Introduction
Tarsus hyperflexion is a sign of biomechanical alteration in several clinical conditions affecting the pelvic limbs of horses (e.g. bone spavin, suspensory desmitis, alterations in the femorotibiopatellar joint, and classical Australian stringhalt). Therefore, specific diagnostic approaches such as clinical evaluation, anesthetic blocks, radiology, ultrasound, and electromyography are required to determine the origin of this alteration, although in some cases it is considered of idiopathic origin (Duque et al., 2014). However, most clinicians initially relate cases of tarsus hyperflexion to classical stringhalt in the Colombian creole horse (CCH).
Classic stringhalt-related tarsus hyperflexion is one of the exclusive pathologies in CCH, affecting the sports performance of the animals. The appearance of the pelvic limb affected by tarsus hyperflexion has been traditionally considered an inherited condition, although it has not been demonstrated. Stringhalt causes economic losses due to rejection from competition and reproduction. The almost unmistakable clinical sign of classic stringhalt is an involuntary hyperflexion of the tarsus.
The etiology of classic stringhalt is still unclear and, in many cases, it remains unknown. Neuropathic muscle atrophy of the lateral digital extensor (LDE) muscle has been reported as a possible cause (Valentine, 2003; Araujo et al., 2008). Other researchers consider pathologies affecting tarsus or hock joints, such as osteoarthritis, may induce this condition (Crabill et al., 1994). Tarsus injuries, specifically those affecting its lateral aspect (femorotibiopatellar joint), nerve lesions (degeneration of the sciatic nerve and peroneus nerves) and foot lesions can cause spasmodic contraction of the muscles, flexing the pelvic extremity and, therefore, induce the condition (Cahill et al., 1985; Crabill et al., 1994; Araya et al., 1998).
Ultrasonography is a widely used diagnostic imaging technique to evaluate injuries to soft tissues and synovial structures (Whitcomb, 2006; Garret, 2014). However, its use has not been standardized for the study and diagnosis of stringhalt in horses. The tarsus is a complex anatomical region with a large number of tendons, ligaments, and synovial structures (Dik, 1993). Therefore, comprehensive knowledge of the regional anatomy and ultrasound technique are required to successfully perform tarsus ultrasonography (Whitcomb, 2006).
The LDE muscle, its tendon, and the associated synovial sheath can be accessed by ultrasonography from the lateral side of the pelvic limb (tarsus) and the proximal third of metatarsus III, respectively, through transverse and longitudinal sections (Reef, 1998; Whitcomb, 2006; Garret, 2014). Under normal conditions, LED muscle and tendon are observed using a cross-section in an oval segment, with well-defined borders and homogenous echogenicity. In a longitudinal section, the tendon shows a parallel fibrillar pattern and well-defined edges throughout its length (Dik, 1993).
The cause of classic stringhalt in the CCH has been diagnosed with ultrasonography on alterations to the LDE muscle and tendon, and synovial sheath adhesions. The treatment described for classic stringhalt is tenectomy of the LDE and partial myectomy (Sullins, 2002). Adherences can be prevented with medical procedures that improve the condition. Although results are not always immediate, prognosis is favorable (Stashak, 2004; Torre, 2010).
To the best of our knowledge, no studies have related stringhalt to alterations of either LDE muscle, its tendon, or adhesions of the synovial sheath. The objective of this study was to establish the presence of LDE muscle, tendon, and synovial sheath alterations using an ultrasonographic technique in the CCH with clinical signs of tarsus hyperflexion, comparing the affected structures with those observed in healthy horses. Additionaly, adhesions between the tendon and its synovial sheath were studied and histopathology of the LDE muscle and tendon was conducted.
Materials and Methods
Horses
Thirty adult CCHs from commercial stalls were monitored, regardless of weight, age, or sex. Two experimental groups were analyzed by ultrasound. The first group (control) consisted of 15 clinically healthy horses, with no history of locomotion alterations. This status was confirmed by a general and specific physical examination of the locomotor system (in static and dynamic), as referred by Stashak (2004). These specimens were evaluated by a cross-sectional and longitudinal ultrasound examination of the LDE muscle and tendon (Garret, 2014). This group was considered as reference for the normal findings. The second group consisted of 15 horses with clinical signs of hock hyperflexion, confirmed by physical examination as proposed by Stashak (1987). Tarsal hyperflexion was categorized according to the grading described by Huntington et al. (1989). These specimens were also subjected to ultrasound examination, evaluating the structures of LDE muscle, tendon, and synovial sheath, in order to compare their results with those of Group 1. Radiographic evaluation was also performed to this group. No anesthetic diagnostic blocks were used to dismiss other causes of hyperflexion.
