Introduction
Cryopreservation of in vitro-produced (IVP) embryos plays an important role protecting genetic material in traditional cattle breeding systems (Ha et al., 2014). Approximately 9.5% of the 385,000 IVP bovine embryos transferred worldwide in 2012 were cryopreserved (Perry, 2012). The low number of cryopreserved embryos could be explained by the fact that IVP embryos are, to some extent, developmentally compromised as compared to their in vivo-derived (IVD) counterparts. As IVP embryos have poor survival rates after cryopreservation (Rizos et al., 2003), they are associated with reduced pregnancy rates (Hasler, 2003). Hence, if such embryos are to be used for commercial bovine reproduction, cryopreservation techniques must be improved (Xu et al., 2006).
During cryopreservation, cells are suspended in a suitable solution, then cooled, stored in liquid nitrogen, warmed to room temperature, and returned to a physiological solution. During each step of this process, cells are at risk of various injuries, such as formation of intracellular ice during cooling (Pollard and Leibo, 1994) which affects the relative abundance of developmentally important gene transcripts (Wrenzycki et al., 2007) and fetal viability after embryo transfer (ET; Schmidt et al., 1996).
Slow-rate freezing and vitrification have been used to cryopreserve IVP bovine embryos. The principles and methodologies for slow-rate freezing and vitrification have been described elsewhere (Saragusty and Arav, 2011; Vajta et al., 2013). The slow freezing method has been widely used for cryopreserving IVP and IVD embryos; however, this method requires special equipment and a longer freezing time (Naik et al., 2005). In contrast, vitrification does not require expensive or sophisticated equipment, but it does require technical expertise to do it properly (Vajta and Nagy, 2006).
In terms of embryo survival after thawing/ warming, vitrification of IVP embryos has been proved to be at least as efficient (Inaba et al., 2011) or better (Vajta and Nagy, 2006; Trigal et al., 2012; Vajta, 2013) than slow-rate freezing, so it is not easy to decide for one methodology over the other; each laboratory should decide which one adapts better to their conditions (Caamaño et al., 2015).
The objective of the present study was to estimate whether differences in composition of vitrification solutions affect in vitro viability of bovine IVP embryos as compared to that of embryos cryopreserved using a conventional slow-freezing method.
Material and methods
Ethical considerations
The study followed the guidelines for animal care and use of Universidad de la Salle, Colombia (Bogotá; Protocol 002, from March 13, 2012).
Chemicals
All chemicals were provided by Sigma-Aldrich (St. Louis, MO, USA) unless otherwise stated.
In vitro production (IVP) of bovine embryos
In vitro oocyte maturation (IVM). Bovine ovaries were obtained from a local abattoir within 15 min post-slaughter and transported to the laboratory at 36-38 °C within 3-4 h in saline solution (0.9% NaCl). The ovaries were rinsed twice in phosphate-buffered saline (PBS) and follicular fluid aspirated from 3 to 6 mm follicles using a 21 gauge needle and a 10 mL syringe. Cumulus oocyte complexes (COCs) were collected from the follicular fluid and those with three or more layers of cumulus cells and homogeneous cytoplasm were randomly distributed in groups of 20-25 COCs and cultured in 100 µL droplets of IVMmedium. IVM consisted of tissue culture medium 199 (TCM-199) supplemented with 2.5 mM Na-pyruvate, 100 IU/mL penicillin, 100 µg/mL streptomycin, 1 mg/ mL estradiol -17 β, 0.01 IU/mL follicle stimulating hormone (FSH), 0.1 IU/mL luteinizing hormone (LH), and 10% fetal bovine serum (FBS) for 24 h in a humidified atmosphere of 5% CO2 in air at 39 °C.
In vitro fertilization (IVF). Frozen-thawed semen from a Holstein bull of proven fertility was used for IVF. Straws were thawed in a water bath at 37 °C for 30 sec and sperm suspension layered onto a two-layer (45:90%) discontinuous Percoll gradient solution (Amersham, Pharmacia Biotech AB, Sweden) by centrifugation (500 x g) for 10 min. The supernatant was removed and the sperm pellet was re-suspended in Tyrode’s Albumin-Lactate-Pyruvate (TALP) IVF medium. For IVF, motile spermatozoa (~1 x 106/mL) were co-incubated with COCs (6 to 7) in 5% CO2 in air at 39 °C under mineral oil in 25 µL droplets of TALP- IVF medium for 18 h.
