and LC99 are indicative of a very heterogeneous response to increasing concentrations of IVM in all three populations.
Discussion
The objective of this study was to investigate farmer reports of failure to control tick infestation at three farms in Antioquia; in each case IVM formulations were no longer able to eliminate tick infestations from cattle. The evaluation of R. microplus resistance to IVM was performed using the LIT bioassay with larvae arising from ticks collected at the sampled farms, and further tested using in vivo studies that are reported elsewhere (López et al., 2015). Although the LIT has been used as a successful method to detect resistance (Sabatini et al., 2001; Klafke et al., 2006), ideally, it should be run in parallel to a susceptible strain to calculate resistance ratios and determine discriminating doses. Despite the limitation of not having a susceptible strain in this study, the results showed LC and concentration-mortality slopes similar to those reported for the most resistant tick populations from Veracruz (Fernández-Salas et al., 2012), Yucatán (Pérez-Cogollo et al., 2010a; 2010b) and Sao Paulo (Klafke et al., 2012). For example, comparison of LC50 values to those of a study that determined the status of resistance to IVM in 53 randomly selected field populations of R. microplus in Mexico (Fernández-Salas et al., 2012) demonstrates that the level of resistance was equal to that of the four most resistant populations, with LC50s in the 30-50 ppm range. In general, low slope values (1.2-1.9) are related to high LC50 (>35 ppm) and high LC99 (>600 ppm) values, as reported here, and are hallmarks of very high resistance compared to values for susceptible R. microplus strains (slopes ≈ 5, LC50s ≈ 5 ppm for the Deutch strain). Low -slopes for the concentrationmortality lines could be interpreted as a result of a large heterogeneity with different levels of resistance in the field populations studied. To our knowledge, no studies of a highly resistant IVM strain have been described with high-slopes that would indicate complete loss of heterogeneity and consequently of the remaining susceptible genes in a population.
To relate the results of the LIT bioassay to the field situation, a long-acting formulation of IVM was used on two of the sampled farms. That clearly showed that the efficacy of IVM in terms of clearing the initial infestation load and protecting against reinfestation was markedly reduced from target expectations (López et al., 2015). At 10 days postinjection, a reduction in the number of adult standard ticks was only 40 and 75% at the San Jeronimo and Tarso farms, respectively (López et al., 2015). These results are worrisome compared to field studies showing that 3.15% ivermectin formulations had a therapeutic and persistent efficacy of 95% at 56 days post-treatment (Arieta-Román et al., 2010). In a study conducted at an USDA-ARS quarantine facility, the therapeutic efficacy of a similar longacting ivermectin formulation was 99.9% against all stages of a susceptible strain of R. microplus at the time of treatment (Davey et al., 2010). Furthermore, the protective period against larval re-infestation was 14 d if a level of control ≥ 99% was desired, and dropped to 70.4% for animals exposed artificially to larvae at 28 d post-treatment. Because ticks are unable to complete development in <18 d, the study concluded that cattle could be treated with this longacting IVM at 31 d intervals without the risk of viable ticks detaching from the infested animals. In the present study, the high degree of resistance based on the LIT was clearly demonstrated by the ineffective elimination of existing infestations and the loss of protection against reinfestations.
In conclusion, the present study confirmed the presence of R. microplus ticks that are highly resistant to IVM to the point of having lost efficacy for eliminating existing tick infestations. The sampled farms completely relied on chemical control to fight tick infestations. Therefore, it is imperative to apply integrated approaches for parasite control in farms that now have multiresistant strains of R. microplus.
Acknowledgements
The authors thank the Biogénesis Research group Sustainability Project 2014-2015 (Estrategia de Sostenibilidad CODI 2014-2015).
Conflicts of interest
The authors declare they have no conflicts of interest with regard to the work presented in this report.
Notes
¤To cite this article: Villar D, Puerta J, López A, Chaparro JJ. Ivermectin resistance of three Rhipicephalus microplus populations using the larval immersion test. Rev Colomb Cienc Pecu 2016; 29:51-57.
