Introduction
Equine gastric ulcer syndrome (EGUS) can affect any area of the stomach mucosa in addition to the distal esophagus and proximal duodenum, although it is more prevalent in the non-glandular mucosa, particularly in areas adjacent to the margo plicatus. This higher prevalence indicates differences between the protection mechanisms of different areas of the gastric mucosa. Several external factors and particular physiological aspects that influence the presentation of ulcerative lesions in horses have been identified (Luthersson et al., 2009; Orsini et al., 2009; Tamzali et al., 2011).
Mechanisms related to the caustic effects of hydrochloric acid, associated with mechanical phenomena and poor defense barriers are involved in lesions of the non-glandular mucosa. In addition, inflammatory and ulcerative lesions in the glandular mucosa have been associated with collateral effects of non-steroidal anti-inflammatory drugs (NSAIDs) in which inhibition of prostaglandin E2 (PGE2) and oxidative stress are within the pathophysiology of the primary lesions (Naito et al., 1998; Tomisato et al., 2004).
PGE2 deficiency increases susceptibility of the mucosa to the formation of ulcers (Kobayashi and Arakawa, 1995). The exogenous administration of prostaglandin (PG) decreases the risk of EGUS, but it is expensive (Cryer, 2001). Studies with dietary supplements rich in linoleic acid as a precursor of arachidonic acid were performed with the aim of obtaining more economical endogenous sources of PG (Grant et al., 1988; Cargile et al., 2004). However, there are few studies in the literature evaluating the benefits of these supplements in the ulcerated gastric mucosa of horses.
The treatment of EGUS requires several strategies and long periods of medication, which increase treatment costs. In addition to the pharmacological approach, changes in the nutritional and environmental management are important to prevent recurrence of ulcers. Studies that aim to find new approaches and more economical products that contribute to both the prevention and the treatment of this syndrome are relevant. The objective of this study was to evaluate the effects of corn oil (CO) on the gastric mucosa of horses with induced ulcers and the possible mechanisms of action related with the repair of the damaged mucosa.
Material and methods
Ethical considerations
The present experiment was approved by the Federal University of Minas Gerais (UFMG) Animal Research Ethics Committee under protocol number 234/2009.
Animals
A group of 15 mixed-breed horses (11 nonpregnant mares and 4 geldings) raised on pasture and aged between 5 and 20 years, with body scores of three to four (Speirs, 1997) and weighing between 270 and 465 Kg, were used for 21 days, including 7 days for the induction of ulcers and the remaining 14 days for the administration of treatments. Clinical and laboratory evaluations testing for infectious anemia and ectoparasite control were performed during the two-week adaptation period.
The horses were divided into three groups with five animals each. Animals were placed in individual stalls and were fed coast cross (Cynodon dactylon) hay and water ad libitum. A 13% crude protein commercial diet was supplied twice a day at a proportion of 1% body weight, and 60 g of a mineral supplement/ animal/day. All animals were equally managed during the two experimental phases, with access to paddocks for one hour on alternate days.
Gastric ulcer induction
The EGUS induction model was based on the use of phenylbutazone (PBZ) associated with confinement during an experimental period of seven days, according to the protocol described by Martinez et al. (2015). Endoscopic evaluations of the gastric mucosa were performed on days zero and seven of the induction period.
Therapeutic protocols
After ulcers were induced in horses of each group, the following treatments were orally administered over 14 days: group I received sucralfate (SA) 30 mg/ Kg three times a day and was used as the control group, and animals from groups II and III received CO at doses of 70 and 90 mL/100 Kg, respectively, divided into two administrations per day. Gastroscopic evaluations were performed on days 14 and 21 in all treated groups during the experimental phase.
Gastroscopic evaluation
The gastric mucosa of the horses was examined using a flexible videoendoscope (PortaScope , 1800PVS, Bradenton, FL, USA) introduced nasogastrically after solid (12-14 h) and liquid (4 h) fasting and sedation using detomidine 10 µg/Kg IV (Dormosedan , Zoetis, New York, NY, USA). The lesions were recorded on video and gastric lesions were scored following recommendations by MacAllister et al. (1997). Gastroscopic evaluations preceded the collection of samples for analysis.
