Introduction
Bovine in vitro embryo production (IVP) has contributed to develop the Brazilian cattle herd (Rodrigues, 2014). However, the percentage of oocytes that reach the blastocyst stage is low (35-45%). Evidence suggests the oocyte quality is the main factor for blastocyst yield, thus the best oocytes should be selected (Lonergan and Fair, 2016).
The criteria for cumulus-oophorus complexes (COCs) selection have been based on morphology, not on the complete evaluation of oocyte competence (Van Den Hurk and Zhao, 2004). Therefore, several studies have been looking for methods to demonstrate oocyte competence in a non-invasive manner. Currently, only invasive methods that damage the oocyte can predict its quality, precluding its use for IVP.
The search for non-invasive biomarkers was reviewed in an earlier paper (Goovaerts et al., 2010). The brilliant cresyl blue (BCB) staining is one of these methods. It determines glucose-6-phosphate dehydrogenase enzyme (G6PDH) activity, which is increased in growing oocytes and decreased in full-grown ones (Rodriguez-Gonzales et al., 2002). Thereby, oocytes with lower enzymatic activity do not degrade BCB, turning blue (BCB+), and thus have greater developmental competence. Furthermore, researchers have identified genes that could be used as biomarkers of oocyte quality (O’Shea et al., 2012). Markers of oocyte quality were proposed in oocytes selected by BCB, with BCB+ oocytes showing different gene expression profile (Ashry et al., 2015). Several authors (Alm et al., 2005; Wu, 2007; Mirshamsi et al., 2013) have evaluated selection of oocytes via BCB. In these studies, they concluded that oocytes could be stained by BCB without changing their competence and favorable results are obtained in selection of competent oocytes, when associated with morphological selection.
Some Brazilian regions have marked seasonality, characterized by temperature and humidity variations that impose different environmental and nutritional standards to cattle throughout the year (Nicoloso et al., 2006). Studies relating seasonality to oocyte quality have focused on morphological analysis, or used harmful substances, which impede oocyte fertilization (Castaneda et al., 2013). Thus, the present study adopted BCB staining to relate bovine oocyte maturation capacity with seasonal variations. Although studies have demonstrated the effect of seasons on feed restriction and cattle reproduction, to the best of our knowledge this is the first attempt of using this method to select oocytes that are more competent for IVP in different seasons, since BCB+ oocytes have a higher maturation rate.
Materials and methods
Location
The experiment was conducted at the Laboratório de Reprodução e Melhoramento Genético Animal Northern Rio de Janeiro (UENF), located in Campos dos Goytacazes, RJ, Brazil.
Climatic conditions
The animals originated from the Northern region of Rio de Janeiro, where the climate is tropical wet-dry (Aw) according the Köppen classification, with a rainy summer and a dry winter. The annual average temperature is 24 oC. The driest quarter occurs in the June/July/August period and the rainy season in December/January/February, being the annual average rainfall 1,055 mm (Mendonça et al., 2012). In summer (December to March) the days are longer than the nights and the solar radiation favors the rise in temperature, precipitation and relative humidity. Winter (June to September) has the lowest rainfall index and the lowest temperatures. In general, rain is five times lower in wintertime and air humidity is low (Emidio, 2016).
Collection and morphological selection of COCs
The COCs recovered from ovaries obtained at slaughterhouses in the city of Campos dos Goytacazes (21°45’15” S latitude, 41°19’28” W longitude) were transported to the laboratory in thermal bottles, where the ovaries were washed in saline solution (0,9% NaCl) with antibiotics (100 IU/mL penicillin and 100 µg/mL streptomycin). Ovarian follicles of 3 to 8 mm in diameter were aspirated and the follicular fluid was placed in a 50-mL conical tube. After sedimentation, the pellet was transferred to a Petri dish (Corning, 100 × 20 mm) with 3-5 mL of handling medium [199 with Earle’s salts plus 25 mM Hepes and antibiotics (100 IU/mL penicillin and 100 µg/mL streptomycin) supplemented with 10% fetal calf serum (FCS)] to search, retrieve, and classify the oocytes. Only degree I and II oocytes (Loos et al., 1989) -with three or more compact layers of cumulus cells and uniform cytoplasm- were used.
