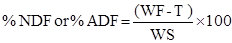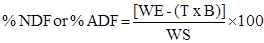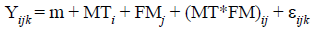Introduction
There is an increase challenge to develop efficient and low-cost analytical methodologies (Bialowas et al., 2006; Gerbase et al., 2006). To make sure that an analytical method generates reliable information from a sample, it must be validated through a process starting from the planning of the analytical strategy and goes on through its practical development (Inmetro, 2007). Analytical determination of neutral detergent fiber (NDF) and acid detergent fiber (ADF) contents are important variables analyzed in a ruminant laboratory. The conventional method by Van Soest has a large feedstuffs database with precise results (Jung, 1997; Silva and Queiróz, 2002). However, reagents are costly and laboratory routine requires long runtimes due to manual steps. In an attempt to optimize this methodology, several alternative methods have been adopted in laboratory routines, but often without ensuring reliability of the results, which requires a systematic evaluation of the analytical procedure to demonstrate its precision and accuracy. The use of autoclaves instead of the conventional digester is an alternative method recommended for NDF and ADF analyses (Deschamps, 1999; Pell and Schofield, 1993; Senger et al., 2008). Through this system, it is possible to analyze the samples collectively, without requiring individual control of the samples. In this system, samples can be weighed both in filter crucibles or small bags, and the analyses can be conducted in a sequential or non-sequential form (Komarec, 1993). Some authors have suggested that fiber analyses should be performed sequentially on high-pectin forages (Van Soest et al., 1991). Additionally, TNT (non-woven textile) bags have been preferentially used to reduce the costs of analysis (Casali et al., 2009). The aim of this study was to evaluate different methodologies for NDF and ADF quantification by assessing the analytical data.
Materials and methods
The experiment was conducted at the Laboratory of Animal Nutrition of UNESP, Campus Jaboticabal - SP, Brazil. Six feedstuffs were evaluated: five roughages - Tifton 85 hay (Cynodon spp.), sugarcane (Saccharum officinarum L.), corn silage (Zea mays L.), xaraes grass (Brachiaria brizantha cv. Xaraés) and marandu grass (Brachiaria brizantha cv. Marandu) - and one protein concentrate - babassu meal (Orbignya phalerata). Except for babassu meal and Tifton 85 hay, all samples were pre-dried, following the procedure described by Silva and Queiroz (2002).
The NDF and ADF solutions were prepared following the methodology proposed by Van Soest et al. (1991). Decalin and sodium sulfite were not used. For measuring amylase-treated NDF of corn silage, 50 μL/g of DM of term stable alpha-amylase were used (Novozymes, Araucária, PR, Brazil). In each analytical method, fifteen liters of each detergent solution were prepared separately and at once, aiming at the precision of results and minimizing possible errors during quantitative determinations. Acetone and deionized water were used as solvents for washing the samples. Repeatability was calculated to verify precision of the analyses, representing the concordance between results from consecutive measurements of the same method performed under the same measuring conditions (repeatability conditions): same procedure, same analyst, same instrument used under the same conditions and place (Inmetro, 2000).
The analyses developed by the conventional methodology followed the method modified by Van Soest et al. (1991). In each determination, around half gram of sample was weighed, adding 100 mL of detergent solution (acid or neutral) in every digestion step, and lead to boil for one hour. The analytical results were obtained considering sample weight -being a gravimetric quantitative determination- through the following formula (1) for the determination of the NDF or ADF contents:
Where:
WS = dry matter weight of the sample in grams;
WF = weight (grams) of the crucible plus detergent fiber residue after digestion and drying; T = tare (initial weight) of the crucible (grams).
The three alternative methodologies using autoclave (Deschamps, 1999; Pell and Schofield, 1993; Senger et al., 2008) are simplifications of the original analytical procedures, without altering the principles of the method proposed by Van Soest (1963, 1967). The alternative methods differed as to the material used for conditioning the samples during analysis. For the purpose of organization, the alternative methods were named as follows:
Alternative method 1 - autoclave/ANKOM bags
Alternative method 2 - autoclave/TNT (non-woven textile) bags
Alternative method 3 - autoclave/filter crucibles
Alternative methods 1 and 2, using small bags for sample conditioning and autoclave as digester, followed the recommendations by Komarek (1993) as to use bags instead of filter crucibles, and by Pell and Schofield (1993), Deschamps (1999) and Senger et al. (2008), concerning the use of autoclave. The time and temperature in the autoclave followed the best result proposed by Senger et al. (2008).
