Introduction
The Brazilian tick fauna is currently represented by 70 species, of which 46 are classified in the Ixodidae family (Krawczak et al., 2015), and 24 in the Argasidae family (Barros-Battesti et al., 2015; Labruna et al., 2016; Wolf et al., 2016). Among this diversity, one species of Ornithodoros (Ramos et al., 2015) and several species of Amblyomma, Haemaphysalis and Ixodes have been reported parasitizing wild birds. Nevertheless, insights derived from the latest investigations suggest that this diversity is probably much higher than currently known (Luz and Faccini, 2013). The Amazon forest, the world’s largest tropical rainforest, harbors an immense biodiversity of plants and animal species (Kress et al., 1998; Mittermeier et al., 2003). More than 1,500 bird species are found in the Amazon basin (Sick, 1997; Mittermeier et al., 2003; Del Hoyo et al., 2013), representing 40% of all bird species that occur in the Neotropical Zoogeographic Region (Stotz et al., 1996). Despite this high diversity, the fauna of ticks associated with avian hosts is poorly known within this ecosystem. Knowledge of ticks infesting wild birds under natural conditions in the Amazonian biome is resumed to three studies, two of them from Brazil (Amazonas and Pará states; Ogrzewalska et al., 2010; Martins et al., 2014) and one from Peru (Ogrzewalska et al., 2012).
A series of studies have put in evidence the occurrence of a great diversity of rickettsial agents infecting ticks hosted by different vertebrate groups (amphibians, reptiles, birds, and mammals) in the Amazon biome (Labruna et al., 2004a; 2007; Parola et al., 2007; Ogrzewalska et al., 2010; 2012; De Barros-Lopes et al., 2014; Soares et al., 2015). Hence, knowledge of the diversity of ticks infesting Amazonian birds is of great public health concern because many of these birds have the potential to transport Rickettsia-infected ticks to other New World biomes, including into the Nearctic region (Mukherjee et al., 2014; Cohen et al., 2015). The present study evaluated the diversity of ticks and their rickettsial infection in wild birds from a forest fragment at the state of Acre, located in the southwestern region of Brazilian Amazon.
Materials and methods
Ethical considerations
Bird capture was authorized by the Chico Mendes Biodiversity Conservation Institute (ICMBio) of the Brazilian Environment Ministry via SISBIO license no. 23269-1 and “Centro Nacional de Pesquisa e Conservação de Aves Silvestres” (CEMAVE/ ICMBIO) of the Brazilian government through Project 1099 developed under the coordination of Edson Guilherme. This study was approved by the ethical Committee of Animal use, according to ethical principles in animal research adopted by the “Animal ethic committee” of the Federal University of Acre (protocol number 55/2015).
Study site
The Brazilian state of Acre (152,581 Km2 in area) is situated in the extreme Southwestern portion of the Northern Region of Brazil, which is primarily composed by the Amazon biome. The Northern and Eastern borders of Acre include the Brazilian states Amazonas and Rondônia, whereas the Southern and Western borders include Bolivia and Peru. The capital of Acre, Rio Branco, with 370,550 inhabitants, is the most populous city of the state (IBGE, 2015). The city has hot and humid equatorial climate, with mean annual temperature of 26.1°C. The rainy season is long and spans from October through May, and a relatively short dry season covers the remaining four months of the year. The average annual precipitation is 1,951 mm. Acre state is located entirely within the Amazon biome and within the Inambari center of bird endemism (Cracraft, 1985; Silva et al., 2005) with 667 registered bird species (Guilherme, 2012).
Bird capture and tick collection
Capture of birds was performed between February 2013 and August 2014 in an Amazon forest fragment situated within the campus of the Federal University of Acre, located within the city of Rio Branco. More than 200 bird species have been previously registered in the area of the university (Guilherme, 2001). Birds were caught by mist nets (12 m long × 2 m height, 36 mm mesh) with a total capture effort of 796,464 net- hours. Birds were identified with the help of bird field- guide handbooks (Hilty and Brown, 1986; Ridgely and Tudor, 1989; 1994) and the species nomenclature followed the classification of the official Brazilian bird committee, the “Comitê Brasileiro de Registros Ornitológicos” (CBRO, 2014).
