Introduction
Abnormal and stereotypic behaviors in horses have been widely studied around the world and several epidemiological situations have been described (Sarrafchi and Blokhuis 2013; Tadich et al., 2013). Susceptibility of stabled horses and horses subjected to stressful activities to develop behavioral disturbances is frequently reported. Therefore, the presence of behavioral disturbances in horses is an indicator of poor welfare. Domestication and practices derived from the process of stabling are known as unnatural measures, where animals need to adapt to the demands of an artificial environment (Hothersall and Casey 2012; Dezfouli et al., 2014; D’Almeida et al., 2014).
When a horse is subjected to confinement, its normal behaviors are interrupted, including grazing, social contact, exercise intensity and feeding practices, and all activities that were carried out for a long time in their natural environment (Waters et al., 2002; Hothersall and Nicol, 2009). This change of environment leads horses to express behavioral patterns as adaptation mechanisms to reduce the impact of anxiety and frustration derived from spatial restriction (Hothersall and Casey 2012; Freymond et al., 2015).
Abnormal behaviors include unusual episodes with a particular purpose. On the other hand, stereotypies are unusual, repetitive behaviors without a definite purpose (Pell and McGreevy, 1999). Despite the absence of an explanatory etiology for these disturbances, several theories have sought to elucidate the mechanisms involved for different breeds (Tadich et al., 2012; Tadich et al., 2013; Dezfouli et al., 2014; Navarrate et al., 2015). Disturbances of dopaminergic, serotonergic, and endogenous opioid systems have been implicated as possible causes for these attempts of adaptation to chronic stress (Dodman et al., 1994; Hugo et al., 2003; McBride and Hemmings. 2005).
Horses used for urban patrol are susceptible to stress generated by activities conducted in a very different environment from their natural one (including stall environment), which has been reported in several latitudes (Leal, 2007).
This study aimed to determine the frequency of abnormal and stereotypic behaviors in police horses through 48 hours of continuous observation, considering ethological and endocrinological evaluations.
Materials and methods
Ethical considerations
This study was approved by the Ethics Committee for Animal Experimentation of Universidad de Antioquia, Colombia (September 30, 2014).
Animals and study design
Twenty clinically healthy castrated male horses were chosen. Animals averaged 12 ± 4.8 years of age and 500 ± 50 Kg. Breeds were Argentine crossbreed, Selle Français, Silla Argentino, and their crosses, property of the Metropolitan Police of Valle de Aburrá (Medellín, Colombia). These horses were part of a group of 50 animals used at least three times per week for urban patrolling and security-related activities in social, sporting, and anti-riot events. General clinical examination was performed to all horses at the beginning six in the other. Each stall had a rectangular opening with a 5 m2 of vertical separation that allowed contact with neighboring animals. Additionally, each stall had one 4 m2 window looking outdoors. The free area of each stall was 11 m2. There was also a paddock where horses exercised one hour daily.
Ethological evaluation
Animal behavior was continuously recorded during 48 h using Sony CCD dome security cameras (model UC-03, with 520 TVL resolution, 20 m image range and 24 PCS infrared led; Unitec-elite®, China) installed in the top of the stalls and connected to a recording device (DVR). Each camera recorded two horses simultaneously in Stall 1, whereas one camera was used per horse in Stall 2. Additionally, the animals were observed directly and individually by the researchers for 60-min periods (every 5 min until the period was completed) between 2:00-3:00, 5:00- 6:00, 8:00-9:00, 11:00-12:00, 14:00-15:00, 17:00-18:00, 20:00-21:00, and 23:00-24:00 h. Results are presented in Table 1, including normal, abnormal and stereotypic behaviors. Behavioral findings were recorded in a digital format using Excel 2013®, to subsequently classify stress levels according to the ethogram described by Young et al. (2012).
Table 1 Normal, abnormal, and stereotypic behaviors observed in 20 patrol horses property of Valle de Aburrá Police Department, Medellin (Colombia).
| Normal behaviors | Abnormal behaviors | Stereotypic behaviors |
|---|---|---|
| Drinking | Bed eating | Aerophagia (cribbiting or sucking) |
| Eating | Coprophagia | Weaving |
| Sleeping | Head-pressing | Stall walking |
| Alert state | Feeder licking | Rolling |
| Distracted state | Lignophagia | |
| Looking outside | Random movements | |
| Neighing | Bed sniffing | |
| Body shaking | Kicking | |
| Pawing | ||
| Self-scratching | ||
| Scratching against objects | ||
| Lying down | ||
| Rearing on feeders |
Horses were continuously monitored inside individual stalls during 48 h. Some horses were absent during 2 h while they were developing patrolling activities.
