Introduction
Dietary phosphorus (P) is essential for good performance and mineralization of bones in pigs, but its high cost imposes the need for appropriate strategies regarding its inclusion in formulations. Monocalcium, mono-dicalcium, dicalcium and tricalcium phosphates are inorganic P sources, and are all used in the formulation of pigs diets. The use of these sources varies according to its physical form, chemical composition and presence of contaminants (Rostagno et al., 2005). Rock phosphates are alternative sources of P in animal nutrition due to their low cost. In fact, rock phosphates are not subjected to manufacturing processes, which is a relevant problem, considering the difficulties to maintain the purity and chemical composition of this product.
In the adult pig, approximately 25% of the total P is encountered in soft tissues, and the remaining P is stored in the bones, which provide structural strength and stability to the animal (Reese et al., 2010). Because of its tridimensional arrangement, mechanical and structural properties of bone tissue are continuously adapting, and its main mineral components are crystals of calcium (Ca) and P (Nikodem et al., 2012).
Mineral supplementation of animals should be optimized for proper bone tissue integrity, prevention of fractures and other lesions during the development, transport, slaughter and conditioning of the carcasses. However, few studies (Teixeira et al., 2004a; Souza et al., 2009) have been published analyzing bone parameters affected by different sources of P in pig diets. Changing the inorganic P source for pigs can cause a change in bone metabolism, altering the characteristics of bone tissue and the incidence of damage, such as fractures. Thus, the objective of this study was to evaluate the effect of different P sources on bone characteristics of pigs.
Material and methods
Ethical considerations
This experiment was approved by the Ethics Committee of the Department of Animal Production of Universidade Federal de Viçosa (UFV), in Viçosa, Minas Gerais State, Brazil (code number 50405449982). All procedures used in this study were in compliance with ethics principles of the Brazilian College of Animal Experimentation (COBEA, 2014).
Husbandry and diets
One-hundred and twelve pigs with 28.65 ± 2.82 Kg initial average weight were distributed into an 8×2 factorial arrangement (eight sources of phosphorus × two sexes), in blocks, in a completely randomized design. Pigs were placed in 56 growth and finishing stalls according to sex and weight, with four replicates for males and three replicates for females, containing two animals per experimental unit.
Diets were formulated with different P sources, as follows: control diet without inorganic P (CTR); control diet + dicalcium phosphate (DCP); control diet + mono-dicalcium phosphate (MDCP); control diet + triple superphosphate (TSP); control diet + single superphosphate (SSP); control diet + rock phosphate (ROCK); control diet + phosphoric acid (PPA); and a diet called “MIX”, constituted by the control diet + a mixture of sources (65% TSP + 30% ROCK + 5% PPA; Table 1). Samples of P sources were analyzed according to the method described by Teixeira et al. (2004a).
Table 1 Analyzed mineral composition.

CTR: control diet; DCP: dicalcium phosphate (CaHPO4); MDCP: mono-dicalcium phosphate (Ca(H2PO4)2); TSP: triple superphosphate (Ca(H2PO4)2H2O); SSP: single superphosphate (Ca(H2PO4)2.H2O + CaSO4.2H2O); ROCK: “Catalão”-rock phosphate (Ca5F(PO4)3); MIX: mixture of sources; PPA: phosphoric acid (H3PO4). nd: not determined.
Calcium:phosphorus (Ca:P), and phosphorus:fluorine (P:F) ratios of the phosphorus sources
The diets for the growth (30 to 60 Kg) and finishing (60 to 90 Kg) phases were formulated to attend the requirements (Rostagno et al., 2005; Table 2), with ad libitum access for the animals. Diets were isoproteic, containing 17.9 and 16.8% crude protein (CP) for the growth and finishing phases, respectively, and isocaloric (3,400 Kcal/Kg digestible energy). The experimental diets were formulated on the basis of total P values, containing practically equal amounts of P, with 0.56 and 0.42% in the growth and finishing phases, respectively.
Mineral composition of bone and morphometric analyses
At the end of the finishing phase, after a 24-hour feed-deprivation period, one animal with weight close to the average of the block was slaughtered per experimental unit. Subsequently, the third metacarpal bone of the forelimb was selected to weight and measure its length and diameter. Bone strength was analyzed using the same material, with the methodology adapted by Saraiva et al. (2009).
After breaking, bones were defatted in a Soxhlet extractor and dried in a ventilation oven at 55 °C for 72 hours. Subsequently, they were ground in a ball mill through a posterior drying in an oven at 105 °C for 24 hours.