Ultrasound, radiographic, and histopathological evaluations
Horses from both groups were subjected to physical restriction. Before scanning, trichotomy of the area was performed if the owner authorized the procedure; otherwise, the coat was removed using scissors. An Aquila VET ultrasound scanner, manufactured by Pie Medical, from Maastricht, The Netherlands, equipped with a linear 8.0 MHZ ultrasonic transducer, attached to a standof pad, was used. The examination of the LDE muscle and tendon was carried out when these structures were in tension or supporting their weight, because relaxation of the tendons causes a reduction in the gray scale of the image, producing hypoechoic artefacts that could be confused with lesions (Wood et al., 1991; Boswell and Schramme, 2000).
Ultrasound evaluations were performed in two planes of the right angle. Transverse plane sections were performed to evaluate the size and echogenicity of the section. By slightly rotating the back of the transducer, the optimal angle of presentation of the ultrasound was defined. When the image was brighter than the previous ones, the recommended angle for the study of the muscle and tendon in the examined area was established. Subsequently, longitudinal plane sections were performed to establish the length and morphology of the muscular and tendinous fibrils. Image enhancement was achieved by slowly moving the transducer to the lateral and medial sides, comparing the relative echogenicity and internal alignment of the muscle and tendon fibers under study. Once the transducer was located, measurements of the LDE tendon thickness were taken, as well as of the space between the tendon synovial sheath where it crosses the lateral border of the tarsus.
Specimens from Group 2 were also subjected to radiographic and histopathological examination. Radiographic studies from the knee to the third phalanx were taken with a Donmun-100P, manufactured by Dongmun, from Goyang City, South Korea, considering four projections: Medial-lateral (ML), plantar-dorsum (PD), lateral-lateral or medial-oblique (LLMO), and medial dorsal-plane or oblique-lateral (MDOL). The examination was carried out to determine if there were pathologies other than those of soft tissue that would alter the possible results of the study. In such case, a new patient was admitted to the group fulfilling the inclusion criteria.
Samples of the LDE muscle and tendon were obtained by myotenectomy and transported in a 10% formalin solution. Samples were fixed in paraffin and stained with hematoxylin and eosin to determine mixed polyneuropathy with axonal degeneration and demyelination of nerve fibers (Armengou et al., 2010). Histopathological studies were carried out on muscle and tendon samples to correlate these with the ultrasound findings.
Statistical analysis
The data obtained from echographic and histopathological examinations were processed by descriptive statistics and presented in percentages with respect to all the horses in Group 2 with signs of evident tarsus hyperflexion. These results were compared with Group 1, considered normal (no signs of hyperflexion). A t-test was used to compare tendon thickness and the space between the tendon synovial sheath where it crosses the lateral border of the tarsus. Both measurements were done for each limb and contralateral, by group, and between groups (1 and 2). A p<0.05 was consider significant.
Results
Group 1 (control) did not show any pathological findings on physical and clinical examinations. The transverse plane echographic evaluation of this group showed an oval section with well-defined borders and a homogenous echogenicity. A well-defined parallel fibrillar pattern in the longitudinal plane was also observed (Figure 1A).
Five horses in Group 2 showed signs of bilateral hyperflexion (with 4/5 to 5/5 degrees), and 10 horses showed unilateral hyperflexion (with 1/5 to 3/5 degrees), according to Huntington et al. (1989). An 86% of the horses in Group 2 showed echographic alterations. A 53.3% (8/15) showed compatible findings of LDE tendon adhesions (Figure 1B) and a 33.3% (5/15) showed an increase in LDE tendon synovial sheath fluid (Figure 1C). Two horses with increased sheath fluid presented bilateral hyperflexion and 4/5 degrees of flexion. The three horses remaining showed unilateral hyperflexion and 3/5 degrees of flexion. Finally, no echographic alterations were found in 13.3% (2/15) of the horses that presented unilateral hyperflexion with 1/5 degrees of flexion. The equines with the greatest alterations were those showing the highest degree of flexion. No radiographic alterations were found in this group.
Measurements, by animal and group, of the LDE tendon thickness as well as space between tendon synovial sheath where it crosses the tarsus lateral border are shown in Table 1. The comparison of means (SD±) of the variables in each group did not show significant differences. However, a significant difference (p<0.05) was found when comparing the two variables between groups.
