Introduction
Australian Stringhalt (AS) is an equine disease that occurs worldwide and it is characterized by a sudden onset of involuntary and exaggerated hyperflexion of one or both hind limbs. In severe cases, the flexed leg may hit the lower abdomen and may cause the horse to walk in a “bunny hopping” manner. Some cases may show muscular atrophy of the affected limbs and laryngeal hemiplegia. It has been reported that all four limbs can be affected but the hind limbs are usually the ones involved prominently (Cahill et al., 1985; Cahill and Goulden, 1992; Armengou et al. 2010).
Two Stringhalt types have been describedmonomelic and bilateral. The classical monomelic type usually affects only individuals and has a tendency to involve only one hind limb. It is often thought of as idiopathic in origin and in some instances it is accompanied by a history of or evidence of recent trauma to the area around the tarso-metatarsal joint (Armengou et al., 2010). The second type refers to AS that has a seasonal presentation in summer and autumn. The AS affects usually the two hind limbs bilaterally (Cahill and Goulden, 1992; Gay et al., 1993). The etiology of AS is still unknown, outbreaks of this disease have been associated to grazing pastures where Hypochaeris radicata predominates and other plants such as Taraxacum officinale and Malva parviflora may be present (Huntington et al., 1989; Cahill and Goulden, 1992). There have been reports of outbreaks of AS in New Zealand, Australia, USA, Chile, Brazil, France, and probably Japan (Cahill et al., 1986; Huntington et al., 1989; Cahill and Goulden, 1992; Slocombe et al., 1992; Gay et al., 1993; Dixon et al., 2001; Takahashi et al., 2002; Domange et al., 2010). The AS has not been reported in Colombia before.
Patient examination
Anamnesis
Five imported 48-month-old Silla Argentina horses (four geldings and one mare) with a history of gait abnormalities in the hind limbs, skin problems and weight loss, were evaluated by the authors at a quarantine facility near Bogotá (Colombia) by request of the importers. Previously, there was a history of two horses affected with similar signs that initiated right on arrival from Argentina in one horse but in the other, it started later on. One of these two horses died and it was sent for post-mortem examination, but the cause of death was not determined. The surviving horse was one of the five horses presented for clinical evaluation in the field. The onset of the gait abnormalities was variable among the horses, with variation between 15 days and 1 month before the signs were presented as well as the skin problems and weight loss. They had arrived from a quarantine center, where they stayed for a month, in the Argentinean province of Buenos Aires, in an importation of 56 horses. The affected five horses along with nine more were acquired from the same farm in same province. Once they arrived in Colombia, they were kept in individual stalls, but some were allowed to stay out on paddocks around the stables at a quarantine facility near Bogota. They were fed Pangola grass hay (Digitaria eriantha) or Angleton bluestem hay (Dichanthium aristatum) and water ad libitum.
Clinical findings
Three of the examined horses on the field had a low body condition, being one mare more affected. All affected horses had skin lesions of different severity and extension such as alopecia, hyperkeratosis and desquamation (Figures 1 and 2). The skin lesions were located mainly on the face, neck, thorax, rump, and limbs. Three horses were very nervous and hyperesthetic, making management difficult. The respiratory rate (RR), heart rate (HR), and temperature (T) were slightly elevated in four horses. At walking forward and backwards, circling and at trotting, there was a marked hyperflexion of both hind limbs in all subjects (Figures 3, 4, and 5). Based on these findings, a working diagnosis of the AS was proposed and the severity was classified according to the scale proposed by Domange et al. (2010; Table 1).

Figure 5 Clinical appearance of skin lesions characterized by alopecia, scales, and crusts over the head and thorax
Blood samples were taken for complete blood count (CBC), measure total plasmatic protein (TPP) fibrinogen, Gama-glutamil transferase (GGT), aspartate aminotransferase (AST), creatinine kinase (CK), alkaline phosphatase (AP), urea, and creatinine. The results indicated slight hyperfibrinogenemia in all cases, hemoconcentration and mild eosinophilia in two horses, the rest of parameters were within normal ranges. Thiamine at 10 mg/Kg intramuscular administration IM and/or phenytoin at 15 mg/Kg PO/ SID oral administration once a day were recommended with a high-energy diet. However, therapy was not implemented and since other affected animals appeared, it was agreed for the horses to be referred to the Large Animal Hospital at Faculty of Veterinary Medicine and Animal Science, Universidad Nacional de Colombia a few days later.