The diet was composed of Kingrass (Saccarum sinense; 40 Kg/d) offered at 3:00 and 10:00 am, commercial feed concentrate (5 Kg/d) offered immediately after the grass, Pangola-grass hay (Digitaria decumbens; 5 Kg/d) offered at 4:00 pm, and commercial mineralized salt (30 gr/d) formulated for horses. Water was offered twice a day.
Endocrinological evaluation
Blood samples were collected through jugular venipuncture in vacuum tubes without additives and centrifuged at 1,500 rpm for 10 min for serum collection. Samples were subsequently kept frozen at -20 °C until analysis. All samples were collected with minimal handling of the horses, between 8:00 and 9:00 h, and then between 16:00 and 17:00 h during day 1 and 2 to evaluate cortisol index (CI).
Serum cortisol was determined using a sandwich ELISA commercial kit (AccuBind®, Monobind Inc., Lake Forest, CA, USA) with kit calibrators and commercial cortisol controls (SURE® Multiligand control-tri level, Monobind Inc., Lake Forest, CA, USA). Readings of ELISA plate were conducted at 450 - 630 nm wavelength in a conventional reader (STAT FAX 303® Plus Microstrip Reader, Awareness Technology Inc., FL, USA). The kit can detect concentrations of serum cortisol from 0.366 µg/dL.
Cortisol values obtained in the morning and afternoon were used to estimate CI to assess the circadian cycle rhythm of each horse. The method used to determine CI was proposed by Douglas (1999) and employed by Leal et al. (2011) and D´Almeida et al. (2014). The CI was calculated as:
The highest cortisol value (HcV) - the lowest cortisol value (LcV) / the highest cortisol value: (HcV-LcV)/HcV
The CI is considered normal when the final value is greater than 0.30. The CI value was then compared with the stress classification for horses proposed by Young et al. (2012).
Statistical analysis
The variables evaluated were analyzed through descriptive statistics using the SPPS V9.0 software (SPSS Inc., Chicago, IL, USA). Additionally, bivariate association analysis was carried out to evaluate the risk of gastrointestinal disease, chronic infectious disease, anxiety or nervousness and stereotypic behaviors when the CI was below the cut value. All the information was taken from the clinical history anamnesis. The results are presented in frequency charts created in Excel® 2013 (Microsoft Corporation, Redmond, WA, USA).
Results
The variables evaluated during the clinical examination were within the normal reference values for all the horses. At least 96 direct observations per animal were recorded manually. Video-recording allowed researchers to verify the efficiency and accuracy of the direct observations.
Ethological evaluation confirmed behavioral disorders in most of the animals (65%), and 55% of these disorders were considered stereotypic behaviors. Not all the expected behaviors described in Table 1 were observed. The behaviors that occurred most frequently in two or more individuals are reported according to the number of animals in Figure 1. Some behaviors had a very low frequency (one individual) so they were not graphed (i.e. neighing, body shaking, and rolling behaviors). The animals did not show behaviors such as coprophagia, feeder licking, kicking, and rearing on feeder during the study. Some behaviors such as head-pressing, random movements, bed sniffing, lying down, and bed eating were complex to evaluate because they were difficult to differentiate from others.
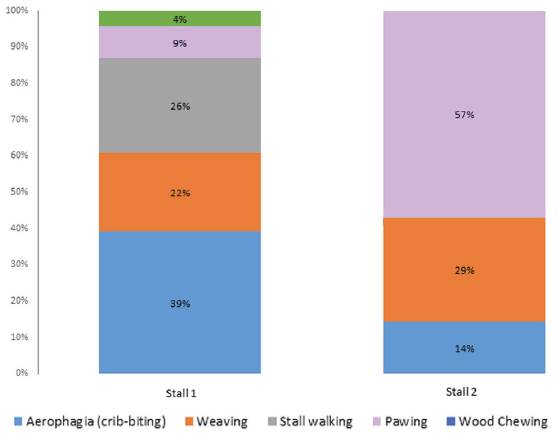
Figure 1 Distribution of relevant abnormal and stereotypic behaviors observed in 20 patrol horses property of Valle de Aburrá Police Department, Medellin (Colombia).