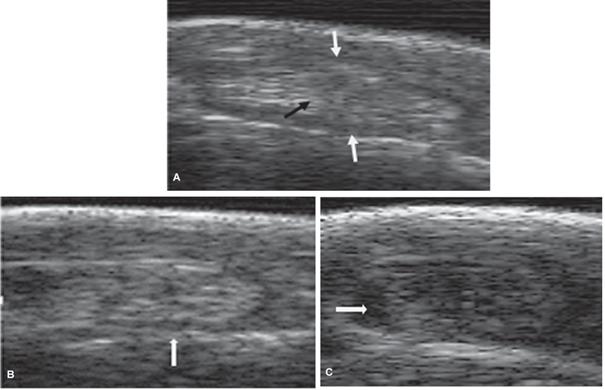
Figure 1 Ultrasound images (linear 8.0 MHZ ultrasonic transducer with coupling) of LDE tendon and synovial sheath where it crosses the lateral border of the tarsus in a CCH. A) Transverse ultrasonographic image of a normal LDE tendon (black arrow) and its synovial sheath (white arrows). B) Transverse ultrasonographic image of an abnormal LDE tendon of tarsus hyperflexion, showing adhesions between the tendon and the synovial sheath (white arrow). C) Transverse ultrasonographic image of an abnormal tarsus (hyperflexion) showing liquids between the synovial sheath and the LDE tendon (white arrow).
Table 1 Ultrasound measurements of the LDE tendon thickness as well as space between tendon and synovial sheath where it crosses the lateral border of the tarsus, of both pelvic limbs of healthy horses (Group 1) and of horses affected by tarsus hyperflexion (Group 2).
| Limb evaluated | Mean | Standard error | SD± | Variance | Minimum | Maximum |
|---|---|---|---|---|---|---|
| Healthy horses (Group 1) | ||||||
| Thickness of the LDE† tendon (mm) | ||||||
| RPL* | 4.89Aa | 0.16 | 0.63 | 0.40 | 3.9 | 5.9 |
| LPL** | 4.84Ac | 0.16 | 0.62 | 0.38 | 4.1 | 5.9 |
| Space between the LDE† tendon and the synovial sheath (mm) | ||||||
| RPL | 1.23Ba | 0.04 | 0.15 | 0.02 | 1 | 1.5 |
| LPL | 1.21Bc | 0.04 | 0.16 | 0.03 | 1 | 1.4 |
| Horses affected by hock hyperflexion (Group 2) | ||||||
| Thickness of the LDE† tendon (mm) | ||||||
| RPL | 4.17Ab | 0.27 | 1.06 | 1.12 | 2.7 | 5.4 |
| LPL | 4.12Ad | 0.20 | 0.78 | 0.61 | 2.7 | 5.4 |
| Space between the LDE† tendon and the synovial sheath (mm) | ||||||
| RPL | 0.63Bb | 0.05 | 0.20 | 0.04 | 0.3 | 1.1 |
| LPL | 0.54Bd | 0.03 | 0.13 | 0.02 | 0.4 | 0.8 |
SD±: Standard deviation. Same capital letters show no difference between limbs of the same group for each variable. Different lowercase letters show significant difference (p<0.05) between limbs of different groups for each variable. †Lateral digital extensor tendon. *Right pelvic limb. **Left pelvic limb.
Regarding the histopathological study, only samples from eight horses from Group 2 (53.3%) showed shape and size multifocal irregularities of fascicular muscle fibers.
Discussion
Tarsus hyperflexion has been linked to other pathologies that alter the biomechanics of the related joint (bone spavin, suspensory desmitis, stifle joint pathologies), being some of the structures reported elsewhere (described as secondary or incidental findings in some cases) the ones evaluated in this study. Consequently, tarsus hyperflexion cannot be only limited to the stringhalt types described in the literature. Therefore, specific diagnostic aids are needed to differentiate and dismiss other etiologies. In the present study, anesthetic blocks and electromyography were not performed, so we described clinical cases as tarsus hyperflexion instead of classic stringhalt.
Control status was assigned to Group 1 due to absence of lameness or clinical signs of hyperflexion at initial examination. In addition, those animals had no history of treatments for recent locomotion conditions. Likewise, normality of structures was verified by echographic evaluation, as described by Garret (2014), so results obtained in the group with tarsus hyperflexion were comparable.
Clinical evaluation of Group 2 verified variability between hyperflexion degrees, and unilateral or bilateral presence of hyperflexion. Absence of abnormalities (as determined by radiological evaluation) dismissed other pathologies that, according to state of evolution and severity, could lead to gait disorders similar to hyperflexion as reported in classic stringhalt. However, no anesthetic diagnostic blocks (to tibial or peroneal nerves) were used to dismiss other causes of tarsal pain. This could be considered a limitation of the present study.