Eight horses that included the cases examined at the quarantine facility were then sent to the hospital. Horse 1 became recumbent, died before reaching the clinic, and was sent for post-mortem examination. All horses were numbered according to chronological hospital admission.
On clinical examination, all horses were very nervous but one was docile. The nervous ones were more difficult to handle and were hyperesthetic. Five horses were in mild to low body condition, the rest were in good body condition (Table 2), The stringhalt severity was also graded using the score proposed by Domange et al. (2010). All the horses had an increase in severity of the disease, over a period of 20 days, except horse 5 that remained stable (Table 2). All of them presented moderate muscle atrophy that involved lateral digital extensor, cranial tibial and lateral tibial muscles.
Table 2 Clinical findings and other features of the horses on first examination at the Large Animal Clinic, Universidad Nacional de Colombia.
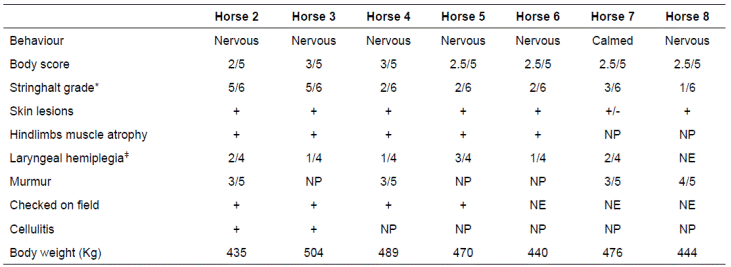
*Domange et al. (2010). ǂ Dixon et al., (2001)in contrast to the usual 4 grades. The RLN cases had a median grade 4 laryngeal paralysis, of which 96% were left-sided, 2% right-sided and 2% bilaterally affected. RLN cases included 204 (58%). NP: Not presented. NE: Not examined.
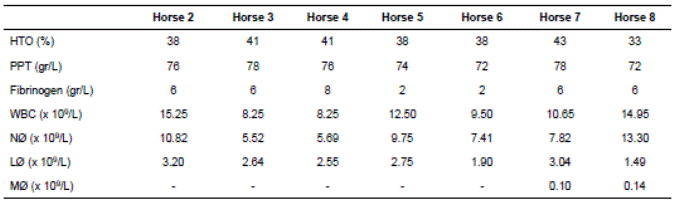
Table 3 Laboratory findings on the horses examined at the Large Animal Clinic, Universidad Nacional de Colombia.
The skin lesions observed on the animals during quarantine examination remained unchanged in all hoses, however horses 6, 7, and 8 that were not examined in the field, had similar lesions also distributed all over the body (Figures 6 and 7). It was perceived that the horses with the higher AS scores also had the most severe skin lesions.
Horses 2, 4, 7, and 8 had a systolic heart murmur that was classified 3/5 for the first three and 4/5 for the latter. Horses 2 and 3 also presented left hind limb swollen, from the fetlock up to the stifle region, with edema and painful over all the affected area, those signs were compatibles with cellulitis. Horse 5 showed a tendinitis of the deep digital flexor tendon on the left hind limb that aggravated the lameness and became more anorectic.
Diagnostic aids used
A CBC was performed on all affected horses and results were unremarkable, except horses 2 and 8 that presented slight neutrophilia (Table 3). All horses underwent standing upper airway endoscopy except for horse 8 that was intractable. The LH severity in each horse can be seen in Table 2. Three of the horses had skin biopsies performed from the different affected areas and all of them were sent to culture to rule out different skin conditions or infectious agents such as Dermatophilus congolenseis. In two horses showed a superficial pyoderma and slight eosinophilic nodular dermatitis and in one horse Staphylococcus aureus was isolated. No lesions compatible with dermatophilosis or any other skin condition was reported, neither D. congolensis was isolated. Liver ultrasound and biopsy were performed in horse 2, but no abnormalities were detected. Surface EMG was performed on three affected horses (2, 3, and 4) and one control that had no neuromuscular disease. In all three tested horses, the long digital extensor muscle of both hind limbs during flexion and standing was evaluated. Fibrillation patterns with positive waves were observed on the mentioned muscle. During flexion, the myoelectric pattern lasted longer and took more time to return to basal levels when compared to the control horse.