Material collection and processing
Samples of blood and gastric content as well as biopsies of the gastric mucosa were collected by endoscopy four times at day 0 (before the treatments) and every seven days up to the 21st experimental day. Blood samples were processed to perform complete blood count and serum biochemistry. The pH and PGE2 levels of the gastric contents were determined, and oxidant and antioxidant variables of the biopsies were measured.
Gastric content
With the endoscope placed in the stomach lumen, approximately 10 mL of gastric juice was aspirated using a urethral probe (5 x 7 mm, 1.50 m length) coupled to the gastroscope. The pH values of the samples were immediately measured and the samples were divided into aliquots for analysis. Samples were filtered, titrated at pH 7 with 0.1 N NaOH and frozen at -20 °C for later determination of PGE2 concentration using a commercial kit (Enzo Life Sciences, Miami, FL, USA).
Gastric biopsies
Biopsies were performed on the glandular mucosa after the stomach was distended with air and the mucosa was rinsed with water jets, which were guided visually by using forceps inserted through the gastroscope.
Seven biopsies were collected per animal in each evaluation period. Six samples were placed in individual cryogenic tubes and preserved in liquid nitrogen to determine enzymatic activity of myeloperoxidase (MPO), n-cetylglucosaminidase (NAG), superoxide dismutase (SOD) and catalase (CAT), and the amounts of nitric oxide (NO) and malondialdehyde (MDA), according to specific protocols for each variable. The remaining biopsy was immediately fixed in 10% buffered formalin to be subsequently processed according to conventional techniques for inclusion in paraffin (Rocha et al., 1989) and then evaluated under a light microscope to detect spiral bacteria.
SOD activity analysis
The method used to evaluate SOD activity follows Dieterich et al. (2000) and is based on the capacity of SOD to remove O2 -, thus decreasing the auto-oxidation rate of pyrogallol. The reaction was read in an enzyme-linked immunosorbent assay (ELISA) reader at 570 nm wavelength. The results are expressed in SOD units/mg total protein of the homogenate (U/mg protein).
CAT activity analysis
The principle of the method used is the oxidation of H2O2 to molecular oxygen and the reduction to water (Aebi, 1984). The samples were homogenized in phosphate buffer, and phosphate buffer with peroxide was then added; the samples were read in a spectrophotometer at 240 nm at 0, 30, and 60 seconds. The results are expressed in CAT units/mg protein of the homogenate.
MPO activity analysis
The MPO activity was determined by the method by Belo et al. (2004). Biopsies were weighed, grinded, homogenized in buffer and centrifuged for 10 min. After the supernatant was removed, it was re-suspended and homogenized with NaPO4 buffer. The activity of MPO was determined by measuring optical density (OD) changes at 450 nm. The results are expressed as OD changes per mg of ground tissue.
NAG activity analysis
The method used was by Carollo et al. (2001) and adapted by the Laboratory of Angiogenesis of the Biological Sciences Institute of the Federal University of Minas Gerais. The results are expressed as changes in OD per mg of ground tissue.
NO analysis
NO was analyzed indirectly performed using the Griess colorimetric reaction adapted by Miranda et al. (2001). The samples were incubated with nitrate reductase, flavin adenine dinucleotide disodium salt hydrate (FAD) and β-nicotinamide adenine dinucleotide 2’-phosphate reduced tetrasodium salt (NADPH). Subsequently, samples were deproteinated with 30% zinc oxide. The Griess solution was added to the supernatant and, after 10 minutes, it was read spectrophotometrically at 540 nm. To construct the standard curve, a 1 mM sodium nitrite base solution was used.
MDA analysis
MDA was analyzed using high-performance liquid chromatography (HPLC), according to the method by Karatas et al. (2002) with modifications. Each sample was ground, HClO4 was added, and the samples were then centrifuged at 4500 g for 5 minutes. The moving phase was 30 mM KH2PO4-methanol (65 + 35, % v/v). TEP (1,1,3,3-tetraethoxypropane) was used as the standard solution. The results are expressed in µg/mL-1.