Every captured bird was rigorously examined for the presence of ticks by observing the whole body by naked eyes. When present, ticks were manually removed with forceps and stored in tubes containing 70% ethanol. Then, birds were marked with numbered rings (provided by “Centro Nacional de Pesquisa e Conservação de Aves Silvestres”, Brazil) and released at the same capture site.
Tick identification
In the laboratory, ticks were morphologically identified following Martins et al. (2010; 2013) for species of Amblyomma nymphs. Tick prevalence (number of infested birds/number of captured birds × 100) was calculated following Bush et al. (1997).
In order to confirm taxonomic identification of nymphs, and to perform species-level identification of Amblyomma larvae, 27 nymphs and 12 larvae were individually submitted to DNA extraction by the Guanidine Isothiocyanate technique (Sangioni et al., 2005). Subsequently, attempts to amplify a ≈460-bp fragment of the tick mitochondrial rRNA gene were performed as described elsewhere (Mangold et al., 1998). When amplification was successful, PCR products were purified with ExoSAP- IT® and sequenced in an ABI automated sequencer (Applied Biosystems/Thermo Fisher Scientific, model ABI 3500 Genetic Analyser, Foster City, CA). Obtained sequences were assembled with Geneious R9 software (Biomatters Ltd., New Zealand) and then submitted to BLAST analyses (www.ncbi.nlm.nih.gov/blast) to determine maximal similarities with available sequences in GenBank.
Detection of Rickettsia in ticks
Tick-extracted DNA samples from larvae and nymphs were individually tested by different PCR protocols targeting rickettsial DNA. The first screening PCR was performed using primers CS-78 and CS-323, which target a 401-bp fragment of the gltA gene that occurs in all Rickettsia species (Labruna et al., 2004b). Positive samples with expected-size fragments were then tested with primers Rr190.70F and Rr190.701R targeting a fragment of ≈632-bp of the rickettsial ompA gene, present only in Rickettsia species belonging to the spotted fever group (SFG; Regnery et al., 1991), and with primers 120-M59 and 120-807, which amplify a ≈832-bp fragment of the rickettsial ompB gene (Roux and Raoult, 2000). PCR products were sequenced and submitted to BLAST analyses as described above.
Results
Overall, 1,322 birds belonging to 16 orders and 37 families were captured. Of these, 79 (6.0%) bird specimens corresponding to 28 species were infested by 521 specimens of the following tick species: Amblyomma nodosum Neumann, 1899 (72 nymphs), Amblyomma longirostre (Koch, 1844; seven larvae, 13 nymphs), Amblyomma humerale Koch, 1844 (four nymphs), Amblyomma geayi Neumann, 1989 (two larvae, two nymphs), and 421 larvae of Amblyomma spp (Table 1). PCR amplification of the mitochondrial 16S rRNA gene was successful for 31 of 39 ticks submitted to DNA extraction. However, high quality sequences were obtained for only 26 specimens, confirming the morphological diagnoses of 17 nymphs and providing the taxonomic identification of nine larvae. The remaining tick larvae were regarded as Amblyomma spp. Voucher tick specimens have been deposited in the “Coleção Nacional de Carrapatos Danilo Gonçalves Saraiva” tick collection at the University of São Paulo, SP, Brazil (accession numbers: CNC 2799-2807).
Table 1 Ticks infesting wild birds in Acre state (Brazil) from February 2013 to August 2014.