The ANKOM bags were acquired ready for use, whereas the non-woven textile (TNT) bags were produced manually, using 100-micron (µ) TNT and a mold with the dimensions of the ANKOM bags (5 x 5 cm).
The digestion step in alternative methods 1 and 2 was done in autoclave for 40 minutes, at a temperature of 110 ºC (Senger et al., 2008). In this stage all sealed bags containing the samples were conditioned in a single plastic beaker (capacity of 2 L) and immersed in 600 mL detergent solution (neutral or acid). All bags were washed in a beaker (2 L capacity) with hot (90-100 ºC) water three times (5 min each). Bag residues were soaked two times in acetone for 5 min. All bags were collectively washed. Concentration of NDF and ADF was calculated using the following formula (2):
Where:
WS = dry matter weight of the sample (grams);
WE = weight (grams) of the bag plus residue of detergent fiber after digestion and drying; T = tare (initial weight) of the bag (grams);
B = blank value (grams; final weight of the bag after drying/initial weight of the bag).
The alternative method 3 used filter crucibles for sample conditioning (Van Soest et al., 1991). Around half gram of each sample was weighed in the filter crucibles in triplicate, which were coupled to individual plastic beakers, adding 600 mL detergent solution (neutral or acid), inside the autoclave containing water for the digestion process. Digestion occurred in 40 minutes. Then, crucibles were immediately washed. Washing, drying and weighing the crucibles with the residue followed the same procedure as the conventional method. NDF and ADF contents were calculated as the difference between the tare of the crucible and the crucible weight plus detergent fiber residue after digestion and drying, using the formula defined in (1).
The analyzes performed by the conventional or alternative methods were conducted considering two different laboratory sequences: the first, known as non-sequential order, involves two weighings of the same sample and proceeding to the NDF and ADF analysis separately; while in the second sequence, known as sequential, a single sample is weighed to determine NDF. Then, we used the NDF residue to determine ADF content by washing, filtering and oven-drying.
The design was completely randomized, in a 4 x 2 factorial arrangement (4 methodologies and 2 sequences of analysis). The statistical model was:
Where:
Yijk = NDF and ADF contents;
(= overall effect of the mean;
MTi = effect of method i;
FMj = effect of sequence j;
(MT*FM)ij = effect of the interaction between method i and sequence j;
(ijk = residual error.
The normality test of error used was Cramer-von-miser’s (α=5%), and the homoscedasticity test was Levene’s (α=5%). The data were subjected to analysis of variance through General Linear Models (GLM) of the Statistical Analysis System software (SAS version 9.1®, Statistical Analysis System Institute, Cary, NC, USA, 2002). Means were compared by Dunnett’s test, having the conventional method as “control” (CMT, α = 5%).
Results
After obtaining the analytical results, the means by each alternative method were compared with those obtained by the conventional method. There was significant difference between methods for all the studied feedstuffs (Table 1; (p<0.01)). The sequence of analytical procedure (non-sequential or sequential) did not differ (p(0.05). No significant interaction was observed (p(0.05) between method and sequence of analysis for all feedstuffs (Table 1). Variation observed in NDF content in hay was 78.84% (alternative method 1) and 81.82% (alternative method 3). NDF content in sugarcane varied from 49.73% (alternative method 2) to 53.68% (alternative method 3). NDF in corn silage was different from the conventional method (NDF=42.9%, p=0.01) compared to alternative method 1 (NDF=55.11%), alternative method 2 (NDF=48.21%), and alternative method 3 (NDF=49.54).
Table 1 Means observed in the analysis of variance in feedstuffs, sequence (SE) and methods studied for evaluating NDF contents.
