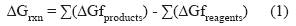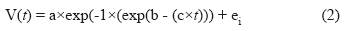Introduction
Fatty acids (FAs) metabolism has a major influence on the FAs composition of ruminant-derived food products. Unsaturated FAs, including linoleic acid (LA; c9,c12-18:2) and alpha-linolenic acid (LN; c9,c12,c15-18:3), are abundant in grass and certain other ruminant feedstuffs (Buccioni et al., 2012). However, LAand LN are present at low concentrations in milk and meat, because they are transformed into numerous isomers, including trans-vaccenic acid (VA; t11-18:1) and conjugated linoleic acid (CLA; c9,t11- 18:2) (Hur et al., 2017). Consumption of CLA has been associated with human health benefits (Ferlay et al., 2017).
The main ruminal biohydrogenation (BH) pathways of LA and LN have been described since the 60s (Kepler and Tove, 1967) and researchers have focused on better understanding their intricate pathways (Laverroux et al., 2011; Ferlay et al., 2017). Researchers have also studied which microbe communities (Kim et al., 2003; Maia et al., 2010) and dietary FAs (Buccioni et al., 2012; Prieto et al., 2013) play major roles in increasing VA synthesis in the rumen. The main interest in VA is because it is the main endogenous precursor of CLA in milk and meat (Kay et al., 2004; Rezamand et al., 2014).
Some in vitro studies suggested that increasing levels of LN decrease the BH rate of VA, resulting in enhanced levels of VA in rumen fluid (Jouany et al., 2007; Honkanen et al., 2012). On the other hand, Ribeiro et al. (2007) reported that BH rate of LA is twice as fast as BH rate of LN, consequently producing more VA from LA than from LN. These contradictory results may be due to the use of LA and LN in esterified forms (Hoffmann et al., 2015) and the presence of other FAs in fat supplements, which could interfere LA and LN ruminal BH (Pashaei et al., 2016).
The scientific literature is still inconclusive regarding the effects of LA or LN levels on VA ruminal concentration. Therefore, the aim of this study was to evaluate the effects of LA:LN ratio in lipid supplements on the rumen biohydrogenation kinetics of LA and LN, as well as on the trans-vaccenic acid (VA) production, using an in vitro system.
Material and Methods
Ethical considerations
All procedures were approved by the Bioethics Committee of Facultad de Medicina Veterinaria y de Zootecnia, Universidad Nacional de Colombia (Act 001 of 2010).
Forage collection, animal, and preparation of rumen inoculum
Samples of Kikuyu grass (Cenchrus clandestinus) with 60 days of regrowth were harvested by hand plucking (Cook, 1964). Forage samples were dried at 60 °C and ground to pass a 1-mm sieve (Romer Labs, Getzersdorf, Austria).
One rumen-fistulated Holstein steer was used as donor of rumen fluid. The animal was kept in a pen during nighttime and received kikuyu grass, clean fresh water, and mineral block ad libitum for 30 d before the rumen fluid was collected. In day 30 (at 07:00 h), 1,500 mL of rumen fluid were obtained from the steer before feeding. The rumen fluid was filtered through four layers of gauze, placed into a flask preheated at 39°C, transferred to separator funnels, and gassed with CO2, recovering the intermediate phase. The collected phase was mixed with McDougall buffer 1:4 (McDougall, 1948), gassed with CO2, and added with urea (1 g/L buffer), producing the rumen inoculum.
Diets, incubation procedures, and sample collections
Fifty milliliters of rumen inoculum pre-warmed at 39 °C were placed into 100 mL incubation tubes containing 500 mg ground kikuyu grass (1 mm) plus 16.3 mg/tube (3.3% of forage quantity to avoid toxic effects of excessive unsaturated FAs addition on cellulolytic population (Messana et al., 2013)) of different LA:LN ratios (100:0, 75:25, 50:50, 25:75 or 0:100). Then, the filled tubes were gassed with CO2, sealed with one-hole rubber stoppers (Fisherbrand, Pittsburgh, PA, USA) and incubated in a water bath at 39°C (Blue Sland Illinois, USA) (Tilley and Terry, 1963). Extra kikuyu tubes without the addition of LA:LN ratio mixtures were assigned as control treatment (CTRL).