The experiment was conducted in the summer and winter of 2010 and 2011. In 2010, the study was carried out in January, February, March, June, July, and August, while in 2011 it took place in February, March, June, and August.
Selection of COCs by BCB test
After selection, COCs were incubated in incubation solution (199 medium supplemented with bicarbonate with antibiotics and 5% FBS) containing 26 µM of BCB (860867, Sigma-Aldrich Brasil Ltda, São Paulo, Brazil).
The COCs were maintained for 90 min in 5% CO2, at 38.5 °C (Manjunatha et al., 2007). Oocytes incubated with BCB were separated according to the cytoplasm staining: Oocytes with blue staining in the cytoplasm (BCB+) and oocytes with colorless cytoplasm (BCB−). The COCs in the control group were kept under the same conditions except for the absence of BCB. Next, COCs were washed twice in PBS and placed in the incubator for in vitro maturation.
Oocyte culture
The COCs were transferred to 100 µL drops of maturation medium [199 medium with Earle’s Salts (M-5017), plus 20 mM sodium bicarbonate solution (S6297), 5.0 mg/mL LH (Lutropin-V, Bioniche, Beleville, Canada), 0.5 µg/mL FSH (Folltropin-V, Bioniche, Belevile, Canada), 0.2 mM pyruvate (P4562), 100 IU/mL penicillin (P3032), and 100 µg/mL streptomycin (S1277). Twenty oocytes per drop remained under mineral oil, for 22 h, at 38.5 °C, in 5% CO2.
Evaluation of oocyte maturation
To determine the effect of seasonality on nuclear competence of oocytes, the meiosis stage was evaluated at 22 h of maturation. After IVM, COCs were denuded mechanically by pipetting in PBS solution (0.01 g/mL PVA), subsequently placed on slide and coverslips and fixed for 72 h in acetic acid and ethanol (1:3, v:v). Afterwards, the material was stained with 2% acetic orcein. Oocytes were evaluated by light microscopy. The stages of nuclear maturation were defined according to Hewitt and England (1997), based on the morphology of chromatin, such as germinal vesicle (GV, prophase I stage), germinal vesicle breakdown (GVBD), metaphase I (MI), and metaphase II (MII).
Experimental design
Experiment one. The COCs were incubated in the BCB solution and then divided into two groups according to the staining of cytoplasm. The COCs from complexes from control group underwent the same treatment as the others, except for the absence of the BCB in the incubation medium. Those with blue cytoplasm were classified as BCB+, and those with colorless cytoplasm were classified as BCB−. Thus, the proportion of these two groups was determined in different seasons of 2010 and 2011.
Experiment two. The exposure of COCs to BCB and the treatment of control group were performed as described in Experiment I. After selection of COCs by the cytoplasm staining (BCB+, BCB−, and control) they were set for in vitro maturation for 22 h and subsequently evaluated for nuclear maturation stage.
Statistical analysis
The rates of oocytes matured in different treatments and the proportions of BCB+ and BCB− oocytes in summer and winter were compared by the Chi-square test at 95% confidence level. All data were analyzed using BioEstat 5.0 software (2007). Four replicates were used in each season of 2010 and 2011 to evaluate the proportion of BCB+ and BCB− oocytes, and five replicates to evaluate the nuclear maturation rate.
Results
Proportion of BCB+ and BCB− oocytes
Results showed a higher percentage (p<0.05) of BCB+ oocytes obtained in the summer of 2010 compared with those obtained in the winter of the same year. Additionally, the percentage of BCB− oocytes was lower (p<0.05) in the summer compared with the winter (Table 1).
Table 1 Percentage of BCB+ and BCB− oocytes obtained in summer and winter of 2010.

Values followed by different superscripts in the same column differ significantly by the chi-square test (p<0.05)
In the year 2011, there were no differences (p>0.05) between the percentages of BCB+ or BCB− oocytes obtained in summer and winter (Table 2).