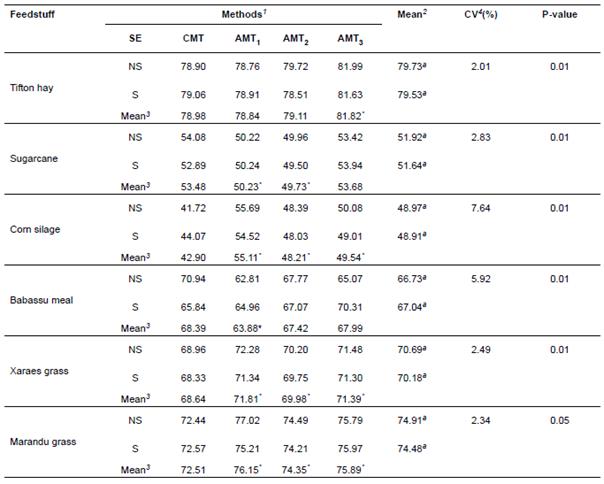
1 SE = Sequence (NS = Non-sequential; S = Sequential); CMT = Conventional method (block digester/filter crucibles); AMT 1 = Alternative method 1 (autoclave/ANKOM); AMT 2 = Alternative method 2 (autoclave/TNT); AMT 3 = Alternative method 3 (autoclave/filter crucibles). 2 Means in column of sequence (NS and S) followed by the same letter do not differ by the F test (α=0.05). 3 Means in rows of methods AMT 1 , AMT 2 and AMT 3 followed by asterisks (*) differ from CMT by Dunnett’s test (α=0.05).
ADF content differed among methods in all feedstuffs studied (p<0.01), but did not differ in the analytical procedure (non-sequential or sequential) for all feedstuffs (p<0.01). As for the interaction between method and sequence of analysis only corn silage was different (p<0.01).
The means of ADF content obtained in the analyses of the six feedstuffs studied were calculated, considering the analytical methods and sequences (Table 2).
Table 2 Means obtained in the analysis of variance of feedstuffs, sequences (SE) and methods studied at the evaluation of ADF contents.
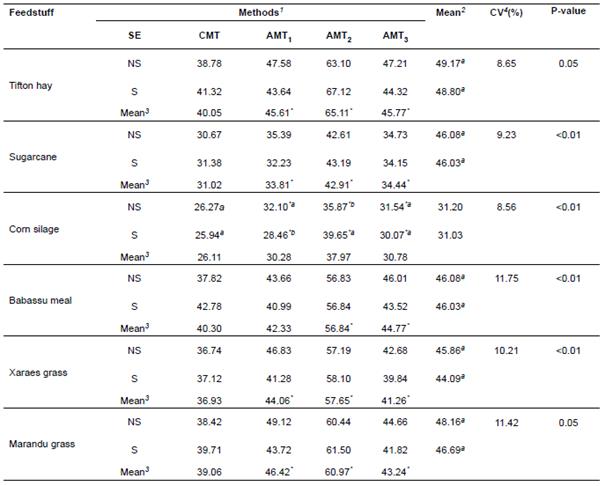
1 SE = Sequence (NS = Non-sequential; S = Sequential); CMT = Conventional method (block digester/filter crucibles); AMT 1 = Alternative method 1 (autoclave/ANKOM); AMT 2 = Alternative method 2 (autoclave/TNT); AMT 3 = Alternative method 3 (autoclave/filter crucibles). 2 Means in column of sequence (NS and S) followed by the same letter do not differ by the F test (α=0.05). 3 Means in rows of methods AMT 1 , AMT 2 and AMT 3 followed by asterisks (*) differ from CMT by Dunnett’s test (α=0.05).
The same analytical pattern was observed in ADF determinations for Tifton hay, sugarcane, xaraes grass and marandu grass. All alternative methods differed (p<0.05) from the conventional method, especially alternative method 2, which had greater mean compared to the other methods. Alternative method 1 was recommended for determining ADF contents in babassu meal, with no loss of analytical precision.
Corn silage was the only sample presenting significant interactions between method and sequence of analysis (p<0.05). In all non-sequential and sequential analyses there was significant difference (p<0.05) between alternative and the conventional method. There was no difference (p>0.05) as to the sequences of analysis within alternative method 3, but there was loss of analytical precision. Therefore, the use of alternative methods (1 and 2) is not recommended for the assessment of ADF contents in corn silage.
The results from all analyses did not differ as to the sequence (non-sequential or sequential), except for ADF determination in corn silage by alternative methods 1 and 2, which use autoclave digestion, and ANKOM and TNT bags, respectively.
Discussion
We observed that alternative methods depend on the feedstuff analyzed when compared to the conventional method. For NDF determinations, alternative method 1 is recommended for the analyses of Tifton hay; alternative method 2 for analyses of Tifton hay and babassu meal; and alternative method 3 for sugarcane and babassu meal. NDF in hay was similar to that reported by Rodrigues et al. (2006) in plants at 28 days (80.80%) and 70 days of age (80.70%). NDF content in sugarcane was similar to that reported by Santos et al. (2008) at different cutting ages, 11 months (48.60%) and 24 months (56.88%), and by Rodrigues et al. (1997), who found variations from 45 to 56% in 11 sugarcane isolates.