Incubation times were 0, 2, 4, 6, 8, or 16 hours. Extra tubes with only 50 mL of pre-warmed rumen inoculum were used as blanks. Three replications were prepared per treatment. Incubations were stopped by adding 500 µL of 20 g/L mercury (II) chloride (Merck®, Kenilworth, NJ, USA) solution, and placing the tubes in an ice bath. The content of the tubes was freeze-dried (Alpha 1-4 Christ® plus LO lyophilizer), ground (Romer Labs, Getzersdorf, Austria), and stored at -60 °C until analyses were performed.
Laboratory analysis
Forage samples were analyzed for crude protein (182 g/kg DM) (AOAC, 2006, method: 984.13), ash (117 g/kg DM) (AOAC, 2006, method: 942.05), and ether extract (30.0 g/kg DM) (AOAC, 2006, method: 930.09). Neutral detergent fiber (609 g/kg DM) was determined using alpha-amylase without sodium sulfite addition, according to Van Soest et al. (1991).
Acid detergent fiber (322 g/kg DM) was determined using the method described by Goering and Van Soest (1970).
The FAs in forage samples and incubated tubes were extracted and methylated according to Garcés and Mancha (1993). The FAs in the LA:LN ratio mixtures were methylated using a 0.2 N solution of (m-trifluoromethylphenyl) trimethylammonium hydroxide (MethprepTM I) (AlltechTM, Nicholasville, KY, USA). Methylated FAs of forage and LA:LN ratio mixtures (Table 1) as well as the methyl esters of principal FAs intermediaries of LA and LN ruminal BH were quantified by GLC- FID using a Shimadzu GC-2014 gas chromatograph (Shimadzu Manufacturing, Inc., Canby, OR, USA). The column was a fused silica capillary (Rt-2560, 100 m x 0.25 mm i.d. x 0.2 μm film thickness; Restec®, Inc, Belefonte, PA, USA). Helium was used as the carrier gas. Detector and injector temperatures were 260 and 270 °C, respectively, and the split ratio was 30:1. Oven temperature was 140 °C for 5 min, increased by 4 °C/min to 220 °C, held for 5 min, increased by °C/min to 240 °C, and held for 10 min. The FAs in samples were identified by comparison of their retention times with those observed in commercial standards as Nu-Chek® Prep (Elysian, MN, USA), and quantified by direct comparison of the peak areas.
Thermodynamic analyses of LA and LN ruminal BH by computational chemistry
Thermodynamic analyses of LA and LN ruminal BH were performed using the Molecular Orbital Package Software (MOPAC) (Version 8.289L). The optimization method (PM6) parameter was used to establish the Gibbs free energy of formation (ΔGf) at 39 °C of the FAs produced during LA and LN ruminal BH. TheseΔGf values were used to calculateΔGrxn of the main steps of LA and LN ruminal BH (Stewart, 2007). The ΔGrxn for each step was calculated as follow:
Where:
∑(∆Gfproducts) and ∑(∆Gfreagents) are the sum of ∆Gf of products and reagents, respectively.
Calculations and statistical analysis
Ruminal BH of LA and LN for each treatment (with exception of CTRL treatment because FAs concentration of BH intermediaries were very low) was modeled by using a multi-compartmental model (Figure 1). The rates of transfer between FAs pools were estimated using the SAAM II software (SAAM, 1997). Trans-9 octadecenoic acid (t9-18:1), trans-6 octadecenoic acid (t6-18:1), cis-11 octadecenoic acid (c11-18:1), and cis-6 octadecenoic acid (c6- 18:1) (i.e., sum 18:1) were not included in the model as independent pools because their concentrations were very low. Therefore, just one pool was used to represent t9-18:1, t6-18:1, c11-18:1, c6-18:1, according to Ribeiro et al. (2007). Rumelenic acid (RU; c9,t11,c15-18:3) (i.e., produced by the cis-12 double bond isomerization of LN) was not determined in the incubation systems, thus it was not included in the model. The rate of transfer from stearic acid (SA; 18:0) to oleic acid (OA; c9-18:1) (dehydrogenation) was also included in the model because its inclusion produced the lowest AIC (Akaike information criterion) and because ruminal fungus P. communis can dehydrogenate SA to OA using a ruminal ∆9-desaturase (Kemp et al., 1984; Ferlay et al., 2017). The Rosenbrock integrator method (SAAM, 1997) was used and optimized with a variance model based on relative data and the forward derivatives.
