Introduction
Handling of fish during sampling or clinical procedures requires the use of anesthetics (Ross and Ross, 2008). Additionally, anesthetics prevent or minimize suffering of fish in routine practices of fish farming. Activities such as biometrics, transportation, tagging, removal of gametes and egg collection are associated with acute stress for the animals. Anesthetics decrease trauma and undesirable effects linked to stress (Okamoto et al., 2009; Vidal et al., 2008). Stress can lead to high incidence of disease, decrease of both appetite and weight gain, changes in behavior, and mortality -in critical cases (Corredor and Landines, 2009; Park et al., 2009). In fish, stress triggers the hypothalamus-pituitary-inter-renal axis functions, resulting in increased blood levels of catecholamines and cortisol (Wendelaar Bonga, 1997). Additionally, alterations in levels of glucose, lactic acid and free fatty acids are expected. Both levels of cortisol and plasma glucose are acute-stress indicators in fish (Corredor and Landines, 2009; Park et al., 2009).
The choice of anesthetics depends on availability, cost, ease of use, safety for the operator and the environment, rapid induction, low initial hyper-activity, adequate immobilization, effective analgesia and fast recovery, without prolonged ataxia or other undesirable effects (Ross and Ross, 2008). Several chemical anesthetics are currently utilized for these purposes, the most common being tricaine methanesulfonate (MS-222), benzocaine, quinaldine, quinaldine sulphate, 2-phe- noxyethanol, ethyl aminobenzoate, etomidate and metomidate, amongst others (Readman, 2013).
In search of new anesthetics for fish, new natural products like eugenol have emerged. Eugenol is obtained from distillation of Eugenia aromatica and Eugenia caryophyllata trees (Soto, 1995). Eugenol (2-methoxy-4- (2-propenyl) phenol) is slightly soluble in water, being necessary a dilution in 95% ethanol to prepare a 100 mg/ml stock solution. However, the dilution may be irritating to skin or mucous membranes, suggesting the use of protective measures (Ross and Ross, 2008; Noga, 2010). Eugenol has several uses in addition to anesthesia: ingredient in food industry, analgesic, antibacterial, antifungal agent and local anesthetic in dentistry, the latter being approved by the Food and Drug Administration in the US (Pereira-da-Silva et al., 2009; Stoskopf, 1993). The use of commercial preparation of eugenol is legal in fish without withdrawal time in Australia, New Zealand, Korea, Chile, Costa Rica, and Honduras (Noga, 2010).
On the other hand, benzocaine is poorly soluble in water, which makes it necessary to prepare a stock solution of 100 g l-1 with 95% ethanol (Ross and Ross, 2008). Benzocaine is derived from benzoic acid and is also used as a local anesthetic in mammals (Noga, 2010). In South America, benzocaine is the anesthethic most widely used in fish species such as Trachinotus marginatus, a common species in Brazil, Uruguay and Argentina (Okamoto et al., 2009).
Eugenol has been investigated in species like Colossoma macropomum, Brycon cephalus, Oreochromis niloticus, among other fish species widely cultivated in countries of Central and South America (Roubach et al., 2005; Vidal et al., 2007; Vidal et al., 2008). In Colombia, eugenol is used in several fish species but reports on the characteristics of anesthesia are scarce. The aim of this study was to determine the anesthetic efficacy of eugenol and benzocaine in juveniles of red tilapia Oreochromis sp.
Material and methods
Ethical considerations
Acclimation and experimental procedures followed guidelines by the Bioethics Committee of the School of Veterinary Medicine and Animal Science, Universidad Nacional de Colombia, Bogotá.
Animals
Thirty juveniles of red tilapia (Oreochromis sp.) from La Terraza fish farm (Villavicencio, Colombia) were used. The animals weighed 56.3 g on average and measured 14.3 cm.
Experimental conditions
The fish were acclimated to the conditions of the Aquatic Toxicology Laboratory, School of Veterinary Medicine and Animal Science, Universidad Nacional de Colombia (Bogotá, Colombia). The animals were housed in 40 gallon-glass aquaria equipped with a filtration system, constant aeration and temperature control (25 oC) for two weeks. During this time, water syphoning and refills of 30-50% were performed two or three times per week. The fish were fed with commercial feed (32% protein) until one day before starting the experiment.
Experimental substances
Commercially available eugenol (2-methoxy-4- (2-propenyl) phenol) and benzocaine were used. Eugenol and benzocaine stock solutions were prepared by dissolving 85% eugenol or benzocaine in 99.9% ethanol, respectively, obtaining working solutions of each anesthetic (100 mg/ml).
Anesthetic evaluation
After acclimation, fish were randomly distributed in three groups of 10 animals. Eugenol was evaluated at two concentrations: 50 mg/l (G1) and 100 mg/l (G2), whereas benzocaine was used at 100 mg/l (G3). These concentrations were chosen according to results reported by Deriggi et al. (2006) and Vidal et al. (2008). In all three groups, water pH was measured before and after dissolving the anesthetic solutions.