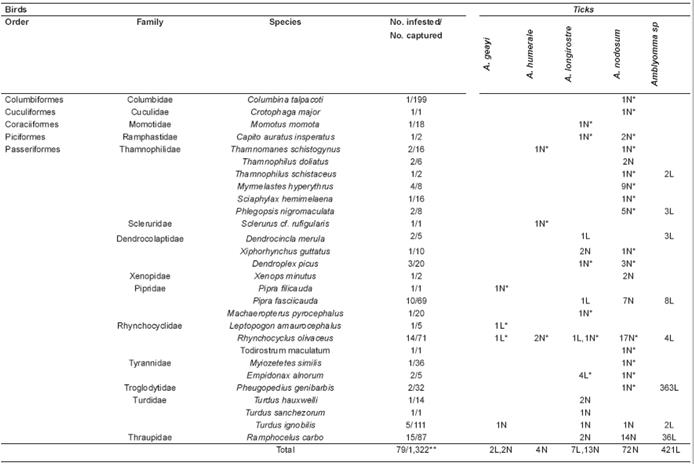
*First record of this tick species on this specific bird species. **Total number of captured birds. Larvae: L; Nymphs: N
Infestations in each bird specimen usually consisted of few ticks (mostly one tick per infested bird; range: 1-18). The only exception was a sole individual of Pheugopedius genibarbis (Swainson, 1838; Passeriformes: Troglodytidae) infested with 363 larvae of Amblyomma spp. No ticks were found on the following bird species (number of captured individuals in parentheses): Tinamiformes, Tinamidae: Crypturellus undulatus (Temminck, 1815; 1); Anseriformes, Anatidae: Dendrocygna viduata (Linnaeus, 1766; 1); Pelecaniformes, Ardeidae: Tigrisoma lineatum (Boddaert, 1783; 1), Ixobrychus exilis (Gmelin, 1789; 1), Butorides striata (Linnaeus, 1758; 4), Pilherodius pileatus (Boddaert, 1783; 1), Accipitriformes, Accipitridae: Rupornis magnirostris (Gmelin, 1788; 2); Gruiformes, Aramidae: Aramus guarauna (Linnaeus, 1766; 1), Rallidae: Porphyrio martinicus (Linnaeus, 1766; 5); Charadriiformes, Charadriidae: Vanellus chilensis (Molina, 1782; 3), Scolopacidae: Tringa solitaria (Wilson, 1813; 6), Jacanidae: Jacana jacana (Linnaeus, 1766; 27), Columbiformes, Columbidae: Leptotila verreauxi Bonaparte, 1855 (15), Leptotila rufaxilla (Richard and Bernard, 1792; 13), Leptotila sp (1), Geotrygon montana (Linnaeus, 1758; 9); Cuculiformes, Cuculidae: Coccycua minuta (Vieillot, 1817; 2), Crotophaga ani Linnaeus, 1758 (31); Strigiformes, Strigidae: Megascops choliba (Vieillot, 1817; 1); Caprimulgiformes, Caprimulgidae: Hydropsalis albicollis (Gmelin, 1789; 2); Apodiformes, Apodidae: Chaetura meridionalis Hellmayr, 1907 (1), Chaetura brachyura (Jardine, 1846; 1), Tachornis squamata (Cassin, 1853; 28); Trochilidae: Glaucis hirsutus (Gmelin, 1788; 18), Phaethornis ruber (Linnaeus, 1758; 6), Phaethornis hispidus (Gould, 1846; 4), Anthracothorax nigricollis (Vieillot, 1817; 1), Thalurania furcata (Gmelin, 1788; 3), Amazilia lactea (Lesson, 1832; 11); Coraciiformes, Alcedinidae: Chloroceryle amazona (Latham, 1790; 5), Chloroceryle aenea (Pallas, 1764; 4), Chloroceryle americana (Gmelin, 1788; 2), Chloroceryle inda (Linnaeus, 1766; 1); Galbuliformes, Bucconidae: Bucco macrodactylus (Spix, 1824; 4), Monasa nigrifrons (Spix, 1824; 9); Piciformes, Picidae: Picumnus rufiventris (Bonaparte, 1838; 3), Veniliornis affinis (Swainson, 1821; 5), Veniliornis passerines (Linnaeus, 1766; 4), Colaptes punctigula (Boddaert, 1783; 4), Celeus spectabilis Sclater and Salvin, 1880 (2), Campephilus melanoleucos (Gmelin, 1788; 1); Psittaciformes, Psittacidae: Aratinga weddellii (Deville, 1851; 2), Brotogeris sanctithomae (Statius Muller, 1776; 3); Passeriformes, Thamnophilidae: Myrmotherula axillaris (Vieillot, 1817; 10), Thamnophilus murinus Sclater and Salvin, 1868 (3), Taraba major (Vieillot, 1816; 1), Myrmelastes schistaceus (Sclater, 1858; 4), Hypocnemis subflava Cabanis, 1873 (2); Dendrocolaptidae: Dendrocincla fuliginosa (Vieillot, 1818; 9), Campylorhamphus trochilirostris (Lichtenstein, 1820; 5); Furnariidae: Furnarius leucopus Swainson, 1838 (1); Pipridae: Neopelma sulphureiventer (Hellmayr, 1903; 9); Rhynchocyclidae: Corythopis torquatus Tschudi, 1844 (2), Hemitriccus flammulatus Berlepsch, 1901 (6), Tyrannidae: Elaenia spectabilis Pelzeln, 1868 (5), Elaenia parvirostris Pelzeln, 1868 (13), Tyrannulus elatus (Latham, 1790; 1), Phaeomyias murina (von Spix, 1825; 2), Pitangus sulphuratus (Linnaeus, 1766; 22), Philohydor lictor (Lichtenstein, 1823; 1), Myiodynastes maculatus (Statius Muller, 1776; 2), Tyrannopsis sulphurea (Spix, 1825; 1), Megarynchus pitangua (Linnaeus, 1766; 1), Myiozetetes cayanensis (Linnaeus, 1766; 24), Tyrannus melancholicus Vieillot, 1819 (39), Pyrocephalus rubinus (Boddaert, 1783; 4), Cnemotriccus fuscatus (Wied, 1831; 1); Vireonidae: Cyclarhis gujanensis (Gmelin, 1789) (1), Vireo olivaceus (Linnaeus, 1766; 2), Vireo chivi (Vieillot, 1817; 5), Vireo flavoviridis (Cassin, 1851; 1); Hirundinidae: Stelgidopteryx ruficollis (Vieillot, 1817; 18); Troglodytidae: Troglodytes musculus Naumann, 1823 (13), Cantorchilus leucotis Swainson, 1831 (16); Turdidae: Turdus amaurochalinus Cabanis, 1850 (1); Icteridae: Cacicus cela (Linnaeus, 1758; 4); Thraupidae: Tangara mexicana (Linnaeus, 1766; 1), Tangara episcopus (Linnaeus, 1766; 18), Tangara palmarum (Wied, 1821; 8), Volatinia jacarina (Linnaeus, 1766; 2), Sporophila lineola (Linnaeus, 1758; 1), Sporophila castaneiventris Cabanis, 1849 (11), Sporophila angolensis Cabanis, 1847 (1), Sporophila sp (1), Saltator maximus (Statius Muller, 1776; 4), Saltator coerulescens Vieillot, 1817 (4), Saltator azarae d’Orbigny, 1839 (2); Fringillidae: Euphonia chlorotica (Linnaeus, 1766; 1), Emberizidae: Ammodramus aurifrons (Spix, 1825; 23).
Rickettsia DNA was detected in 29.0% (9/31) of the tested ticks, from which fragments of the gltA, ompA, and ompB genes were obtained. The eight ticks with negative results for the tick mitochondrial 16S rRNA PCR were excluded from this analysis. Sequences from three A. nodosum nymphs were 100% identical to Rickettsia sp strain NOD (GenBank accession numbers EU567177, EU567180, EU567179 for gltA, ompA, ompB, respectively). Sequences from four larvae and one nymph of A. longirostre, and one larva of A. geayi were 100% identical to Rickettsia amblyommatis (=“Candidatus Rickettsia amblyommii”; GenBank accession numbers JX867425, KF702333, JX867427 for gltA, ompA, ompB, respectively). GenBank accession numbers for the partial sequences of the tick mitochondrial 16S rRNA obtained in the present study were deposited in GenBank and received the accession numbers KX999294 (A. geayi, larva), KX999295 (A. longirostre, larva), and KX999296 (A. nodosum, nymph). GenBank accession numbers for the partial sequences of the rickettsial genes gltA, ompA and ompB are KY008392, KY008394 and KY008396 for Rickettsia sp strain NOD, and KY008393, KY008395 and KY008397 for R. amblyommatis.