Table 1 Fatty acid composition of forage, and c9,c12-18:2 (LA) and c9,c12,c15-18:3 (LN) ratio mixtures.
| LA:LN ratios | ||||||
|---|---|---|---|---|---|---|
| Fatty acid composition (g/100 g FAs) | Forage | 100:0 | 75:25 | 50:50 | 25:75 | 0:100 |
| 12:0 | 0.9 | -1 | - | - | - | - |
| 14:0 | 1.4 | - | - | - | - | - |
| 16:0 | 33.4 | - | - | - | - | - |
| 18:0 | 5.1 | - | - | - | - | - |
| 20:0 | 1.6 | - | - | - | - | - |
| c9-18:1 | 2.5 | - | - | - | - | - |
| c9,c12-18:2 | 11.6 | 100.0 | 75.7 | 49.3 | 23.9 | 0.30 |
| c9,c12,c15-18:3 | 43.0 | - | 24.3 | 50.7 | 76.1 | 99.7 |
| c5,c8,c11,c14-18:4 | 0.5 | - | - | - | - | - |
1Not detected.
The VA accumulation rate (r-VA) for each treatment was calculated by fitting the Gompertz model (Gompertz, 1825) using the NLIN procedure of SAS, and their initial parameters were determined by Curve Expert Basic (Version 1.4). The full mathematical model used was:
Where:
V is the VA concentration at t hours.
a is the concentration at 16 hours.
b is the difference between VA concentrations at 0 and 16 hours.
c is the r-VA.
ei is the residual error of the model.
The values of LA, LN, VA, and SA concentrations were analyzed as a completely randomized design in a 6×6 factorial arrangement of treatment and time using the MIXED procedure of SAS. The statistical model included fixed effects of treatment, hour of sampling, and hour of sampling × treatment (Snedecor and Cochran, 1989). The r-VA and the rates of transfer of the multi-compartmental models were analyzed as a completely randomized design. Differences between treatments were significant at p<0.05.
Results
The LA and LN concentration decreased (Figure 2(a) and (b)) in all treatments, whereas VA and SA increased (Figure 2(c) and (d)) as incubation time increased (p<0.01). In addition, VA concentration decreased (Figure 2 (c)), whereas SA concentration increased (Figure 2 (d)) from 100:0 to 0:100 LA:LN ratios, at 16- hour incubation (p<0.01). It was noteworthy that LA and LN concentrations unexpectedly increased from 2 to 4 h, whereas SA concentration unexpectedly decreased in the same period for treatments in which LA:LN ratios mixtures were added.
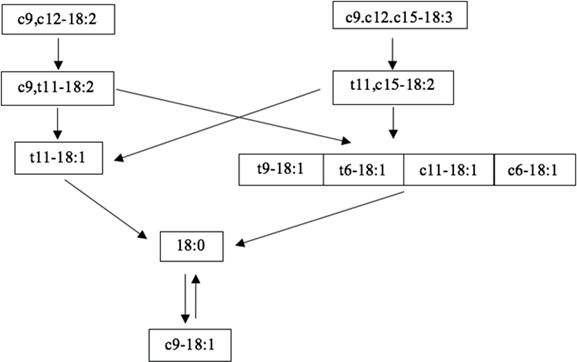
Figure 1 Proposed main pathways of linoleic (LA; c9,c12-18:2) and alpha-linolenic (LN; c9,c12,c15-18:3) acids biohydrogenation.