Statistical analysis
The statistical design was completely randomized with a split plot arrangement. The plots were the groups (I, II, and III) and the subplots the time periods (0, 7, 14, and 21 days), with five replicates (animals) per treatment. Lilliefors and Bartlett tests were performed to evaluate normality and homoscedasticity of all variables studied. However, variables such as neutrophils, aspartate alanine transferase (ALT), MPO, NAG, and MDA were log-transformed; PGE2 was log+4 transformed, and pH data were square root transformed to attain normality and homoscedasticity. Tukey’s test (coefficient of variation [CV] below 15%) and Student’s t test (CV above 15%) at 5% error probability were used to compare the means of these variables. The variables that did not exhibit normality and homoscedasticity according to the tests previously mentioned (i.e., leukocytes, eosinophils, monocytes, basophils, band cells, chloride, potassium, aspartate aminotransferase (AST), and SOD were evaluated within groups using the Friedman test (p<0.05) and between groups in each period using the Kruskal- Wallis test (p<0.05). When there were differences according to these tests, Dunn’s comparison test was used (p<0.05).
Results
There were no relevant changes in clinical parameters, complete blood count and serum biochemistry analysis of horses from all groups.
Presence of ulcers after induction was associated with the amount of PBZ used. Both groups induced with this NSAID started the treatment with CO when the gastric lesion scores were between 1 and 2 for the number of lesions and 2 for the intensity of the lesions in the glandular mucosa, with score 2 for both the number and intensity of lesions in the non-glandular mucosa. The group that did not receive NSAID in the induction period and that later received SA only had horses with score one (1) for number and intensity of lesions in the glandular mucosa. After 14 days of treatment and two gastroscopic evaluations, all ulcers from the glandular mucosa had healed in the three groups. Group II experienced earlier effects, as the ulcers were completely healed after seven days. However, the ulcers present in the non-glandular mucosa only decreased by 60 and 80% in groups II and III, respectively, and the number and intensity score increased during the treatment in these groups.
Tables 1 and 2 show the values of antioxidant and oxidant parameters, respectively. These parameters exhibited variable response among the times evaluated in each group, but the groups subjected to ulcer induction with PBZ exhibited greater variations in these parameters. At the end of the experiment, these parameters exhibited a decreased concentration or activity according to the variable. However, NO in groups I and II, NAG in the three groups and MDA in groups II and III exhibited slight but non-significant increases.
The variables CAT, NO, and MPO had no interactions with groups and time. The SOD activity decreased significantly only in groups I and II in the periods evaluated. However, NAG increased significantly in the groups treated with oil. MDA differed significantly among treatment periods in all groups but with greater increases in those groups treated with CO.
The variables analyzed in the gastric content are presented in Tables 3 and 4. The PGE2 concentrations in the three treatments and within the periods evaluated were not significantly different, although they increased in the groups treated with corn oil. The pH of the gastric content exhibited the opposite pattern, but the differences were also not significant.
Table 1 Means (± SD; CAT y ON) and medians (SOD) for the oxidant parameters of the glandular gastric mucosa of 15 horses submitted to gastric ulcer induction protocol and treated with corn oil (70-90 mL/100Kg/VO/bid, Groups II and III, respectively) and sucralfate (30 mg/Kg/VO/TID, Group I).
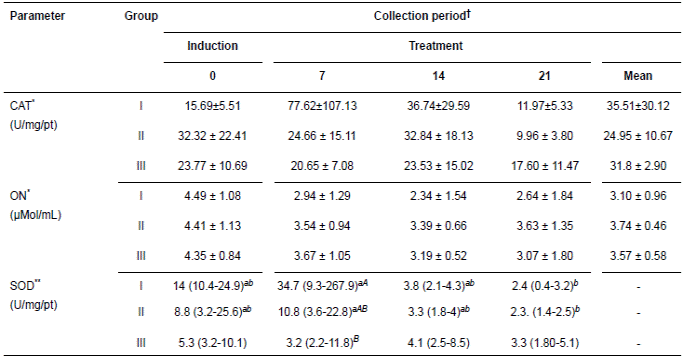
†Periods day: Induction of ulcers (day 0) and treatment (7, 14, 21). *Means followed by different capital letters in the line represent difference by t test (p<0.05).**Medians followed by different lower case letters representing the difference in line by Dunn’s test (p<0.05) groups compared in Friedman’s test (p<0.05); median followed by capital letters distinct difference in the column represent the Dunn test (p<0.05) compared to groups in Kruskal-Wallis test (p<0.05). CAT = catalase; ON = nitric oxide; SOD = superoxide dismutase; GSH = total glutathione; Uni. = Units; mg/Pt = milligram of total protein; mg/t = milligram of tissue.