Evaluation of nuclear maturation in different seasons
We also evaluated the effect of seasonality on the ability of oocytes selected by the BCB method to reach MII after 22 h of in vitro maturation. Data related to nuclear maturation of different experimental groups in summer and winter of 2010 and 2011 were not significantly different (Tables 3 and 4).
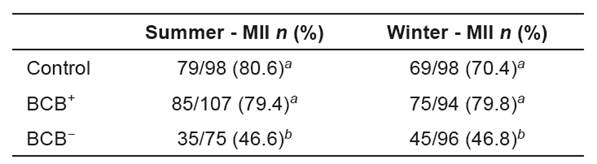
Table 3 Percentages of BCB+ bovine oocytes matured in vitro that reached metaphase II during the summer and winter of 2010 and 2011.

Data were analyzed by the chi-square test at the 5% significance level.
Evaluation of nuclear maturation in BCB+ and BCB− oocytes
The percentage of metaphase II in BCB− oocytes was lower (p<0.05) than those of control and BCB+ groups (Tables 5 and 6).
Table 5 Meiotic configuration of bovine oocytes matured in vitro obtained during the summer and winter of 2010.
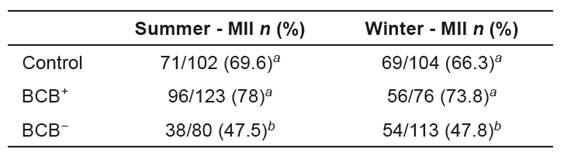
1 MII: Metaphase II. Values followed by different superscripts in the same column differ significantly by the chi-square test (p<0.05).
Discussion
Success in in vitro bovine embryo production is directly related to the quality of the gametes used, which is largely influenced by nutrition (Sales et al., 2015). In general, oocyte donors receive a controlled diet without significant changes in quantity and composition throughout the year, which makes it a negligible factor in the variation of the process. However, in vitro embryo production from ovaries obtained from slaughterhouses is dependent upon the forage supply, which may be influenced by the effects of seasonality on forage production. (Sartori and Guardieiro, 2010).
Due to its extension, Brazil has pronounced climatic variability across regions. The regions nearest to the equator have lower temperature variations throughout the year, but seasonality is mainly due to the volume and distribution of rainfall during the year (Sansigolo et al., 2010). Therefore, due to the high spatial variability in the rainfall regime these data should be validated in other regions and countries (Alvares et al., 2013).
To evaluate the effect of seasonality on the quality of bovine COCs, we used BCB, a vital stain, substrate from glucose-6-phosphate dehydrogenase (G6PD). The BCB has been used as a noninvasive method for the selection of oocytes that reach a stage of development consistent with the ability of these gametes to be fertilized and reach embryonic and fetal development. Glucose-6-phosphate dehydrogenase is active in growing oocytes, but has low activity in those oocytes that have completed this phase. Since the latter have a low G6PD activity, they do not degrade the BCB and turn blue (BCB+; Alm et al., 2005). Oocytes considered BCB−, by contrast, have a higher G6PD activity and thus degrade the stain, remaining colorless. These oocytes have a lower ability to complete maturation and to be fertilized in vitro since they are still growing and have a smaller pool of mRNA, proteins, lipids, and sugars (Mota et al., 2009).
Oocyte maturation is a crucial event comprising the progression of meiotic cycle. The major parameter to know oocyte has achieved nuclear maturation is a meiotic configuration of MII (Fulka et al., 1998). In this stage oocytes can be fertilized and support embryonic development (Smitz et al., 2004).
In this study, values found during the summer of the two years assessed are similar to those described in 2005 by Alm et al. (57.9% BCB+) and in 2009 by Mota et al. (60.37% BCB+), who used the BCB method to select more competent oocytes for IVM. The total annual rainfall in the municipality of Campos dos Goytacazes in 2010 and 2011 was 597 and 739 mm (Evapotranspiration Station at CCTA/ UENF -unpublished data), respectively. These volumes were lower than the historical annual average rainfall volume (1,055 mm).