References
Arieta Román RJ, Rodriguez Vivas RI, Rosado Aguilar JA, Ramirez Cruz GT, Basto Estrella G. Persistent efficacy of two macrocyclic lactones against natural Rhipiceplalus (Boophilus) microplus infestations in cattle in the Mexican tropics. Rev Mex Cienc Pecu 2010; 1: 59-67. [ Links ]
Castro Janer E, Rifran L, Gonzalez P, Niell C, Piaggio J, Gil A, Schumaker TTS. Determination of the susceptibility of Rhipicephalus (Boophilus) microplus (Acari: Ixodidae) to ivermectin and fipronil by larval immersion test (LIT) in Uruguay. Vet Parasitol 2011; 178:148-55. [ Links ]
Davey RB, Pound JM, Miller JA, Klavons JA. Therapeutic and persistent efficacy of a long-acting (LA) formulation of ivermectin against Rhipicephalus (Boophilus) microplus (Acari: Ixodidae) and será concentration through time in treated cattle. Vet Parasitol 2010; 169:149-56. [ Links ]
FAO. Working Group on Parasite Resistance. ''Acaricide resistance: diagnosis, management and prevention'', in Guidelines Resistance Management and Integrated Parasite Control in Ruminants 2004, pp. 25-77. [ Links ]
Fernández Salas A, Rodríguez Vivas RI, Alonso Diaz MA, Basurto Camberos H. Ivermectin resistance status and factors associated in Rhipicephalus microplus (Acari:Ixodidae) populations from Veracruz, Mexico. Vet Parasitol 2012; 190:210-5. [ Links ]
García Paz JL. Evaluación de las propiedades acaricidas de Piper crassinervium Kunth. Piper aequale Vahl. (Piperaceae) sobre larvas de Rhipicephalus (Boophilus) microplus. Trabajo de grado para optar a título de maestría. Universidad Nacional. Colombia. 2011 [ Links ]
Grise L, Leite RC, Martins JR, Barros AT, Andreotti R, Perez de Leon AA, Pereira JC, Silva H. Reassessment of the potential economic impact of cattle parasites in Brazil. Braz J Vet Parasitol Jaboticabal 2014; 23(2):150-6. [ Links ]
Holdsworth PA, Kemp D, Green P, Peter RJ, De Bruin C, Jonsson NN, Letonja T, Rehbein S, Vercruysse J. World Association for the Advancement of Veterinary Parasitology (W.A.A.V.P.) guidelines for evaluating the efficacy of acaricides against ticks (Ixodidae) on ruminants. Vet Parasitol 2006; 136:29-43. [ Links ]
Klafke GM, Sabatini GA, Albuquerque TA, Martins JR, Kemp DH, Miller RJ, Schumaker TTS. Larval immersion tests with ivermectin in populations of the cattle tick Rhipicephalus (Boophilus) microplus (Acari: Ixodidae) from State of Sao Paulo, Brazil. Vet Parasitol 2006; 142:386-90. [ Links ]
Klafke GM, Albuquerque TA, Miller RJ, Schumaker TTS. Selection of an ivermectin-resistant strain of Rhipicephalus microplus (Acari: Ixodidae) in Brazil. Vet Parasitol 2010; 168:97-104. [ Links ]
Klafke GM, Castro Janer E, Mendes MC, Namindome A, Schumaker TTS. Applicability of in vitro bioassays for the diagnosis of ivermectin resistance in Rhipicephalus microplus (Acari: Ixodidae). Vet Parasitol 2012; 184:121-220. [ Links ]
López Arias A, Villar D, Miller R, Chaparro J, Pérez de león, A. Reduced efficacy of commercial acaricides against populations of resistant cattle tick Rhipicephalus microplus from two municipalities of Antioquia, Colombia. Libertas Académica, 2015 (in press). [ Links ]
Martins JR, Furlong J. Ivermectin resistance of the cattle tick Boophilus microplus in Brazil. Vet Rec 2001; 149:64. [ Links ]
Pérez Cogollo LC, Rodríguez Vivas RI, Ramírez Cruz GT, Rosado-Aguilar JA. Survey of Rhipicephalus microplus resistance to ivermectin at cattle farms with history of macrocyclic lactones use in Yucatan, Mexico. Vet Parasitol 2010a; 172:109-113. [ Links ]
Pérez Cogollo LC, Rodríguez Vivas RI, Ramírez Cruz GT, Miller RJ. First report of the cattle tick Rhipicephalus microplus resistant to ivermectin in Mexico. Vet Parasitol 2010b; 168:165-9. [ Links ]
Sabatini GA, Kemp DH, Hughes S, Nari A, Hansen J. Tests to determine LC50 and discriminating doses for macrocyclic lactones against the cattle tick, Boophilus microplus. Vet Parasitol 2001; 95(1):53-62. [ Links ]