Discussion
Four tick species, A. geayi, A. humerale, A. longirostre and A. nodosum, were found parasitizing wild birds in Acre state, similarly to two previous studies in eastern and western Brazilian Amazon, where these four tick species were also found parasitizing passerine hosts (Ogrzewalska et al., 2010; Martins et al., 2014). While the number of studies about ticks of passerine birds is still very low for the Amazon biome, current data suggest that A. geayi, A. humerale, A. longirostre and A. nodosum are at least among the most common bird-associated ticks of the Amazon. In addition, for the first time, the present study reports 27 bird species parasitized with at least one of these four Amblyomma species (Table 1).
Amblyomma longirostre and A. nodosum were the most prevalent tick species in the present study. Adults of A. longirostre feed primarily on porcupines (Rodentia, Erethizontidae), whereas adults of A. nodosum are commonly found on anteaters, Myrmecophaga tridactyla Linnaeus, 1758 and Tamandua spp (Guglielmone et al., 2014). Larvae and nymphs of these two tick species seem to feed primarily on birds (reviewed by Luz and Faccini,2013; Ogrzewalska and Pinter, 2016), an ecological association reinforced by results of the present study. These species were also found migrating to the North.
While adults of A. humerale feed primarily on tortoises (Chelonoidis spp), adults of A. geayi feed on sloths (Bradypus spp and Choloepus spp; Guglielmone et al., 2014). Based on these records of mature stages, established populations of both tick species are known to be almost restricted to the Amazon biome, yet hosts records for their immature stages are scarcely documented and currently limited to passerine birds and medium-sized mammals (Labruna et al., 2002; Ogrzewalska et al., 2010; Martins et al., 2013; 2014; Soares et al., 2015). Our results, in conjunction with these previous reports, suggest that passerine birds may also play an underestimated role as hosts for immature stages of A. humerale and A. geayi.
According to literature data (Aragão, 1936; Guimarães et al., 2001; Souza et al., 2016), the following nine tick species have been previously reported in Acre state: Amblyomma calcaratum Neumann, 1899, Amblyomma coelebs Neumann, 1899, Amblyomma dissimile Koch, 1844, A. geayi, Amblyomma incisum Neumann, 1906, A. longirostre, Amblyomma oblongoguttatum Koch, 1844, Amblyomma ovale Koch, 1844, and Rhipicephalus microplus (Canestrini, 1888). According to Labruna et al. (2005), previous reports of A. incisum from the Amazonian region require confirmation, as they could be misidentified with Amblyomma scalpturatum Neumann, 1906 or Amblyomma latepunctatum Tonelli-Rondelli, 1939; hence, we do not consider valid the report by Aragão (1936) of A. incisum in Acre.
Herein, we report two species, A. humerale and A. nodosum, for the first time in Acre. In addition, the CNC tick collection contains the following unpublished records of ticks in Acre: A. scalpturatum (CNC-1663: One male ex. vegetation, Xapuri, 17 July 2008); Amblyomma rotundatum (CNC-1667: One female ex. Rhinella sp, Assis Brasil, December 2009), and Ixodes luciae (CNC-1665, 1666, 2870: 10 males, eight females, ex. Didelphis marsupialis, Assis Brazil, December 2009). These whole data indicate that the tick fauna in Acre is currently represented by 13 tick species.
We report two SFG rickettsial agents, Rickettsia sp strain NOD in A. nodosum ticks, and R. amblyommatis in A. longirostre and A. geayi ticks. These Rickettsia- tick associations have been previously reported in other areas of Brazil (Ogrzewalska et al., 2009; 2010; Soares et al., 2015), reinforcing that these are not just occasional associations. In these previous studies, R. amblyommatis was reported as R. amblyommii or ‘Candidatus R. amblyommii’. However, a recent study reassessed the taxonomic status of this agent, which is now validated as R. amblyommatis (Karpathy et al., 2016). Despite a nearly widespread distribution of tick infection by these two rickettsial agents in South America, including the Amazon region, their role as human pathogens remains unknown. Finally, the present study provides a basic background occurrence of immature stages of Amblyomma spp in wild birds at the southwestern Brazilian Amazon, and for the dispersion of Rickettsia-infected ticks.