Table 2 Means (± SD) for the antioxidant parameters of glandular mucosa of 15 horses submitted to gastric ulcer induction protocol and treated with corn oil (70-90 mL/100 Kg/VO/bid, Groups II and III, respectively) and sucralfate (30 mg/Kg/VO/TID, Group I).
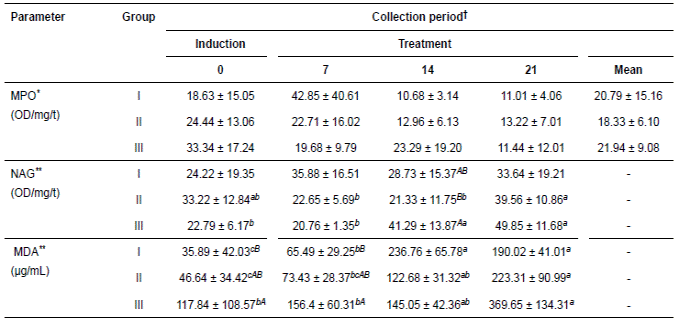
†Periods day: Induction of ulcers (day 0) and treatment (7, 14, 21). *Means followed by different capital letters in the line represent difference by t test (p<0.05). **Means followed by different lowercase letters in the row and column capitals represent difference by Tukey test (p<0.05). MPO = myeloperoxidase; NAG = N-acetylglucosaminidase; MDA = malondialdehyde. OD/mg/t = optical density per milligram of tissue.
Table 3 Mean (± SD) PGE2 concentration (pg/mL) of the gastric content of 15 horses submitted to gastric ulcer induction protocol treated with corn oil (70-90 mL/100 Kg/VO, Groups II and III respectively) and sucralfate (30 mg/Kg/VO/tid, Group I).
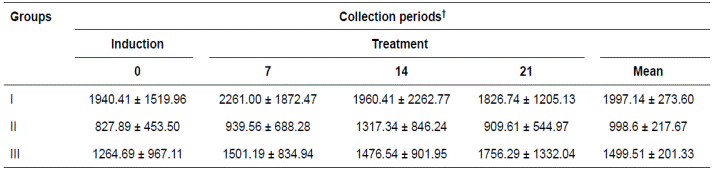
†Periods days: Gastric ulcer induction (day 0) and treatment (7, 14, 21). Means without differences by Tukey test (p>0.05).
Table 4 Mean (± SD) of pH (pg/mL) of the gastric content from 15 horses submitted to gastric ulcer induction protocol treated with corn oil (70-90 mL/100 Kg/VO, Groups II and III respectively) and sucralfate (30 mg/Kg/VO/tid, Group I).
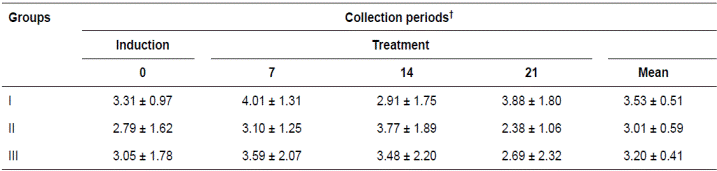
†Periods days: Gastric ulcer induction (day 0) and treatment (7, 14, 21). Means without differences by t test.
Discussion
NSAIDs are widely used in horses, and the toxic effects in this species are also widely described (MacAllister et al., 1993; Tomlinson and Blikslager, 2003; Andrews and McConnico, 2009). Despite evidence of this side effect by depletion of PG, a recent study concluded that the expression of COX1-2 genes does not change after oral administration of PBZ (Nieto et al., 2012).
However, in the induction phase of the present study, PBZ administration contributed to increasing the intensity of ulcers and to the induction of ulcers in both the glandular and non-glandular gastric mucosa in direct association with the amount administered (Martínez et al., 2015).
PGE2 concentration did not decrease after PBZ was administered, and the increase in this prostanoid was likely a result of an overexpression of constitutive and inducible cyclooxygenase (COXs) in both the glandular and non-glandular mucosa due to lesions or inflammatory process (Morrissey et al., 2010). This finding suggests other possible pathways for lesion formation in the gastric mucosa due to the use of NSAIDs (Naito et al., 1998; Polat et al., 2010). Conversely, the ulcerative lesions were not caused only by the PBZ, as the control group also developed lesions. All animals developed ulcerations before the induction treatments, possibly because of the effects of confinement and changes in management and environment, which were common circumstances for all animals and are recognized for their potential ulcerating effects (Jonsson and Egenvall, 2006; Luthersson et al., 2009; Martínez et al., 2015). The combination of all of these factors produced different ulcer classification scores for all animals in the groups.