Behavior dynamics evaluated in detail showed that animals in Stall 1 carried out the “looking outside” behavior at day and night, while animals in Stall 2 did it during daytime only. Aerophagia (cribbing or wind-sucking) was observed in animals in Stall 2 during the morning, while animals in Stall 1 showed this behavior during night and morning.
Weaving and aerophagia were present in animals in Stall 1, which showed these behaviors at night and early morning. Animals in Stall 2 concentrated the weaving frequency in the morning, keeping a shorter time range of occurrence. Additionally, box walking was evident only for animals in Stall 1, without a clear trend of occurrence. Finally, pawing was more frequent during the morning (between 8:00 and 12:00) in both stalls. Wood chewing occurred in just one individual.
The average value for serum cortisol concentration in the morning was 10.10 ± 8.69 µg/dL, while it was 4.61 ± 3.96 µg/dL in the evening. The dynamics of overall mean concentration taken at the two different times showed a circadian rhythm behavior characteristic of the species. Most individuals with CI<0.30 (9/13) corresponded to animals in Stall 1. Additionally, abnormal behaviors in this group, such as aerophagia (crib-biting), stall walking, weaving, kicking and constant alert were also present, in contrast with individuals showing CI greater than or equal to 0.30 (Table 2).
Table 2 Stress levels, cortisol index, and specific behaviors based on stall and individual observations in 20 patrol horses property of Valle de Aburrá Police Department, Medellin (Colombia).
| Stall | Classification * | CI | Specific behavioral observations | |
|---|---|---|---|---|
| Individual 1 | 1 | 2 | 0.05 | Looks repeatedly outside |
| Individual 2 | 1 | 4 | 0.07 | Looks repeatedly outside |
| Individual 3 | 2 | 0 | 0.07 | Looks repeatedly outside and yawns constantly |
| Individual 4 | 1 | 0 | 0.09 | Eventually stall walking, looks repeatedly outside |
| Individual 5 | 1 | 1 | 0.11 | Eventually exhibits crib-biting behavior, remains alert in the middle of the stall |
| Individual 6 | 2 | 5 | 0.12 | Weaving episodes, eventually rests its head against the wall |
| Individual 7 | 2 | 0 | 0.13 | Remains alert in station, coughs with relative frequency |
| Individual 8 | 1 | 3 | 0.17 | Eventually stall walking, looks constantly outside |
| Individual 9 | 1 | 5 | 0.17 | Kicks the door, licks the door, exhibits crib-biting behavior eventually, remains alert when the other horses are sleeping |
| Individual 10 | 1 | 1 | 0.17 | Eventually exhibits crib-biting behavior, eventually rests against the wall |
| Individual 11 | 2 | 0 | 0.18 | Eventually scratches against the walls |
| Individual 12 | 1 | 3 | 0.20 | Weaving episodes, eventually exhibits crib-biting behavior |
| Individual 13 | 1 | 5 | 0.21 | Eventually pawing, bites wooden structures, remains vigilant in station |
| Individual 14 | 1 | 1 | 0.30 | Eventually rests against the wall, remains distracted in station, eventually looks outside |
| Individual 15 | 2 | 0 | 0.48 | Weaving episodes eventually |
| Individual 16 | 2 | 0 | 0.54 | Eventually scratches against the walls |
| Individual 17 | 1 | 1 | 0.56 | Eventually kicks the door with the fore limbs |
| Individual 18 | 1 | 3 | Worked | Patrolled during evening blood sampling |
| Individual 19 | 1 | 1 | Aggressive | It was impossible to handle due to its temperament |
| Individual 20 | 1 | 1 | Worked | Patrolled during evening blood sampling |
*No stress: 0-2; Low stress: 3-4; Medium stress: 5-7; High stress: 8-10. Classification according to Young et al. (2012).
When comparing CI with stress classification, the animals classified as “moderately stressed” were in the low CI group. Some animals classified as “stressed” by CI did not match the stress classification tool.
Association analysis revealed no risk of chronic gastrointestinal disease (p = 0.8), chronic infectious disease (p = 0.80), disturbances of temperament such as aggressiveness, anxiety, or nervousness (p = 0.24) or that of at least one stereotypic behavior (p = 0.91) when CI was less than 0.30.