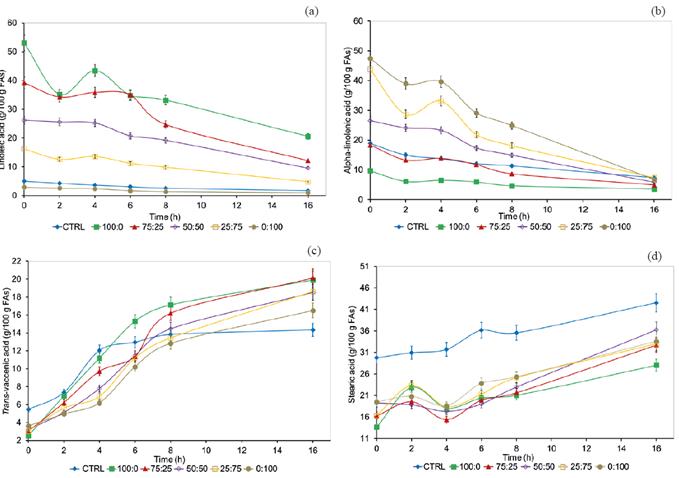
Figure 2 Fatty acids concentration in incubation systems without (CTRL), and with the addition of linoleic (LA; c9,12-18:2), and alpha-linolenic (LN; c9,c12,c15-18:3) acids ratio mixtures (LA:LN: 100:0, 75:25, 50:50, 25:75, and 0:100); (a) c9,c12-18:2; c9,c12,c15-18:3; (c) trans-vaccenic acid (VA; t11-18:1); and (d) stearic acid (SA; 18:0); bars represent the standard error of the mean. The fatty acids concentration differs between treatments (p<0.05).
The r-VA decreased as the LN proportion increased in the LA:LN ratio mixtures (Table 2; p<0.01). However, r-VA did not differ between the following treatments: CTRL and LA:LN 100:0, LA:LN 75:25 and LA:LN 50:50, and LA:LN 25:75 and LA:LN 0:100 (Table 2; p>0.05).
Partial substitution of LA by LN increased the rate of transfer from LA to CLA, from CLA to VA, and from CLA to sum 18:1 (Figure 1; Table 3; p<0.05). Also, the rate of transfer from LN to trans-11, cis- 15 octadecadienoic acid (TA; t11,c15-18:2) differed between 100:0 and 25:75 LA:LN ratio mixture, as well as between 75:25 and 25:75 LA:LN ratio mixtures, and concomitantly, the rates of transfer from SA to OA just increased from 75:25 to 25:75 LA:LN ratios mixtures (Figure 1; Table 3; p<0.01). For the other rates of transfer, comparison between treatments were not signifi cantly diff erent (Figure 1; Table 3; p>0.10).
Table 2 Trans-vaccenic acid accumulation rates (r-VA) when linoleic and alpha-linolenic acid ratios mixtures (LA:LN) plus kikuyu grass were incubated in rumen fluid during 16 h
| LA:LN ratios | ||||||||
|---|---|---|---|---|---|---|---|---|
| CTRL | 100:0 | 75:25 | 50:50 | 25:75 | 0:100 | SEM | p-value | |
| r-VA (%/h) | 0.353a | 0.335a | 0.232b,c | 0.245b | 0.184c | 0.185c | 0.014 | <0.001 |
Values with different superscript letters (a, b, c) within a row differ statistically (p<0.05).
SEM: standard error of the mean.
p-value: differences in parameters between treatments.
The ΔGrxn at 39 °C for the conversion from CLA (c9,t11-18:2) to VA (t11-18:1) (-2.65 kJ/mol) was lower than that from TA (t11,c15-18:2) to VA (-0.29 kJ/mol) indicating that conversion from CLA to VA was more spontaneous than that from TA to VA. In addition, ΔGrxn at 39 °C for the conversion from VA to SA (+15.9 kj/mol) presented low spontaneity under ruminal conditions (Figure 3).
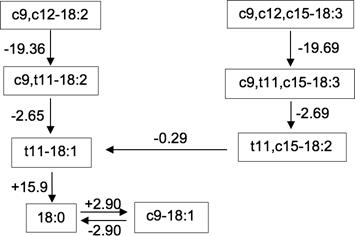
Figure 3 Thermodynamic description of the main steps of linoleic (LA; c9,c12-18:2) and alpha-linolenic (LN; c9,c12,c15-18:3) acids biohydrogenation. The values next to the arrows correspond to changes of Gibbs free energy of reaction (ΔGrxn, kJ/mol) at 39 ºC.