Vegetable oils are widely used in horses as an energy supplement because of its high digestibility and tolerance (Junior et al., 2003). In addition, their anti-inflammatory and anti-ulcerogenic effects on the gastrointestinal tract, based on the increases in endogenous prostanoids, have been extensively described in both rats and humans (Grant et al., 1988; Sammon and Iputo, 2006). In horses, CO decreased the production of hydrochloric acid and increased sodium and PGE2 levels in stomachs with healthy mucosa (Cargile et al., 2004). However, Frank et al. (2005) found that ulcers in the non-glandular mucosa induced with ulcerogenic diets were not prevented. The present study demonstrated that ulcers in this area of the mucosa did not heal, although the induction methods were different and the objective was to resolve rather than to prevent the lesions, as in other studies.
One of the major components of CO is linoleic polyunsaturated fatty acid, found at a high proportion and which has high potential as a precursor of arachidonic acid and, thus, to increase production of intragastric PGE2, a tendency that has been demonstrated in rats (Grant et al., 1988), humans (Sammon and Iputo 2006) and horses (Cargile et al., 2004). In the present study, the gastroprotective effects of this prostaglandin predominated in the healing of ulcers in the glandular mucosa due to its increased concentration in all experimental groups, which indicates similar effects between treatments and control. However, the pH of the gastric content, which is directly related to PGE2, decreased in all groups but also with no significant differences. This finding may be a result of the fasting time established for the gastroscopic evaluations, for which intragastric pH measurements are recommended at shorter time intervals before and after food deprivation.
Conversely, high PGE2 expression levels from precursors such as linoleic acid has been demonstrated in diets with riboflavin and lipid deficiency and low content of omega-3 fatty acid (Sammon and Iputo, 2006). In the present study, the diet components were not analyzed to evaluate possible interactions with the PGE2 concentration induced by the CO, but it is estimated that this oil contains 60% linoleic acid.
The similarity in PGE2 concentration between the groups treated with CO and the control group that received SA may be explained by the demonstrated gastroprotective effects of SA in increasing PG and mucus production and reacting with hydrochloric acid (Gulcan et al., 2012). Thus contributing to higher pH and the resolution of ulcers in this control group. This result validates the healing effect of CO, which is similar to that of SA.
Cargile et al. (2004) used CO, and Frank et al. (2005) used rice and CO; both groups reported utility only for ulcers in the glandular mucosa, results that were similar to those found in the present study. The absence of important clinical and laboratory changes in the treated groups when compared to the control group reinforces the increased margin of tolerability of the oil by horses (Junior et al., 2003), to be used according to the complexity of the EGUS condition.
The decrease in the values of antioxidant and oxidant parameters in all groups at the end of the experiment was related to the healing of glandular mucosa ulcers, and the activities of these enzymes indicate the lesion intensity (Sanchez et al., 2002). In addition, the resolution of these lesions was confirmed gastroscopically in the last evaluation. However, NO, MDA and NAG exhibited different patterns, including the two groups treated with corn oil, perhaps as a result of the treatments. This finding is also related with the degree of the lesion caused by PBZ, which is directly influenced by the dose and frequency of administration. This relationship is associated with the lesion pathway of NSAIDs, which generates oxidative stress in the gastric mucosa (Naito et al., 1998; Martinez et al., 2015).
The pattern of the antioxidant and oxidant variables (CAT, SOD, NO, MPO, MDA, and NAG) of the control group that received SA evidenced the gastroprotective properties of this drug, possibly due to mechanisms already described in the literature, such as inhibition of the microvascular permeability, the decrease in free radicals and the increase in mucus and nitric oxide synthase activity (Abdallah, 2010; Gulcan et al., 2012). Thus, the descending dynamics of MDA in the final period indicated low lesion levels due to free radicals, as it is considered a marker of lipid peroxidation. The pattern exhibited by NAG may be related with the participation of macrophages in the healing process of the gastric mucosa during the last period.