The ash content was determined in a muffle furnace at 600 °C. Calcium (Ca) and magnesium (Mg) concentration in bones was obtained by atomic absorption spectroscopy, while phosphorus (P) was determined by colorimetry. Fluorine (F) analyses were performed by potentiometry. All analytical methodologies were conducted at the laboratory of Rodes Química Cajati Ltda. (Cajati, São Paulo, Brazil). All minerals content was determined according to the methodology described by AOAC (2007).
Table 2 Composition (dry matter basis) and nutritive value of the diets for pigs in the growth and finishing phases.
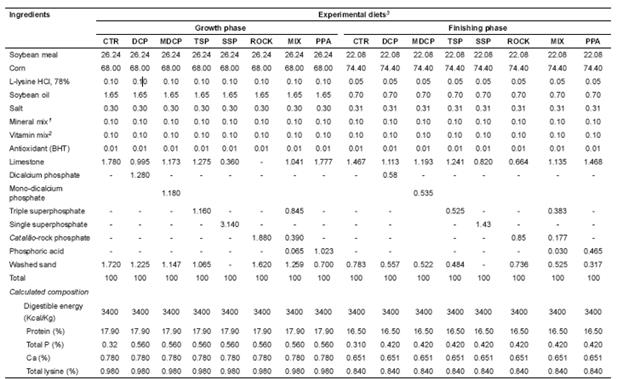
1 Content g/Kg: 100 Fe; 10 Cu; 1 Co; 40 Mn; 100 Zn; 0.3 Se; 1.5 I; 1,000.
2 Content/Kg: 6,000,000 IU/Kg vit A; 1,500,000 IU/Kg D3; 15,000 UI/Kg E; 1.35 g/Kg B1; 4 g/Kg B2; 2 g/Kg B6; 9.35 g/Kg pantothenic acid; 1.5 g/Kg vit K3; 20.0 g/Kg nicotinic acid; 20.0 g/Kg vit B12; 0.6 g/Kg folic acid; 0.08 g/Kg biotin; 1,000 g/Kg
3 CTR: control diet; DCP: dicalcium phosphate; MDCP: mono-dicalcium phosphate; TSP: triple superphosphate; SSP: single superphosphate; ROCK: Catalão-rock phosphate; MIX: mixture of sources; PPA: phosphoric acid.
Histological characterization
A fragment of the sixth rib, approximately 1 cm, was collected from each animal through a cut 10 cm below the costovertebral joint. This procedure was made for posterior histological analysis. Rib fragments were washed in physiological solution (saline), fixed in Bouin solution for 24 hours, dehydrated in ethylic alcohol, demineralized in an ethylenediaminetetraacetic acid (EDTA) solution, diaphanized in xylene and included in paraffin. Seven (7) μm thickness cuts were made with a microtome, and cuts were stained with hematoxylineosin (HE).
For the histological study of rib bones, 140 observations were performed per treatment at 20 distinct sites on the surface of the cortical bone. This procedure was developed to determine periosteum thickness, from the muscle tissue until the surface of the compact layer, and thickness of the compact tissue, which includes the periosteal surface until the trabecular bone (Junqueira, 2004; Figure 1).
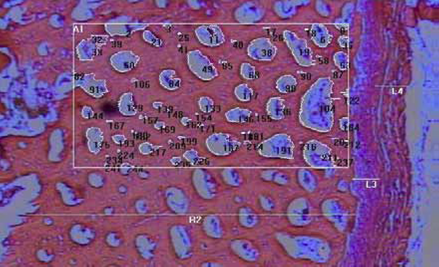
Figure 1 Cross section of a pig rib (pig weight: 90 Kg). Details: (L4) muscle tissue; (L3) periosteum; and (R2) compact tissue, in which an area (A1) and demineralized zones (number) are highlighted. Magnification: 40 X, Color: hematoxylin-eosin (HE).
Porosity was determined by randomized measurements involving five areas on the compact tissue, which furnish the identification of the total areas and the respective areas occupied by Haversian canals and demineralized areas. Subsequently, the specific ratio was established to quantify porosity, according to the following relationship:
Porosity = demineralized area (μm2) x 100 x (total area (μm2))-1.
Histological analyses were performed using a microscope with 40 X magnification coupled to an image analyzer Image-Pro Plus (The Proven Solution; Media Cybernetics Inc., Silver Spring, MD, USA).
Statistical analysis
Variables were analyzed using the varianceanalysis procedures, employing the SAS software, Version 6 (SAS Institute, Inc, Cary, NC, USA). Each experimental unit consisted of two animals. In case of difference, the means of treatments containing different P sources were compared by Student-Newman Keuls’ test (p<0.05). The means of treatments containing different sources of P were compared with the control treatment (CTR) by Dunnett’s test (p> or <0.05).