Treatment approach
Each horse was placed in an individual stall at the hospital and all were treated with thiamine at 10 mg/ Kg IM SID and phenylbutazone at 4.4 mg/Kg SID. The treatment lasted for 15 days with progressively decreasing the PBZ during this time.
During hospitalization, the horses were fed with Angleton hay (D. aristatum), two to three bales daily, complemented with pasture forage (Pennisetum clandestinum), and 4 to 5 Kg of concentrated feed.
The horses affected with cellulitis were treated using a “sweat” bandage for 48 hours. After removing it, four purulent draining fistulas along the cannon bone area were observed. They were also treated with the combination of trimethoprim - sulfadiazine and sodic penicillin for 10 days. For horse 2, the signs of cellulitis improved as the stringhalt signs abated, classified 5/6 at admission to 1/6 on day 20 of hospitalization. However, it continued to lose weight and it was found recumbent on day 25 of hospitalization and died. A necropsy examination was performed. Horse 8 had cellulitis that was treated similarly. Its most prominent skin lesion was treated with a dermatologic ointment made of prednisolone, neomycin, zinc oxide, and clotrimazole, and it was discharged in good condition after 7 days. The stringhalt score remained 1/6 in this horse at time of discharge.
The other five patients were turned out on a small paddock and they were hand walked daily for 20 minutes two times per day from day 8th of hospitalization onward. Horses 4, 5, 6, and 7 showed a slight improvement and on discharge, it had a score of 1/6. Horse 8 did not have any change in the degree of stringhalt. Horse 3 had very fluctuant signs with a score of 4/6 to 5/6. On a follow up at 6 months after being discharged from the clinic, the personal in charge of the horses indicated a major improvement and according to them horses 4, 5, 6, 7, and 8 were working normally.
Necropsy findings
Horse 1, which died and was unable to reach the Clinic, had a moderate difference among the muscle fibers size on the lateral extensors, the long digital extensor and the cranial tibial muscles. Sarcocystis spp. cysts were found in long digital extensor muscle. Lesions indicating peripheral axonopathy with myelin loss (i.e. Wallerian degeneration) were observed in the long nerves such as the recurrent laryngeal, tibial, deep and superficial peroneal nerves. The lesion severity varied among nerves and muscles. No CNS lesions were observed.
The histopathological findings of the long nerves in horse 2 indicated a similar Wallerian degeneration (Figure 8) as in horse 1. Histological examination of horse’s 2 skin lesions showed a chronic dermatitis with abundant granulation tissue and S. aureus was isolated from the skin cultures (Figure 8). As with the skin biopsies on live animals, no other agent or skin disease was determined.
Discussion
The typical clinical and mainly neuromuscular findings observed in AS such as: sudden onsets of signs, outbreak presentation of bilateral hyperflexion during protraction of the hind limbs (stringhalt) with different degrees of severity (Cahill et al., 1985; Huntington et al., 1989; Domange et al., 2010) were exhibited by all the cases in this outbreak. Different hypotheses have been proposed to explain the hyperflexion observed in AS (Cahill et al., 1986; Cahill and Goulden, 1992; Slocombe et al., 1992).