Discussion
The continuous 48-hours observation with video cameras allowed behaviors to be recorded at times other than the morning, afternoon, and evening. Additionally, the method allowed verifying frequencies for some horse activities that are not usually observed during short periods of direct observation. Similar studies have been conducted for different breeds of patrol horses (Vieira, 2006; Leal, 2007; D´Almeida et al., 2014). However, those studies have been performed during only 8-hour periods of direct observation, leading to underestimation of behaviors occurring during the night and early morning, and resulting in greater amount of records for analysis.
The present work found a high prevalence of behavioral disturbances compared with other studies. Other researchers have described incidences between 5 and 32.5% (McGreevy et al., 1995; Johnson et al., 1998) and between 26.7 and 43% (Vieira, 2006; Leal, 2007; D›Almeida et al., 2014). The obvious differences between these studies lie in the type of activities carried out by the animals and differences related to animal husbandry. Additionally, the data-gathering method differed between our study and other reports. As an important difference, our observations were conducted for 48 continuous hours seeking to eliminate as much as possible the subjectivity from surveys applied to owners or handlers. The high prevalence was due to the mechanism of continuous evaluation, since the horses were continuously in the stall. The continuous behavioral assessment showed variability in frequency, time, and individual patterns regarding abnormal and stereotypic behaviors in response to environmental factors (Leal, 2007). This could feasibly distort the resting periods and welfare levels, leading to different degrees of adaptation to the environment, depending on stress intensity (Irvine and Alexander, 1994). There is evidence that horses dedicated to patrolling urban environments show high incidence of behavioral disturbances and colics (Leal, 2007; Leal et al., 2011).
Confinement and some associated management practices such as feeding offered in portions, inappropriate stalls, and restriction or elimination of social coexistence favor the development of abnormal and stereotypic behaviors (Mason, 1991; Hemmann et al., 2013), which predispose animals to somatic and ethological disturbances (Broom, 2006). Therefore, confinement is considered a key factor within the epidemiology of abdominal syndrome in cases of gastric ulceration and small intestine entrapment in the epiploic foramen, and in other disorders (Cooper et al., 2000; Nicol et al., 2002; Archer and Proudman, 2006). Nevertheless, the animals evaluated did not exhibit disturbances or signs of disease at the beginning or at the end of the study.
Studies in police and military horses used for urban patrolling have reported colic incidences between 12 and 95%/year (Vieira, 2006; Leal, 2007; Laranjeira et al., 2009). However, clinical history of animals in this work reported only two colic cases in the past 12 months, similar to results by D’Almeida et al. (2014) who attributed it to good nutrition and management.
The low incidence of colic in this study could also be explained by the type of fiber ingested and the feeding during critical periods when animals require reducing the stomach acid gradient (Husted et al., 2009). In spite of these strategies, presence of behavioral disturbances was observed in the early hours of the morning, which indicates poor welfare. This implies that efforts to offer the animals an artificial environment similar to their natural one are not enough.
Social interaction is fundamental for equines due to their gregarious nature and because they are prey in wild environments, which requires territorial freedom to collect and select their own food (Goodwing et al., 2007). Confinement triggers anxiety and frustration feelings so animals establish mechanisms to reduce them through abnormal or stereotypic behaviors (Mason, 1991). The stall design in the present study allowed horses visual contact with the external environment through large windows, as part of environmental enrichment that functions as a distraction factor (Martinez et al., 2007). For some authors, stall design does not affect the prevalence of stereotypic behaviors (Dezfouli et al., 2014). However, Sarrafchi and Blokhuis (2013) related behavioral disturbances to poor welfare and deficient stalling environment. Stall dimensions in this study were below those recommended for this type of horsesand allowed only visual but not physical contact, which could contribute to prevalence of abnormal and stereotypic behaviors as well as to greater disturbances compared with those allowing both visual and physical contact (Dezfouli et al., 2014).
Although good management practices and stall design were implemented, a correlation with comfort and welfare was not established. The high prevalence of disturbances could also reflect chronic stress generated by patrolling activities. It has been reported that subjecting patrol horses to atypical situations in urban centers predisposes them to behavioral disturbances (Leal, 2007).