The direct inhibition of SOD and the indirect inhibition of CAT by NSAIDs have been reported (Basiviredy et al., 2003; Martinez et al., 2015). The CO used in group II had a similar effect to that of SA in recovering the activity of SOD and CAT, considering that the degree of lesion was lower and the resolution of lesions occurred more quickly. However, the CO used in group III also reestablished the activity of these enzymes in the interim period, as was described by Lastra et al. (2002) and Odabasoglu et al. (2008) when using corn, olive and sunflower oil in the stomachs of rats. Thus, the activities of these enzymes were associated with the healing process of ulcers.
NO participates in several physiological and pathological functions and, along with PGs, contributes to conserve the integrity of the gastric mucosa. The findings of the present study were not conclusive regarding the gastroprotective behaviors of NO, which were similar in all three groups. However, slight recovery in the concentration of NO was observed in response to lesion resolution (group I and II), as NO is involved in gastric repair mechanisms (Wallace and Miller, 2000). However, the depleting effect of PBZ on NO was observed in the group that received the highest amount of NSAID without exhibiting significant recovery at the end of the treatment. The production of NO from the resolution of lesions and its depletion by the NSAID is in agreement with the results obtained by Abdallah (2010). However, the variability of this molecule may be a result of the measuring techniques used, as the production mechanisms and the biological effects depend on the location and forms of NO (Beall et al., 2012).
When comparing oxidizing parameters of the groups treated with CO with those obtained in the control group that received SA, the MPO activity showed similar decreases in the last 14 days of the experiment, with no significance, which could indirectly indicate decreased infiltration of neutrophils in the inflammatory condition, which would result in attenuation of the mucosal damage by activated neutrophils (Odabasoglu et al., 2008). The fluctuation of this enzyme in the interim periods in the groups treated with oil was a result of the inhibitory effect of PBZ, possibly similar to that described with the use of indomethacin (Odabasoglu et al.,
2008). Conversely, in the control group, the increase was a result of the inflammatory process accompanying the lesions. The NAG activity indicated macrophage infiltration in the glandular mucosa, most likely as a consequence of the dynamics of the healing process, despite intermittences during the evaluation periods.
There was a progressive increase in MDA in the groups treated with oil, which suggests lipid peroxidation by free radicals derived from the oxidative stress, as it is considered the main biological peroxidation marker of polyunsaturated fatty acids (Del Rio et al.,
2005). However, the increase in MDA apparently occurred as a result of the chemical reaction between the acid gastric environment and the specific components of corn oil, as it contrasts with the gastric health observed in the last gastroscopic examination and does not agree with the pattern demonstrated by the control group or with results reported in other studies that used olive oil (Lastra et al., 2002; Cicerale et al., 2012).
The decrease in MPO and activity of antioxidant enzymes observed with lesion resolution suggest other pathways or sources of MDA production. Enzymatic and non-enzymatic pathways have been described in the origin of this marker. The synthesis of PGs and thromboxanes may produce MDA (Hecker and Ullrich, 1989) and other hypotheses have been recently published, in which oxidative processes of linoleic acid in acid environments may exacerbate the production of this metabolite (Onyango and Baba, 2010). These mechanisms may explain the increase in MDA in treatments with oil because of its high concentration of linoleic acid, which is a precursor of arachidonic acid.
Finally, similar to all vegetable oils, CO has smaller components, such as α-tocopherol, oleic acid, sitosterol, saturated fatty acids and polyphenols, which have biological functions as they reduce the production of free radicals (H2O2 and O- 2), in addition to inhibiting COX and lipoxygenase activities (Visioli et al., 2002; Moreno 2003). This fact suggests the need of further studies to evaluate the patterns of the variables for a longer period and the effects of interactions with other oil components on the gastric mucosa of horses.
CO only had a positive therapeutic effect on lesions induced in the glandular mucosa, similar to the effect obtained with SA. Among the possible mechanisms, reestablishment of antioxidant parameters and the inhibition of the oxidant enzyme MPO were predominant, but PGE2 concentration had poor influence on the restitution of gastric epithelium. Further studies should be conducted with a greater number of animals to clarify some aspects related to activation of antioxidant defense systems of the gastric mucosa resulting from other NSAIDs used in horses.
Acknowledgements
The authors thank the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and Conselho Nacional de
Desenvolvimento Científico e Tecnológico (CNPq) for their financial support. The authors also thank the Federal University of Minas Gerais, the 2013-2014 of CODI sustainability strategy of the Universidad de Antioquia and CODI financial support for language translate, of Universidad de Antioquia.