Stringhalt is currently thought to be associated with damage to the axons (axonopathy) in the peripheral long nerves (Cahill and Goulden, 1992; Armengou et al., 2010). Despite the existence of reports that indicate that front limbs are affected (Cahill et al., 1985), none of the patients in our report showed any involvement. The hind limb muscular atrophy observed in these cases occurs as a result of myelin loss that follows axonal disease which occurs in the peripheral nerve sensory and motor fibers (Cahill et al., 1985; Huntington et al., 1989; Slocombe et al., 1992). This also explains the presentation of laryngeal hemiplegia in AS, but as occurred in this report not all AS cases present this condition (Cahill et al., 1985; Huntington et al., 1989; Gardner, 2005; Domange et al., 2010). In addition, given the involved breed in these cases along with the prevalence of laryngeal hemiplegia; however, it is not known whether these horses presented it before the AS incident. Five out of the 8 horses referred to the Large Animal Clinic showed a poor body condition despite having a good appetite, this has also been reported by Cahill et al. (1985), but it is not known whether it is due to the toxicity of the putative agent or to the difficulties of the animals to move and procuring.
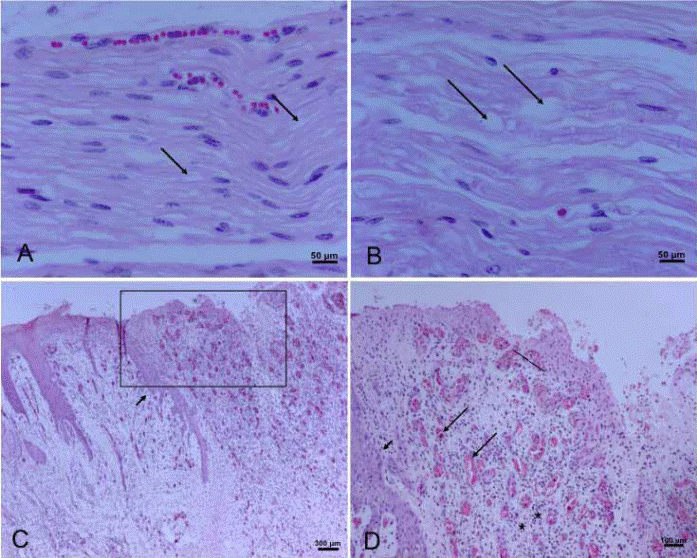
Figure 8 A-B: Peripheral nerve lesions. A) Superficial peroneal nerve. Mild to moderate presence of vacuoles (arrows). B) Recurrent laryngeal nerve. Note bigger vacuoles within the nerve fibers (arrows). C-D: Skin lesion. C) Marked epidermal hyperplasia, with papillae presence (arrow), also severe and extended ulcer with abundant granulation tissue (square). D) At higher magnification note epithelial hyperplasia (short arrow) and abundant granulation with neovascularization processes (long arrows) and inflammatory reaction presence (stars).
Other clinical characteristic of the AS cases is the presentation of behavioral abnormalities such as nervousness, hyperesthesia and inclusive aggressiveness (Cahill et al., 1985; Huntington et al., 1989; Domange et al., 2010), as it was observed in these cases. However, no further examination on neurologic system was done, due to the lack of evidence that might cause those signs. Furthermore, no pathological lesion in CNS has been observed in AS cases that could explain this behavior. These signs have been associated to a degree of incapacity but no pathophysiological explanation exists currently. There is recent evidence of CNS effects in mice fed with Hypochoeris radicata (MacKay et al., 2013).
A striking finding in these cases is the presentation of the peculiar skin lesions in several of the animals and the degree of involvement. These types of skin lesions had not been reported until the report by Domange et al. (2010), who reported two cases with skin lesions that seemed to be associated with AS as occurred in these cases. However, they did not describe what type of lesions was observed. In this outbreak, the skin pathology was characterized as chronic dermatitis with abundant granulation tissue and with some eosinophilic infiltration. There is no explanation as to the origin of the skin lesions and there is not clear association with AS or with its severity. Although clinically, it very much resembled dermatophilosis, histopathology and culture results ruled out this hypothesis.
There are indications that AS is associated strongly with H. radicata consumption, and less with other plants; however, no specific neurotoxin nor any substance has been determined to cause it. Despite that, several trials have been performed to reproduce AS by feeding H. radicata, these trials had not been successful until Araújo et al. (2008) were able to reproduce stringhalt in one horse after being fed a daily amount of 9.8 Kg of fresh H. radicata during 50 days. The main conclusion from that trial was that the toxic substance is not always present in all H. radicata plants or on all premises that have the weed growing.