Table 3 Rates of transfer (%/h) between intermediates of linoleic (LA; c9,c12-18:2) and alpha-linolenic (LN; c9,c12,c15-18:3) acids biohydrogenation when LA:LN ratios mixtures and kikuyu grass, were incubated in rumen fluid during 16 h.
| LA:LN ratios mixtures | |||||||
|---|---|---|---|---|---|---|---|
| Reaction | 100:0 | 75:25 | 50:50 | 25:75 | 0:100 | SE | p-value |
| BH of Linoleic acid | |||||||
| c9,c12-18:2c9,t11-18:2 | 0.059a | 0.057a | 0.054a | 0.068a,b | 0.075b | 0.005 | 0.044 |
| c9,t11-18:2t11-18:1 | 0.76a | 0.58a | 0.59a | 1.81b | 4.17c | 0.261 | 0.001 |
| c9,t11-18:2sum 18:11 | 0.041a | 0.044a | 0.038a | 0.21b | 1.90a,b | 0.013 | 0.037 |
| BH of Linolenic acid | |||||||
| c9,c12,c15-18:3t11,c15-18:2 | 0.076a | 0.076a | 0.076a,b | 0.10b | 0.096a,b | 0.018 | <0.001 |
| t11,c15-18:2t11-18:1 | 0.24 | 0.31 | 0.24 | 0.13 | 0.17 | 0.065 | 0.114 |
| t11,c15-18:2sum 18:1 | 0.10 | 0.020 | 0.24 | 0.039 | 0.040 | 0.054 | 0.466 |
| Other BH pathways | |||||||
| t11-18:118:0 | 0.078 | 0.072 | 0.053 | 0.057 | 0.075 | 0.026 | 0.894 |
| 18:0c9-18:1 | 0.0020a,b | 0.000039a | 0.0027b | 0.32c | 0.080a,b,c | 0.001 | 0.003 |
| c9-18:118:0 | 0.043 | 0.032 | 0.047 | 1.90 | 2.04 | 0.339 | 0.095 |
| sum 18:1118:0 | 0.054 | 0.027 | 0.045 | 0.21 | 0.16 | 0.020 | 0.597 |
Values with different superscript letters (a, b, c) within a row differ statistically (p<0.05).
p-value refers to differences in parameters between treatments.
1Sum 18:1= 18:1-t9 + 18:1-t6 + 18:1-c6 + 18:1-c11.
Discussion
The goal of the current study was to investigate the effects of LA:LN ratio in lipid supplements on the BH kinetics of LA and LN, as well as on the VA production in an in vitro ruminal system. Our results indicated that partial substitution of LA by LN in lipid supplements increased isomerization and biohydrogenation rates of the main steps of LA and LN ruminal BH, and decreased VA production.
Our data showed that, regardless of LA:LN ratio, LN disappears faster than LA. This suggests that, regardless of LA:LN proportion in lipids supplements, LN tends to be biohydrogenated faster than LA. Additionally, we found that LA tends to accumulate in ruminal fluid when its concentration in lipid supplements is greater than LN concentration. This agrees with Jouany et al. (2007), who demonstrated that disappearance rates of LA ranged from 78.7 to 85.9%, whereas that of LN ranged from 80.0 to 91.2%, also showing that LA ruminal BH produces a major diversity of FAs isomers than LA ruminal BH. Thus, regardless of lipid supplement composition, LN tends to escape more easily than LN from ruminal BH. In addition, if lipid supplements are rich in LA, the proportion of LA that escapes from ruminal BH increases. This information may be useful for designing lipid supplements directed to increase LA and LN concentration in ruminant milk and fat.
Factors affecting ruminal VA concentration have been extensively studied (Jenkins et al., 2008; Hur et al., 2017), considering that the majority of CLA in ruminant milk and meat derives from desaturation of VA by stearoyl-CoA desaturase in mammary gland and tissues (Kay et al., 2004; Rezamand et al., 2014). Our data revealed that partial substitution of LA by LN decreased the VA concentration, and increased SA production. Additionally, partial substitution of LA by LN decreased the r-VA. It suggests that an increase in LA concentration in lipid supplements could decrease the conversion from VA to SA (a common step of LA and LN ruminal BH), increasing VA ruminal accumulation with the respective decrease in SA ruminal production. These results are in agreement with Ribeiro et al. (2007), who found that the BH rate of LA is twice as fast as the BH rate of LN, consequently producing more VA from LA than from LN. Thus, supplements rich in LA may be more desirable than supplements rich in LN for enhancing CLA concentration in ruminant milk and meat.