Results
The treatment had no effect on feed intake in any of the periods (p>0.05; data not shown).
Morphometric parameters
No interaction was observed between P source and sex (p>0.05), and P source had no effect on morphometric parameters and bone strength, which were evaluated according to the sex of pigs (p>0.05; Table 3).
Table 3 Morphometric parameters of the third metacarpal bone of finishing pigs fed different phosphorus sources.
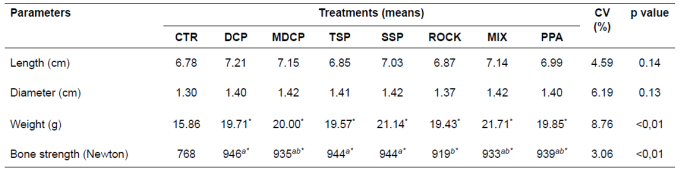
Means followed by different superscript letters (a,b) within rows differ by SNK’ test (p<0.05).
*Different means (> or <) in relation to CTR diet (negative control), by Dunnett’s test at 1%.
CTR: control diet; DCP: dicalcium phosphate; MDCP: mono-dicalcium phosphate; TSP: triple superphosphate; SSP: single superphosphate; ROCK: Catalão rock phosphate; MIX: mixture of sources; PPA: phosphoric acid.
The diets containing supplemental P sources did not affect (p>0.05) length or diameter of the third metacarpal bone. The control diet, on the other hand, resulted in lower (p<0.05) bone weight and resistance compared to the other treatments. However, piglets fed DCP, TSP, and SSP showed more resistant bones than those fed ROCK, whereas the MDCP, MIX and PPA diets did not differ from the other P sources (Table 3).
Mineral composition of bone
There were not treatments by sex interaction and no difference was observed (p>0.05) between male and female in several parameters, such as fat, ash, Ca, P, Mg and F contents in bone of pigs fed different P sources during growth and finishing (Table 4).
The experimental diets did not affect (p>0.05) the percentage of bone fat, but they influenced (p<0.05) ash, P, Ca, Mg, and F concentrations. Ash content in dry matter was greater (p<0.05) in bones of animals fed DCP. Ash content for SSP, ROCK, MIX and PPA diets was intermediate (p>0.05) and mutually similar, with the lowest value for MDCP and TSP. The P content was lower (p<0.05) in animals consuming PPA. The P contents for MDCP, SSP and ROCK were intermediate (p<0.05) and reciprocally similar. The highest value was obtained for DCP, TSP and MIX. Only the bones of pigs fed MDCP and PPA showed similar (p<0.05) Ca content in comparison with the control diet. The other treatments presented higher levels of Ca compared with the control diet.
Table 4 Bone mineral composition (dry matter basis) of the third metacarpal bone of finishing pigs fed different phosphorus sources.
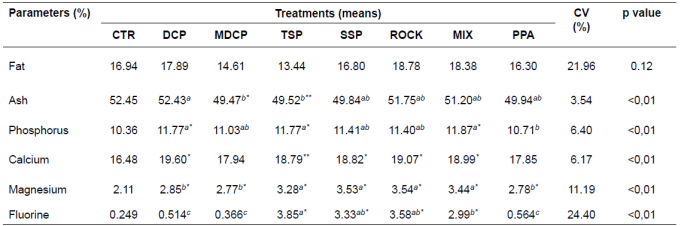
Means followed by different superscript letters (a,b) within rows differ by SNK’ test (p<0.05).
*Different means (> or <) in relation to CTR diet (negative control), by Dunnett’s test at 1%.
**Different means (> or <) in relation to CTR diet (negative control), by Dunnett’s test at 5%.
CTR: control diet; DCP: dicalcium phosphate; MDCP: mono-dicalcium phosphate; TSP: triple superphosphate; SSP: single superphosphate; ROCK: Catalãorock phosphate; MIX: mixture of sources; PPA: phosphoric acid.
Considering bone magnesium, the lowest concentration (p<0.05) was observed in animals consuming DCP, MDCP and PPA, and the highest values (p<0.05) were for animals fed TSP, SSP, ROCK and MIX. In regard to fluorine (F) content in bone, the lowest value (p<0.05) was for animals consuming DCP, MDCP, and PPA, and differed (P<0.05) from those receiving MIX, TSP, SSP, and ROCK.