NDF values in corn silage are in accordance with the 55.44% observed by Valadares Filho et al. (2006). Fox et al. (1990), Van Soest (1994) and Carvalho (1995) reported NDF contents in corn silage varying from 46 to 63.2%, which is a wider variation compared with the present study.
NDF content in babassu meal varied from 63.88% (alternative method 1) to 68.39% (conventional method). The contents observed by Rocha Júnior et al. (2003), Cavalcante et al. (2005) and Vieira et al. (2005) (64.50% to 78.70%) indicate a threshold that comprises the results obtained in this study.
The NDF variation obtained in samples of Xaraes grass was from 68.64% (conventional method) to 71.81% (alternative method 1), which is slightly lower to the content (73.40%) reported by Euclides (2002).
The mean NDF content in marandu grass varied from 72.51% (conventional method) to 76.15% (alternative method 1), differing from the variation reported by Araujo (2005), 69.80 to 74.31%, in a sub-humid region during the dry period with the use of irrigation.
The mean ADF contents for Tifton hay varied from 40.05% (conventional method) to 65.11% (alternative method 2), which is greater than the values reported by Gonçalves et al. (2003): 35.60% in hay at a cutting age of 25 days.
In sugarcane, variation from 31.02% (conventional method) to 42.91% (alternative method 2) was observed. Pate et al.(2001), analyzing the nutritional value of 66 commercial varieties of sugarcane planted in south of Florida, observed a wide variation in ADF (28.30 to 41.50%), which are similar to values observed in our study.
The analyses of corn silage through the non-sequential test revealed ADF variations from 26.26% (conventional method) to 35.87% (alternative method 2). The ADF mean variation was 25.94% (conventional method) to 39.65% (alternative method 2). The mean ADF observed by Valadares Filho et al. (2006) was 30.80%, analyzing 112 samples of corn silage.
The results for babassu meal, 40.30% (conventional method) to 56.84% (alternative method 2), are close to those mentioned by Cavalcante et al. (2005) and Vieira et al. (2005), who reported values between 32.90 and 53.80%. The 48.30% mean ADF was also observed by Moreira Filho (2008), studying the chemical composition of six native species of babassu meal grazed by goats.
The variation of means obtained for xaraes grass ranged from 36.93% (conventional method) to 57.65% (alternative method 2). The mean of the results obtained in alternative method 2 is the highest compared with the means for the other methods analyzed. Pereira et al. (2008) obtained a 38.96% mean value for ADF in xaraes grass, close to the value observed by the conventional method.
In marandu grass, the variation in the means was 39.06% (conventional method) to 60.97% (alternative method 2). The values observed in the alternative method 1 (46.42%) and alternative method 3 (43.24%) were near to those reported (46.42%) by Pereira et al. (2008); whereas, the mean (39.06%) for the conventional method was greater than the mean content (34.20%) reported by Santos et al. (2008), who studied marandu grass cultivars subjected to three different levels of fertilization. We also observed that the average results obtained with alternative method 2 were higher compared with the other methods studied.
During all ADF analysis, alternative method 2 had the greatest means and divergence from the conventional method. Casali et al. (2009) reported that TNT, in spite of being a fabric similar to ANKOM, does not present pores. This characteristic is given to TNT during the manufacturing process, in which part of the surface is sealed by heat. This fabric structure could broaden the estimates of analytical results. Thus, the results obtained allow us to suggest that it is still necessary to better investigate the use of bags fabricated with TNT in NDF and ADF analyses.
Only corn silage presented difference (p<0.05) between sequences of analysis in alternative methods 1 and 2. The validation of alternative methodologies, which use the autoclave as digester system, to analyse NDF and ADF contents was not obtained for all the feedstuffs used; it was proven for Tifton 85 hay, babassu meal and sugarcane for NDF, but only in babassu meal at the determination of ADF.
In conclusion, the non-sequential and sequential forms of analysis and all analytical methods can be applied, with no loss of analytical precision for assessing NDF and ADF in tifton hay, sugar cane, babassu meal, Xaraes and Marandu grass. However, ADF quantification is not appropriate for corn silage using alternative method 2 (autoclave/TNT) and alternative method 3 (autoclave/filter crucible).