Abnormal ultrasound findings in Group 2 were in agreement with those reported by Reef (1998), Stashak (2004), and Garret (2014). Undefined borders characterize these findings, as well as non-parallel fibrillar patterns in most tendons, and no echogenic homogeneity, contrasting with the findings described in Group 1, which did not present the characteristic sign of hyperflexion and other locomotion alterations, as described above. Nevertheless, it is unknown why the LDE muscle ends up involved in a hyperflexion that shows certain degrees of improvement with the tenectomy (Stashak, 2004; Torre, 2010), a fact that encourages further investigation into the cause of this clinical condition.
During the myotenectomy procedure in patients in Group 2, a greater tensile force (up to two people) was required to remove the ruptured tendon in its distal part. This observation was possibly due to the rupture of the adhesions that were formed around the tendon when crossing the tarsus, as it was found echographically in our study (53.3%), and previously described by Stashak (2004). Although there is no elucidated cause of classic stringhalt in the CCH, it has been described as idiopathic (Duque et al., 2014). However, there is a lack of research aimed at considering the inflammatory process in the LDE muscular package derived from injury due to repetitive exercise (Martínez et al., 2007).
Five horses (33.3%) presented alterations in the synovial fluid volume according to the ultrasound examination. These findings are possibly related to biomechanical stress, generating inflammation and adhesions, as previously described (Stashak, 2004). Although the origin of the fluid was not determined, the effusion was present, in contrast to what was observed in Group 1 horses. On the other hand, only two horses (13.3%) with tarsus hyperflexion of lower degree did not show any ultrasound findings. Therefore, it could be inferred that flexion severity is related to the presence of ultrasonographic alterations (Whitcomb, 2006; Garret, 2014).
The LDE tendon thickness and width of the space between synovial sheath and the same tendon were equal between limbs of the same horse, in each of the two groups. These findings contrast when comparing between the two groups. Group 2 showed significantly smaller measurements by both variables when crossing the lateral aspect of the tarsus. This narrowness could be clinically related to horse`s hock stenosing tenosynovitis, leading to the presentation of stringhalt-like manifestations, followed by adhesions reported in our study and by other authors (Stashak, 2004), possibly due to inflammatory processes as a result of repetitive exercise (Martínez et al., 2007).
Histopathological findings were possibly associated with axonal degeneration and vacuolization of nerve fibers, as previously reported in classic stringhalt in CCH (Duque et al., 2014). Similarly, these findings can be associated with axonopathy and neuromuscular deficit (Domange et al., 2010; Pennington et al., 2011; Oliver and Suarez, 2016). Therefore, a localized nerve dysfunction may be involved in the presentation of hyperflexion, which characterizes both stringhalt types.
Electromyographic evaluation was not performed in our study. We consider that this diagnostic approach could have revealed similar findings to those reported by other authors (Duque et al., 2014; Oliver and Suárez, 2016). Similar histopathological findings observed in this study would reaffirm the myoelectric deficits reported for tension and flexion during the LDE evaluation. On the other hand, it is important to emphasize that none of the previously described studies used ultrasound as a diagnostic aid.
In this study, the association observed between clinical, echographic, and histopathological findings reveal a relationship between the presence of alterations and the degree of severity. Horses presenting obvious alterations in the myofibrillar pattern showed the highest degree of flexion, independently of whether it was unilateral or bilateral tarsus hyperflexion.
Finally, 86.6% of horses with signs of hock hyperflexion showed echographic alterations of the LDE tendon and synovial sheath. When confronting these findings with 53.3% of horses showing histopathological changes, inference could be done on the possible cause of stringhalt. However, the way in which histopathological changes are generated in the two types of stringhalt is still not clear. Australian stringhalt focuses on damages caused by a neurotoxin that compromises several long and peripheral nerves, in contrast to the classic stringhalt, where a chronic inflammatory process in the LDE tendon alters tarsus biomechanics (Huntington et al., 1989; Pennington et al., 2011). Nevertheless, the histopathologic results show similar neuromuscular alteration. We hypothesize that neuromuscular damage in classic stringhalt could be secondary to chronic inflammatory processes (adhesions) derived from repetitive injury (trauma), particularly in the CCH.
In conclusion, the ultrasound and histopathological results in this study are indicative of alterations of the LDE tendon and synovial sheath caused by tarsus hyperflexion, possibly related to classic stringhalt in the CCH.