Histological characterization
There was no interaction (p>0.05) between P source and sex. Indeed, no effect of sex was observed in periosteal thickness or thickness and porosity of rib bone compact tissue (Table 5). Periosteum was thicker (p<0.05) in bones of animals that consumed TSP, but it was not different (p>0.05) in those receiving DCP, MDCP, SSP, ROCK, and PPA. The lowest value (p<0.05) was observed in animals consuming MIX. Regarding thickness of the compact bone tissue, the lowest value (p<0.05) was observed in pigs fed DCP, MIX, and PPA, differing (p<0.05) from the animals that consumed SSP, TSP, and ROCK.
Table 5 Histological characterization of ribs in finishing pigs fed different phosphorus sources.
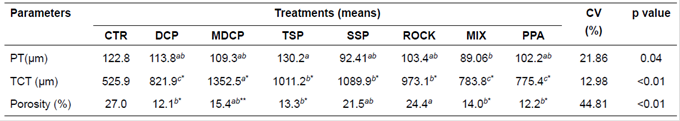
Means followed by different superscript letters (a,b) within rows differ by SNK’ test (p<0.05).
*Different means (> or <) in relation to CTR diet (negative control), by Dunnett’s test at 1%.
**Different means (> or <) in relation to CTR diet (negative control), by Dunnett’s test at 5%.
CTR: control diet; DCP: dicalcium phosphate; MDCP: mono-dicalcium phosphate; TSP: triple superphosphate; SSP: single superphosphate; ROCK: Catalãorock phosphate; MIX: mixture of sources; PPA: phosphoric acid. Parameters: PT: periosteal thickness; TCT: thickness of the compact tissue
Porosity of the compact tissue was greater (p<0.05) in bones of pigs fed ROCK, but was not different (p>0.05) compared with animals fed MDCP and SSP, whereas the lowest (p<0.05) porosity was observed in animals consuming DCP, TSP, MIX, and PPA.
The control diet resulted in the lowest (p<0.05) thickness of the compact bone tissue when compared with the other treatments containing a supplementary P source. Bones of animals that received the control diet showed greater (p<0.05) porosity than those fed DCP, MDCP, TSP, and PPA.
Discussion
Morphometric parameters
Bone weight and resistance was similar for the treatments containing inorganic P sources, which means that P supplementation was, indeed, nutritionally efficient. Bone strength values for treatments with inorganic sources were close to those reported by Saraiva et al. (2009) at the highest P level tested by those authors, and this variable increased linearly with P addition to the diet. In contrast, Viana et al. (2013) evaluated different levels of available P and did not observe effect of supplementation on bone strength of the third metatarsal.
With respect to the bone resistance, the ranking of P sources in the present work resulted similar to that based on P bioavailability values for the same inorganic sources evaluated by Teixeira et al. (2004a). Thus, it is reasonable to consider that although the diets contained the same levels of total P, the animals used different amounts for tissue building. The different bone resistance obtained is related to distinct bioavailabilities associated with each P source. In the same context, Nikodem et al. (2012) compared diets containing dicalcium, monocalcium, and calciumsodium phosphate, and their results indicated greater values for mechanical parameters associated with DCP, as a consequence of Ca accumulation in compact bone. Resistance to fracture correlates with the mineral density of the material. However, the use of data on bone fracture in a specific anatomical region, aiming to estimate the risk of fracture in another anatomical region, is not a trivial task, even if it is in the same animal, thus, it is difficult to predict fracture sites in commercial pigs (Nielsen et al., 2007).
Mineral composition of bone
Except for the pigs fed MDCP and PPA, all treatments generated higher bone Ca content than those receiving no inorganic P supplementation. Therefore, it can be inferred that the greater presence of P favored Ca fixation in bone, and, consequently, produced greater bone resistance in the animals receiving considerable P supplementation. This happens because P atoms in the different chemical compounds can act as Lewis bases for metal cations such as Ca2+ (Moreira et al., 2016). Alebrante et al. (2011) reported an increase in P, Ca and ash contents in bones of growing pigs. In fact, P, Ca and ash levels increased with available P in the diet, provided by the addition of dicalcium phosphate. Varley et al. (2011) reported that ash, Ca, and P contents in metacarpus were greatest in pigs fed high P levels.
The lower P content in bones of animals consuming phosphoric acid (PPA) could reflect that this compound is a weak acid, with pKa values of approximately 2.2, 7.1, and 12.4. Thus, phosphoric acid (H3PO4) would not be triply ionized under physiological pH conditions, meaning that the most abundant species in the physiological medium would be H2PO4- and HPO4 2-, which are in significant concentrations at pH close to neutrality (second pKa value = 7.1). In fact, because HPO4 2- is a divalent ion, it should have an extensive electrostatic interaction with the divalent Ca2+ cation; i.e., it would be an efficient Lewis base for a nucleophilic attack performed by the calcium ion (Moreira et al., 2016).