We observed that LA and LN concentrations decreased from 2 to 4 h, and, unexpectedly, decreased from 4 to 6 h. Concomitantly, SA concentration decreased from 2 to 4 h, and, unexpectedly increased from 4 to 6 h. Similar changes in LA, LN, and SA concentrations at the same hours have been reported in other in vitro studies (Jouany et al., 2007; Sterk et al., 2010), but no explanation has been proposed. Since the best multi-compartmental model (i.e., model with the lowest AIC; data not shown) was obtained when conversion from SAto OAwas included, and the LA:LN ratio in mixtures affected the rate of transfer from SA to OA, we suggest that desaturation from SA to OA may explain the increase in LA and LN, and the decrease in SA concentration between the aforementioned incubation times. Rumen conditions does not favor FAs desaturation (Van Soest et al., 1994). However, it has been observed that fungi (i.e., Piromyces communis) are able to desaturate SA to OA, and form CLA (Kemp et al., 1984; Ferlay et al., 2017). Therefore, considering that FAs concentration patterns and mathematical models have biological coherence, these results may constitute evidence that ruminal microorganisms are capable of desaturate FAs, suggesting that additional studies are needed to identify specific microbial species involved in FAs ruminal desaturation.
Previous studies have identified the FAs intermediates and products of LA and LN ruminal BH, modelling their BH kinetics (Jenkins et al., 2008; Hur et al., 2017). However, no previous studies have explored the effects of different LA:LN ratios in lipid supplements on the LA and LN ruminal BH kinetics utilizing a multi- compartmental model. Our data showed that partial substitution of LA by LN increased the rates of transfer from LA to CLA, from CLA to VA, and from CLA to sum 18:1. On the contrary, the rate of transfer from LN to TA slightly increased, and from TA to VA did not increase as LN increased in the mixtures. In this sense, the increase of LN enhanced BH of LA; however, increase of LA did not enhance BH of LN. This suggest that LA and LN may interact during their BH, which is in accordance with Troegeler-Meynadier et al. (2003), and Jouany et al. (2007), who suggested that LA and LN share linoleate isomerase enzyme (EC 5.2.1.5) in the first step of LA and LN ruminal BH. Thus, not only LAand LN concentration but also their proportion in lipid supplement could affect the rates of transfer of principal steps of LA and LN ruminal BH. Considering that transfer efficiency of FAs from rumen to milk and ruminant meat is limited by ruminal BH (Schmidely et al., 2017), the kinetic parameters derived from this study could be incorporated into mathematical models to explore the effects of FAs composition in lipid supplements on milk and meat FAs concentrations. This information could be useful for designing nutritional strategies to enhance the quality of ruminant-derived products.
In this study, we explored for the first time, the use of computational chemistry to evaluate the spontaneity of the reactions during LA and LN ruminal BH. Our data showed that conversion from CLA to VA is more spontaneous than that from TA to VA. The thermodynamic responses of these reactions were in accordance with their respective kinetics responses, because the rates of transfer from CLA to VA were greater than the rates of transfer from TA to VA, irrespective of the LA:LN ratio used. Also, our data showed that conversion from VA to SA may present low spontaneity under ruminal conditions. This explains why VA tends to accumulate in the rumen, as revealed by the present study, as well as in other studies exploring the effects of lipid supplementation on VA concentration (Sterk et al., 2010; Prieto et al., 2013). Thus, kinetic and thermodynamic approaches were biologically coherent, suggesting that thermodynamics could be useful for interpreting the kinetics of LA and LN ruminal BH.
In summary, this study indicates that the LA:LN ratio can modulate the kinetics of LA and LN ruminal BH affecting VA production. We demonstrated that thermodynamics contributes to the understanding of several kinetics responses during LA and LN ruminal BH. Thus, an integrated overview of kinetics and thermodynamics of LA and LN ruminal BH could help to design future strategies for improving FAs composition of ruminant-derived food products.