Normal behaviors (looking outside), abnormal behaviors (pawing and wood chewing) and stereoty- pic behaviors (crib-biting, weaving, and stall walking) in Stall 1 can be attributed to a higher number of animals in this stall compared to Stall 2. Therefore, it is not appropriate to establish a comparison between both stalls. The same types of behaviors were observed in both stalls with the exception of stall walking stereotypy, which is controversial, as some authors consider it a natural kinetic behavior. The stall walking frequency (26%) was within the 0.25 to 18.1% range reported in other studies (Vieira, 2006; Leal, 2007; D’Almeida et al., 2014) for horses performing similar patrolling activities, and between 1.02 and 8% (Muñoz et al., 2009; Muñoz et al., 2013; Muñoz et al., 2014) in Chilean horses intended for sports. According to some reports, stall walking reflects social needs (Sarrafchi and Blokhuis, 2013; Dezfouli et al., 2014). Although animals in our study had visual contact, physical contact was scarce, which could frustrate the animals when they were confined after patrolling activities. Leal (2007) attributes high prevalence of behavioral disturbs in animals who experiment freedom for a long period and are subsequently confined, generating unrest and frustration. In this case, the horses were subjected to some hours of patrolling before confinement, situation that could recreate the same frustration feeling.
Crib-biting was more frequent in Stall 1. This stereotypy has been linked to stress caused by social isolation and absence of grazing (Albright et al., 2009; Hemmann et al., 2014). In addition, urban patrolling is a highly stressful activity. Stress was confirmed endocrinologically through the CI, which demonstrated chronic stress in these animals. However, causes should be explained specifically (patrolling, handling, stabling). Our results are in contrast with other reports showing less than 8.5% crib-biting frequency (Vieira, 2006; Leal, 2007; Pagliosa et al., 2008).
Prevalence of crib-biting in horses used for other activities has been estimated between 2 and 10.5% (Waters et al., 2002; Albright et al., 2009; Muñoz et al., 2009; Muñoz et al., 2013; Dezfouli et al., 2014; Muñoz et al., 2014). The high prevalence observed in this study could be due to the 48-hours continuous assessment method more than to other factors, as horses were subjected to similar stress factors related to and derived from stabling.
Occurrence of weaving observed in this work was within the range reported in previous studies. Most of the animals with this stereotypy (22 and 29% in Stalls 1 and 2, respectively) exhibited this behavior before feed was offered, indicating anxiety and frustration to receive the ration, as described by Houpt and McDonnell (1993). Possibly, looking outside and pawing were also responses to the anxiety and frustration preceding the feeding, as the stall design enabled animals to observe people offering feed to other horses.
The three stereotypies observed are considered classic and have been reported in other studies with stalled horses used for different activities (Tadich et al., 2012; Dezfouli et al., 2014; Muñoz et al., 2015). Nonetheless, there are differences in the frequency of occurrence, crib-biting being more frequent for some horses and then by stall walking and weaving (Albright et al., 2009; Muñoz et al., 2009), which shows individual variation in animal response to similar situations (Leal et al., 2011). This variability could explain the different results obtained in the literature aimed at elucidating equine behavior.
Abnormal behaviors such as pawing and wood chewing were within the range reported for the same type of horses (Vieira, 2006; Leal, 2007; D´Almeida et al., 2014), but lower than the 8 to 30.3% range for other breeds and activities (Waters et al., 2002; Tadich et al., 2012). The above-mentioned authors recommended studying a possible link between wood chewing and crib-biting and sucking with other management factors. Another study found no association between genes and crib-biting (Hemmann et al., 2014).
Finally, CI showed chronic stress in most of the horses. It could explain the behavioral disturbances observed. These findings confirm the high degree of stress of patrol horses (Leal, 2007). The average CI for the present study was 0.21, similar to that reported in stalled horses with the same activities (Leal et al., 2011) but different from the 0.36 found in a different population, indicating better welfare despite performing the same activity (D’Almeida et al., 2014). Thus, prevalence of behavioral disturbances could be lowered by controlling stress factors.
According to Alexander and Irvine (1998), disruption of the circadian rhythm of cortisol (CI>0.30) indicates a horse inability to confront stress, which stimulates stereotypies (McGreevy and Nicol, 1998; Freymond et al., 2015). This contributes to the development of adaptation because of pressure, chronicity, and nature of the stimuli on the hypothalamic-pituitary-adrenal axis (Broom, 2006). This could explain the abnormal behaviors of some horses with normal CI in this study. In other words, stereotypies could be part of the adaptive process to stress. It was not possible to establish a link between management factors and occurrence of behavioral disturbances, as these conditions were similar for all individuals, thus, follow-up studies under different environments should be conducted.