Anesthetic process
The fish were individually placed in the corresponding anesthetic solution. After immersion, time to achieve the anesthetic stages described by Woolsey et al. (2004) were recorded. Hyper-excitability, if any, upon contact with the anesthetic solution was also registered. When fish reached stage 5 of anesthesia or when ten minutes in the anesthetic solution had elapsed, fish were removed from the tank and observed during ten minutes or until signs of recovery happened before reaching a ten-minute period. The ten-minute period was chosen given that it isusually an ideal time to accomplish various clinical procedures on fish (e.g. blood sampling, biopsies, etc.). Then, the animals were transferred to anesthetic-free aquaria (recovery tanks) allocated for each concentration of the anesthetic solutions, with constant aeration and temperature control. Once fish were transferred to the recovery tanks, times to recovery of the swimming axis (RSA) and total recovery (TR) were registered. The animals were kept under observation for 24 hours.
Blood chemistry
During the anesthetic process in state 5 of anesthesia fish were weighed, measured and blood samples were collected. Blood samples were collected with heparinized syringes from the caudal vein to measure blood glucose and plasma cortisol. Blood glucose was measured immediately using a glucometer (ACCU-CHEK® - Roche, Barcelona, Spain). Subsequently, blood was centrifuged at 5,000 rpm for 10 minutes at 4 °C and plasma was transferred into cryotubes stored at -70 °C prior to cortisol analysis with a commercial ELISA kit (SPINREACT® - Bio-analytics, Madrid, Spain).
Data analysis
Analysis was performed, depending on the normality of the variables, through ANOVA or Kruskal-Wallis tests at 5% significance. Pairwise comparisons were performed using Tukey and Kramer (Nemenyi) tests with Tukey-Dist approximation for independent samples (Pohlert, 2014). The statistical program R (version 2.15.1) was used for this purpose.
Results
The fish in G2 had higher length (p=0.005) and weight (p=0.006) compared to fish in the other two groups. All fish underwent the anesthetic phases described by Woolsey et al. (2004) (Table 1). In G1 and G3, 30% of the animals had hyperactive reaction upon contact with the anesthetic solution, characterized by frenetic swimming against the aquarium walls, whereas 50% of the fish in G2 showed the same behavior. However, these differences were not statistically significant (p>0.05). The fish anesthetized with eugenol (G1 and G2) showed behavior corresponding with a deeper anesthetic plane as compared to the benzocaine group (G3). In the immersion, benzocaine-treated fish had fin movements despite having lost the swimming axis.
Time to loss of swimming axis was higher in G3 compared to the others (p<0.01). On the other hand, time for the first reaction after reaching the anesthetic plane happened later in G2 than in G3 (p=0.03). Total recovery time was also higher in G2 (p=0.04) compared to G1. No significant differences were found among groups with regard to loss of reactivity and recovery of swimming axis.
Table 1 Average time (min.) spent during each phase of anesthesia in juveniles of red tilapia Oreochromis sp. (mean ± S.D.) (n=10 fish/ group)
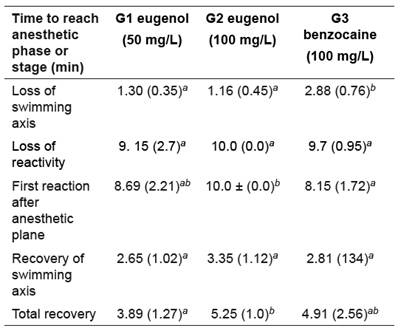
Different letters within the same column indicate significant differences amongst anesthetics (p<0.05). Total loss of reactivity: time for total loss of reaction or when fish reached 10 minutes in the anesthetic solution. Initial reaction: time elapsed until the first reaction of anesthetized fish happened or 10 minutes under observation without reaction.
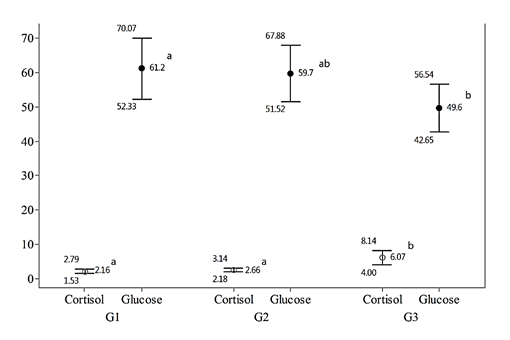
Glucose and cortisol values are shown in Figure 1. Glucose levels were significantly higher in G1 (p=0.03) compared to G3. However, cortisol levels were higher in G3 compared to the other two groups (p=0.0005). In the post-anesthetic, 24-h monitoring period, mortality was observed in G1 (10%), whereas in G2 and G3 this event was not observed. No significant differences were found for water pH values before or after dissolving the anesthetic (p>0.05). Values before and after dissolving the anesthetic were: G1 6.6 and 6.8; G2 6.9 and 7.0; and G3 6.6 and 6.9 respectively, without significant differences between groups (p>0.05).