Despite the low volume of rainfall in the two years under study, in 2011, the rainfall was slightly higher than in the previous year. This situation may have provided a greater supply of forage to animals in the dry season, which may have increased the percentage of BCB+ oocytes obtained in the winter.
Ovaries were obtained from cows from the north region of Rio de Janeiro State, in which the use of pastures for cattle feeding predominates. These pastures are subject to seasonal variations, with maximum yield occurring in the rainy season and a significant decline in production during the dry season. (Euclides et al., 2007; Teixeira et al., 2011).
Thus, cattle are not feed uniformly during the year, and this affects reproductive characteristics, since follicular growth, maintenance of pregnancy, and estrous cyclicity are low-priority activities on a nutrient-distribution scale and only work normally when functions like growth and nutrient reserves are maintained (Zeron et al., 2001).
In this work, BCB− oocytes showed a lower nuclear maturation rate compared with control and BCB+ groups (Tables 5 and 6). As the progression of oocyte meiosis to metaphase II is a requirement for fertilization and subsequent embryonic development, these data support the hypothesis that BCB− oocytes are less able to develop, reinforcing the fact that the BCB method is an important tool to select oocytes for in vitro embryo production. We found no difference in maturation rate between BCB+ and control oocytes, proving the lack of deleterious effect from BCB exposure and reinforcing the fact that BCB is a vital dye (Rodríguez-González et al., 2002).
In this regard, Ashry et al. (2015) demonstrated that abundance of oocyte gene transcripts related to maturation and proper development (such as JY-1 and BMP15) was higher in BCB+ oocytes compared with BCB−. On the other hand, BCB− COCs showed increased expression of cathepsins B, S, and Z of cumulus cells. Thus, the authors correlated the abundance of transcripts of these genes with BCB+ and BCB− oocytes to prove that the BCB method can be used to acquire more competent oocytes (Ashry et al., 2015). Moreover, the use of this colorimetric method in oocytes and zygotes increases the possibility of predicting the early embryonic development potential, since oocytes with blue cytoplasm and unstained zygotes significantly increase embryo production (Mirshamsi et al., 2013).
These data confirm the efficacy of the BCB test in selecting oocytes able to undergo maturation. Oocytes obtained from prepubertal goats (Rodriguez- Gonzalez et al., 2002) and from swine (Wongsrikeao et al., 2006) have shown increased maturation and fertilization rates of BCB+ oocytes in relation to BCB− and control. However, Alm et al. (2005) found similar nuclear maturation rates between BCB+ oocytes and the control group in cattle. Nevertheless, BCB+ oocytes resulted in a significantly higher (p<0.05) blastocyst rate (34.1%) than BCB− (3.9%) and the control (18.3%) groups.
Data from Tables 3 and 4 indicate that the nuclear maturation rates of BCB+ and BCB− oocytes were not affected by seasonality, but the effect of seasonality on the proportion of these oocytes obtained in the summer and winter was remarkable. Adamiak et al. (2005), demonstrated that feeding donors twice their nutritional requirements for maintenance increases the number of follicles from 4 to 8 mm and the number of embryos produced. By contrast, malnutrition due to seasonal issues has negative implications on the resumption of meiosis, number of follicles, and oocyte quality (Santos et al., 2008).
In this work, mainly cattle of Bos primigenius indicus breeds such as Nellore were used. These animals are more resistant to tropical conditions (Wolfenson et al., 2000) like high temperature and humidity than breeds evolved in Europe (e.g. Angus and Holstein; Hansen, 2004; Paula-Lopes et al., 2003). The ovaries were collected at slaughterhouses in Campos dos Goytacazes (RJ, Brazil) where the tropical conditions are intense. Thus, the effect of seasonality influenced by nutrition becomes more evident than the thermal stress during summer.
In view of the above considerations, it can be concluded that the season can influence the percentage of oocytes best suited for in vitro embryo production in situations in which oocyte donors receive feed exclusively on pasture. Additionally, the brilliant cresyl blue method is effective to determine the effect of seasonality on the competence of bovine oocytes to achieve nuclear maturation.