In vitro culture (IVC). After IVF, the remaining cumulus and corona cells were removed by gently vortexing. Then, presumptive zygotes were cultured in 25 µl droplets (1 embryo/µL) of synthetic oviductal fluid medium (SOF) as previously described by Holm et al. (1999), covered with mineral oil in a humidified atmosphere (5% CO2, 5% O2 and 90% N2) at 38.5 °C.
Embryo cryopreservation
On day 6 or 7 of IVC, embryos at the morula, early blastocyst and blastocyst stage were cryopreserved either: 1) at a slow controlled-rate (control group), or 2) through vitrification by using two different vitrification and warming solutions: (1) Protocol V1: a commercial vitrification Kit from Achilles Genetics (of unknown composition), and (2) Protocol V2: vitrification and warming solutions that were prepared in the laboratory. Embryos in both vitrification protocols were cryopreserved by using the vitrification hook WTA system (WTA, Watanabe Applied Technology, WTA, Brazil). The system consisted of a hook device and three pieces of equipment: 1) foam bath, 2) plastic container placed inside the foam bath, and 3) vitrification block placed inside the plastic container for cooling the embryos. The vitrification block was cooled by filling the foam bath with liquid nitrogen to maintain the temperature during the vitrification process.
Vitrification - Protocol V1
Vitrification and warming solutions were carried out in 5 µL micro-drops of each in Petri dishes at 22 °C. One to four embryos were exposed to an equilibration solution for 2 min, and then transferred into a vitrification solution (VS1) for 2 min, to be finally transferred into VS2 for 45 s. Embryos were loaded onto the hook device which was then held against the cooled surface of the vitrification block until the droplet vitrifies into a glassy bead. The hook was placed immediately into pre-cooled sleeves and plunged into liquid nitrogen (components of the system are shown in Figure 1).
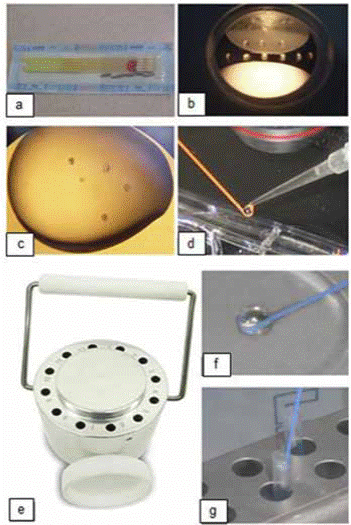
Figure 1 Components used during vitrification. (a) Hooks in the sleeves; (b) Petri dish with vitrification solutions; (c) Embryos in vitrification droplet solution; (d) Droplet with embryos transferred to the hook; (e) Vitrification block; (f) Vitrified droplet; (g) Inserting hook into a chilled sleeve.
After storage, embryos were warmed by immersion of the hook device directly into warming solution (WS1) for 2 min, which was followed by transferring them into a WS2 for 2 min (Figure 2).
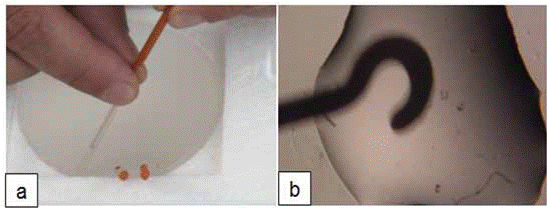
Figure 2 Warming procedure: (a) Unpacking the vitrification hook; (b) Transferring embryos from the hook to the warming solution.
Warmed embryos were cultured in 50 µl drops of SOF medium under paraffin oil (5-7 embryos/droplet) at 39 °C in a humidified atmosphere of 7% O2, 5% CO2, and 90% N2. Embryo viability was recorded at 24, 48, and 72 h after thawing by evaluating the numbers of embryos that re-expanded to their original size and developed to the hatching blastocyst stage.
Vitrification - Protocol V2
Embryo handling was performed at 22 °C while embryo culture media was maintained at 39 °C. The vitrification solution (VS2) consisted of 20% (v/v) ethylene glycol (EG; E9129), 20% (v/v) dimethyl sulfoxide (DMSO; D5879), and 20% (v/v) FBS diluted in TCM-199. One to four embryos were equilibrated in TCM- 199 supplemented with 20% FBS for 5 min. Then, embryos were transferred to VS1 consisting of TCM-199 + 20% FBS, + 10% EG, and 10% DMSO for 3 min, and finally briefly exposed (45 s) to the VS2. Embryo loading and cooling was performed as described in Protocol V1. For warming, embryos were transferred directly into 500 µL TCM- 199 supplemented with 20% FBS and 0.2 M Sucrose (S; S9378). Embryo culture and viability evaluation were performed as described in Protocol V1.