This entity has not been reported up to now in Colombia despite the presence of H. radicata in the country. The possibility that the outbreak had occurred from consumption of these plants in Colombia could be ruled out because the first case showed clinical stringhalt immediately upon arrival in Colombia. All the animals were stabled, and despite the fact that some horses were outside the quarantine stalls, none of these types of plants was observed in the quarantine station in Colombia. Based on these previous points and on personal interview with the manager of the quarantine of these animals in Argentina, he indicated that there was a severe drought spell and a severe proliferation of plants that were similar to H. radicata when a picture of the plant was shown to him.
In the study by Araújo et al. (2008), there is not any conclusive information on the incubation period of the presumed neurotoxicity. Animals also show different severities of the disease in an outbreak, and some animals even start only to show signs after being withdrawn from the triggering pastures. This suggests a very erratic behavior by the possible toxin that along with the lack of accurate knowledge of the conditions in Argentina would make very difficult to ascertain the exact origin of the outbreak (Huntington et al., 1989; Araújo et al., 2008).
The severity of the clinical signs in this outbreak was variable. In some cases, marked deterioration of the patients was observed and they reached the highest degree of severity which was grade 6 as reported only by Domange et al. (2010); but in other cases, the severity of clinical signs remained stable through hospitalization and with time improvement was observed. This variability in signs and severity seems to be very characteristic of AS and has been reported by different authors (Cahill et al., 1985; Huntington et al., 1989; Domange et al., 2010). What made this outbreak different clinically from those previously reported so far in the literature was that several cases developed a clinical severity of grade 6/6 as reported by Domange et al. (2010) and the striking association with severe and multiple skin lesions.
As reported earlier (Araya et al., 1998; Gardner, 2005), clinical laboratory tests did not show any significant abnormalities except for changes resulting from concurrent diseases. The report by Araya et al (Araya et al., 1998) in Chile indicated that one horse that died from AS had hepatic fibrosis; however, in the present case no hepatic involvement was detected by either hepatic function laboratory tests or histopathology of liver tissue obtain by biopsy or at necropsy.
Electromyography has been used previously to evaluate muscle and neurological activity in horses with AS (Gardner, 2005; Huntington et al., 1989). We observed fibrillation patterns with positive waves that indicates that a neuropathy was present as shown in other cases of AS (Huntington et al., 1989; Wijnberg et al., 2009) referred to the Veterinary Clinical Centre (n = 13). It was also observed that the measurements done during limb flexion and standing, is similar to the activity in horses affected with AS that is characterized by an increased tonic activity while standing and the increased in the duration of this pattern after hind limb flexion (Huntington et al., 1989).
Thiamine and its metabolites are an important factor in the energy metabolism in the neurons (Ang et al., 2008) and given the fact that it has been used in other cases (Huntington et al., 1989) it was used in the present outbreak. However, there is no way to ascertain its usefulness, particularly because of the fact that animals with AS improve spontaneously in time over days to months to years (Cahill et al., 1985; Huntington et al., 1989; Domange et al., 2010).
Necropsy findings showed characteristic lesions of AS (Slocombe et al., 1992). However, there is no clear understanding why the two horses died. These horses developed grade 6/6 AS score and Domange et al. (2010) described that horses with grade 6/6 go into recumbence and die.
The 6-month follow up after discharge indicated that four horses were performing up to their intended use as police horses. And such recovery has been reported by others (Cahill et al., 1985; Huntington et al., 1989; Gardner, 2005).
Conclusion
This is the first report of an outbreak of AS in Colombia and it includes a complete description of the clinical picture. The affected horses showed all the clinical degrees described for the first time by Domange et al. (2010). Despite the clinical similarities of the skin lesions with dermatophilosis, this bacterial agent was not isolated nor observed on histopathology. No plausible explanation as to the mechanisms involved in the occurrence of the skin lesions in these AS cases was provided.