(n=10 fish/ group) (mean ± S.D.) anesthetized with eugenol 50 mg/l (G1), eugenol 100 mg/l (G2), or benzocaine 100 mg/l (G3) (n=10 fish/treatment). Different letters indicate differences between groups.
Discussion
The number of fish showing hyper-excitability upon contact with the anesthetic solution was not significantly different among groups. Some authors indicate this may be related to the presence of ethanol as a solvent in the anesthetic solutions (Vidal et al., 2008). However, Readman et al. (2013) reported that zebrafish are aversive to the anaesthetics commonly used in fish, regardless of the ethanol dilution.
Additionally, G1 and G2 induced anesthesia faster than G3, considering the time for loss of swimming axis. Similar results were reported by Vidal and Col. (2008) in Oreochromis niloticus, with induction time of 50 seconds at 100 mg/l eugenol. The time for total loss of reactivity was signaled as the time required for the loss of all reflexes. In the eugenol groups, the fish did not stop ventilatory movements at all. Stoskopf (1993) reported higher frequency of ventilatory rate when benzocaine was used. Future studies should evaluate heart rate as an indicator of anesthetic depth. Further, the initial reaction time was significantly higher in G2 when compared to G3. These findings suggest a lower depth of anesthesia induced by benzocaine as compared to eugenol.
For G1 and G2 groups it took less time to loss the swimming axis compared with G3. However, these two groups spent more time to show initial reaction compared with G3. These results suggest that eugenol may be safer than benzocaine for clinical procedures in laboratory and fish farming. Fish in the three groups showed adequate recovery time (3.89 ± 1.27 minutes), which is within the ideal range (less than 5 minutes; Ross and Ross, 2008). Therefore, eugenol induced deep anesthesia with adequate recovery time and no medullary collapse. Similar results were reported by Vidal et al. (2008) with eugenol at 75 mg/l in Nile tilapia, obtaining a recovery time of 2.38 minutes.
Glucose values were significantly higher in G1 compared to G3. This indicates that eugenol 50 mg/l was not effective enough in inhibiting stress. These findings are similar to those reported in other species such as rainbow trout which showed higher plasma glucose in comparison to those treated with tricaine methanesulfonate MS-222 (Wagner et al., 2003). On the other hand, Roubach et al. (2005) reported no significant differences in plasma glucose concentrations when eugenol and benzocaine in Colossoma macropomum were compared.
In another report, no significant differences were observed in glucose levels between eugenol, MS-222 and 2-phenoxyethanol in perch (Velíšek et al., 2009). Iversen et al. (2003) found no significant differences in plasma glucose levels in salmon using eugenol, isoeugenol and benzocaine. Similar studies in Nile tilapia reported no differences when comparing plasma glucose levels in fish anesthetized with eugenol, benzocaine and sodium chloride (Oliveira et al., 2009).
Cortisol was significantly higher in benzocaine-anesthetized fish when compared with both eugenol groups. Similar findings in Brycon cephalus were reported for animals during transportation with and without eugenol. Animals transported with eugenol had significantly lower plasma cortisol levels (Inoue et al., 2005). During transportation of salmon, higher values of plasma cortisol were observed in the control group compared to eugenol (Iversen et al., 2009). In the same study, higher plasma cortisol concentrations were found in benzocaine-exposed fish as compared to those exposed to eugenol (Iversen et al., 2003). In contrast to the present results, Palic et al. (2006) reported no significant differences in plasma cortisol concentrations of fish anesthetized with MS-222, metomidate and eugenol (Palić et al.,2006). In rainbow trout, no differences were observed in plasma cortisol levels between MS-222, eugenol, and CO2 (Pirhonen et al., 2003). Cortisol concentrations in G1 and G2 suggest that eugenol was more effective in reducing stress (Inoue et al., 2005; Iversen et al., 2003).
All fish recovered and no mortality was reported. However, one fish in eugenol (50 mg/l) was found dead during the 24h recovery period. In this case, it was not possible to identify the cause of death and no reliable necropsy findings, due to autolytic changes, allowed conclusive evidence. Nevertheless, it was observed that the specimen was affected by other fish nipping in the recovery tank. Nipping is characterized by a swimming pattern towards the tank-mates followed by biting of head, caudal fin, median-lateral area of the body, and the ventral area (Giaquinto and Volpato, 1997). According to present results, 100 mg/l eugenol is safe for juveniles of red tilapia, without causing mortality. This study has practical and innovative applications in Colombia regarding the anesthetic management of finfish species. In conclusion eugenol 100 mg/l leads to induction of anesthesia in juveniles of red tilapia in less than 3 minutes and recovery in less than 5 minutes, without causing mortality.