The different bone P contents in this study disagree with results by Teixeira et al. (2004c), who did not find differences in P contents among diets containing different inorganic sources of this mineral. Saraiva et al. (2011) also reported that available P did not have a significant influence on P deposition in bones.
Most P sources provided higher Ca than those diets without inorganic P supplementation. Nevertheless, the rates of Ca and P deposition in bones was similar among treatments (1.60), suggesting that metabolism tends to maintain Ca/P ratio in bones with low quantitative variation, independently of its ratio in the supplement. Consequently, even in phosphates whose ratio is quite far from 1.60, usually found in bones, pig metabolism tends to fix Ca and P so as to maintain a constant ratio. Saraiva et al. (2011) observed greater Ca deposition in bone when available P increased, corroborating our suggestion that a Lewis’ acid-base interaction between the cation of the alkaline earth metal (Ca2+) and the phosphate group present in the additives would be a decisive factor for Ca fixation in bone (Moreira et al., 2016).
It is interesting to note that, just as occurred with calcium, magnesium also had low fixation with phosphoric acid as P supplement. In fact, Mg2+ cation is also an alkaline earth metal, such as Ca2+, displaying a chemical behavior, like Lewis’ acid, similar to that of calcium divalent cation. Furthermore, in this similar chemical behavior, concomitant presence of both cations (Ca2+ and Mg2+) can occur in many biological environments.
The lowest value of fluorine (F) in bones of animals that consumed mono-dicalcium phosphate and phosphoric acid reflects the low levels of this mineral in their sources and diet. Fluorine is an important mineral because it is closely related to mineralized hard tissues, where it replaces hydroxyapatite by fluorapatite (Barbosa et al., 1993). This F fixation in bones occurs because fluoride anion (F-) is a Lewis’ base, considered a “hard” base according to Pearson’s concept, thereby, it is greatly related to “hard” acids (Pearson), such as Ca2+ present in bone hydroxyapatite. It should be noticed that hydroxyapatite is formed by crystalline Ca phosphate (Ca10 (PO4)6(OH)2) and represents 99% of body Ca and 80% of the total P in the organism.
It should be considered that the high levels of F observed in bones of animals fed TSP, SSP, ROCK and MIX can pose a safety limitation due to toxicity risk, according to NRC (2005) recommendations. Ingredients for animal feeds must not contain more than one part fluorine to 100 parts P (Table 1). Souza et al. (2009) tried to assess the relative bioavailability of P with three rock phosphate samples containing 1.7, 1.4 and 3.6% fluorine. They concluded that low animal performance and bone strength related to toxicity should be expected if rock phosphates are used to feed pigs. Moreover, P bioavailability -based on weight gain, bone ash, and breaking strength of metacarpals and metatarsals was 49, 49, and 28%, respectively.
Histological characterization
Bone structures with a high degree of porosity can limit its ability to resist impacts, increasing the risk of fractures during slaughter. Based on the statistical similarity among sources, it can be inferred that porosity (Table 5) is related to P contents in metacarpus (Table 4), which may, in turn, affect bone resistance.
Necrosis was observed in some osteons in the cortical layer of bones from animals fed the control diet. This process generates large resorption cavities containing active osteoclasts, and disorganization of the Haversian system in compact bone, with osteon layers interspersed by cancellous bone layers, showing irregularity in formation of bone tissue.
In terms of mineral concentration in metacarpus as well as histological characterization of rib bones, the differences found among treatments are possibly related to differences in deposition of each anatomical region, as observed by Lopes et al. (2009). According Viana et al. (2013), P mobilization of bone occurs differently under bone type, once spongy bones - ribs, vertebrae and externum - are the first affected during dietary P deficiency, while long bones such as humerus, femur, tibia, and the small bones of the extremities - e.g. metacarpals and metatarsals- are the last reserves used.
Animals fed diets with Catalão-rock phosphate had a tendency to be adversely affected, since results did not differ from the control diet with respect to rib porosity, ash and P in metacarpus. Likewise, Teixeira et al. (2004b) concluded that diets supplemented with rock phosphate negatively affected the rates of mobilization, capture and retention of P in the tissues.
In conclusion, feeding pigs with diets containing dicalcium phosphate, monodicalcium phosphate, triple superphosphate, mix of sources and phosphoric acid results in similar bone characteristics; while the use of less elaborate P sources, such as Catalão-rock phosphate and single superphosphate were less efficient in improving bone integrity.