Slow controlled-rate freezing
Embryos were frozen according to a method previously reported by Lim et al. (2008). Briefly, embryos at each developmental stage were equilibrated in a cryoprotectant solution consisting of 1.5 M EG, 0.1 M S and 10% FBS in D-PBS for 5 min at room temperature. Embryos were individually loaded into 0.25 mL straws and, after sealing the tip, each straw was placed into the chamber of a controlled rate freezing unit (model CL-5500, CryoLogic, Victoria, Australia) equilibrated for 10 min at -7 °C during which time seeding was initiated. Freezing was accomplished at a cooling rate of 0.3 °C/min from -7 to -35 °C, and straws were plunged into liquid nitrogen for storage.
Straws were thawed for 6 to 10 s in air at 20 °C, followed by 15 s in a water bath at 37 °C. Thawed embryos were washed three times for 4 min each in Hepes buffered TCM-199 and then cultured in SOF medium. Embryo viability was recorded at 24, 48, and 72 h after thawing by evaluating the number of embryos that developed to the expanded or hatching blastocyst stage.
Experimental designs
Morulae, early blastocysts and in vitro produced blastocysts were randomly allocated to three freezing protocols: slow controlledrate freezing, vitrification protocol V1, and vitrification protocol V2. Embryo survival was evaluated in terms of re-expansion and hatching at 24, 48, and 72 hours after thawing/warming.
Statistical analysis
Nonparametric Chi Square Test was used for reexpanded and hatching rate using the STATISTICS 8.0. program (Analytical Software in Tallahasee, Florida). For all analysis, p<0.05 was considered significant.
Results
The frequency of embryos that re-expanded at 24 h after warming/thawing was higher for embryos vitrified with protocols V1 and V2 (89.0, 86.2%, respectively), compared with those cryopreserved by slow-controlled rate method (73.6%, p<0.05; Table 1). Similarly, higher percentages of embryos cryopreserved by vitrification hatched at 72 h after warming/thawing, where protocol V2 resulted in higher percentage of hatched embryos (84.3%) compared with protocol V1 (64.0%, p<0.05). Furthermore, hatching rates in both vitrification solutions were higher compared with embryos cryopreserved by the slow-controlled rate method (55.2%; p<0.05; Table 1).
Table 1 Embryo re-expansion and hatching in culture at 24 and 72 h after warming or thawing, respectively.
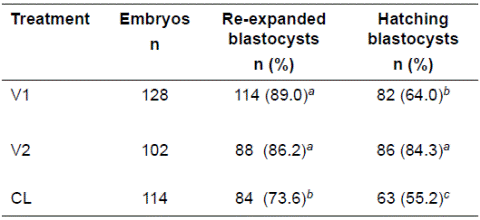
V1: vitrification protocol 1 (commercial vitrification Kit from Achilles Genetics®); V2: vitrification protocol 2 prepared in the laboratory); CL: slow controlled-rate freezing. Different superscripts letters (a, b) within the same column indicate significant differences (p<0.05).
Although re-expansion rates observed after 24 h of warming for embryos vitrified with V1 and V2 protocols were higher than those of embryos in the slow-controlled rate group, re-expansion rates at 72 h for embryos vitrified with protocol V1 (31.2%) were similar to those of embryos cryopreserved by slowcontrolled rate (21.9%), but re-expansion rate in both groups was higher than that of embryos vitrified with protocol V2 (9.8%; p<0.05; Figure 3). In contrast, there were no significant differences in re-expansion rates among treatments at 48 h (Figure 3).
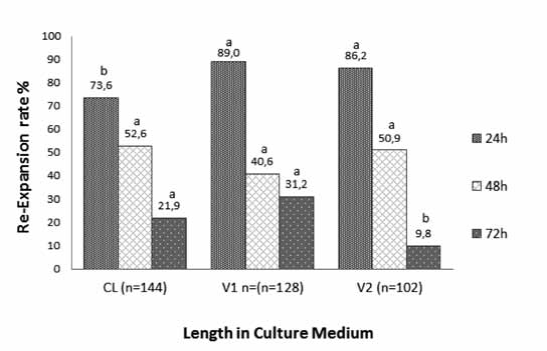
Figure 3 Re-expansion rate of IVP bovine embryos cryopreserved by slow controlled-rate freezing (Cl), vitrification protocol 1 (V1) and vitrification protocol 2 (V2) at 24, 48, and 72 h after thawing or warming. Different superscript letters (a, b) within the same culture time indicate significant differences (p<0.05).
The percentage of vitrified embryos that hatched at 24 h after warming was similar for vitrification protocols V1 (48.4%) and V2 (36.2%), and higher than that of embryos frozen by the slow-controlled rate method (21.5%; p<0.05; Figure 4). The percentages of blastocysts that hatched at 48 h after warming was similar for embryos vitrified with protocol V2 and frozen by slow freezing (41.1 and 30.7%, respectively), but higher than those vitrified with protocol V1 (9.5%; p<0.05; Figure 4). The lowest hatching rates were observed at 72 h after warming/ thawing and no differences were observed among treatments (Figure 4).
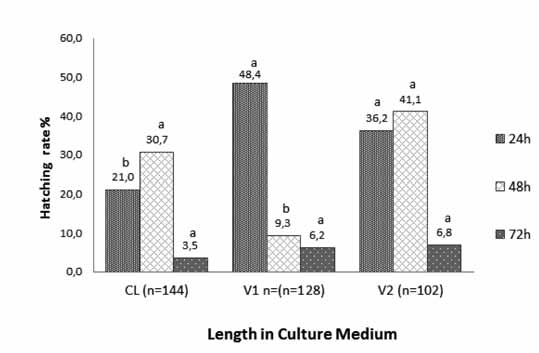
Figure 4 Hatching rate of IVP bovine embryos cryopreserved by slow controlled-rate freezing (Cl), vitrification protocol 1 (V1) and vitrification protocol 2 (V2) at 24, 48, and 72 h after thawing or warming. Different superscript letters (a, b) within the same culture time indicate significant differences (p<0.05).
Discussion
In the present study we evaluated the effect of slow freezing and vitrification techniques on the survival of in vitro bovine embryos produced under the same culture conditions. Results show that there are morphologic differences regarding re-expansion and hatching rates. We observed that bovine IVP embryos survived vitrification at higher rates than freezing by the slow-controlled rate method. Previous studies have shown similar results (Nedembale et al., 2004; Kuwayama , 2007; Rios et al., 2010; Trigal et al., 2012; Zhao et al., 2012), and demonstrated that vitrification is an appropriate method for cryopreservation of bovine IVP embryos (Vajta, 2000).
Additionally, vitrification with a V2 solution prepared at our laboratory resulted in higher percentage of embryos reaching the hatching stage compared with embryos vitrified with the commercially available V1 solution. We infer that the differences could be linked directly with the development stage of the embryo, volume and concentration of the cryoprotectants. It seems that these factors have a severe impact on embryo cryopreservation (Rios et al., 2010; Shirazi et al., 2010; Stinshoff et al., 2011).
On the other hand, it is not clear why embryos cryopreserved by vitrification resulted in higher survival rates than that of slowcontrolled rate freezing. It is possible that the higher embryo survival rates at 24 h post-warming for embryos vitrified in both vitrification solutions (V1 or V2) resulted from inhibition of chilling injury by the ultra-rapid cooling rates and lower cellular damage during cryopreservation (Vajta et al., 1998).
Despite vitrification avoids problems associated with ice crystal formation, this method implies a challenge derived from the high concentration of cryoprotectant. However, the observed low proportion of cells with altered plasma membrane and their relation with expansion and hatching rates may be attributed to the brief exposure to cryoprotectant, as described by Vajta et al. (1998) and Mucci et al. (2006). Membrane stability of in vitro produced bovine embryos might be an important factor determining their susceptibility and viability to freezing (Imai et al., 1997).
A possible adverse effect of cryopreservation is the reduced ability of post-warming embryos to continue mitotic division (Kaidi et al., 1998), or the induction of apoptosis or necrosis in some embryonic cells (Abdalla et al., 2010). The adverse effect of vitrification/warming in this study was consistent with the commonly reported losses of these processes, which is in agreement with results reported for cows (George et al., 2006; Abdalla et al., 2010; Ha et al., 2014).
In conclusion, the method of cryopreservation and the composition of the vitrification solution have a direct effect on the viability of bovine IVP embryos. Results of this work, however, require verification by embryo transfer.
Conflicts of interest
The authors declare they have no conflicts of interest with regard to the work presented in this report.














