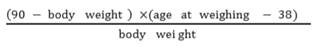Introduction
Pigs raised in the same pen sometimes show aggressive behaviors toward each other. Aggression is acknowledged as a critical problem, especially after mixing, in commercial pig farming (D’Eath et al., 2009; Turner, 2011). Increases in stress levels, physical activity, and injuries generally occur because of aggression after mixing. This aggression is associated with a reduction in the rate and efficiency of body mass gain, poor meat quality, and low carcass grading (Turner et al., 2009). Despite these disadvantages, mixing at various ages is commonly practice in many countries (Turner et al., 2008).
The estimated breeding value based on individual performance and pedigree information is generally used in genetic evaluation and selection of livestock (Muir, 2005). Genetic selection has mainly focused on reproductive traits and production, such as litter size and daily body mass gain in pigs (Løvendahl et al., 2005). New breeding models include not only individual performance, but also social breeding values (SBV) regarding performance with pen mates. The genetic effects of an individual on the phenotypes of its social partners (for example, pen-mates) are known as SBV (Moore et al., 1997). Moreover, SBVs represent specific traits such as growth rate or feed conversion (Bijma et al., 2007; Reimert et al., 2014). Social breeding values have been observed in several livestock species, including pig (Bergsma et al., 2008), chicken (Ellen et al., 2008), quail (Muir, 2005), deer (Wilson et al., 2011), and mink (Alemu et al., 2014).
Social interactions between individuals have been ignored in animal breeding so far. However, behavioral traits of animals have been shown to be partly under genetic control, which raises the possibility that behavior might be altered by genetic selection, resulting in better adapted animals (D’Eath et al., 2009). The individual growth performance of a pig is affected by its own genes and the genes of its pen mates (Grandinson et al., 2003; Ellen et al., 2008; Reimert et al., 2013a). Social breeding value is related to behaviors such as aggression and competition (Muir, 2005; Wilson et al., 2009). Our hypothesis is that pigs can affect the growth of their pen mates by their own social behavior and through social facilitation of feeding and drinking, increasing food intake and growth.
This study evaluated the behavior and growth of finishing pigs and investigated pigs selected for high or low social breeding value (SBV) in relation to social behavior and group growth.
Materials and methods
Ethical considerations
The experimental protocols describing the management and care of the animals were reviewed and approved according to the Guide for the Care and Use of Laboratory Animals (Institutional Animal Care and Use Committee, National Institute of Animal Science, Republic of Korea) on March 7, 2014 (approval number: NIAS 2014-289). Care was taken to minimize pain or discomfort during animal procedures. Drugs commonly administered to deal with animal pain or discomfort -such as tranquilizers, analgesics and anesthetics- were not used in this study.
Location and animals
The study was conducted at the experimental farm of the National Institute of Animal Science in Cheonan (Chungnam Province, South Korea) using Landrace pigs (Chookjin Land/Republic of Korea). Estimated SBVs were based on growth rates during the finishing phase (from approximately 30-90 kg). Social breeding values were estimated with a random regression model for social breeding values in WOMBAT version 1.0 (Meyer, 2007), using the following model that accounted for social genetic effects (Arango et al., 2005; Bijma et al., 2007):
y = Xb + Z D a D + Z S a S + Wl + Vg + e,
where, y is the vector of average daily gain observations; b is the vector of fixed effects, which included batch (year-month), sex (male or female), age at the end of the test, group size, and number of full siblings within the group; a D is the vector of random direct additive genetic effects; a S is the vector of random social genetic effects; l is the vector for the random non-genetic litter effects; g is the vector of random non-genetic group effects (accounting for the group in which the pigs were penned during the finishing period); e is the vector of residuals, and X, Z D , Z S , W, and V are the corresponding incidence matrices.
 Assumptions for the probability distributions were
Assumptions for the probability distributions were 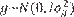 ,
,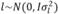 , and
, and 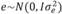 , in which N indicates a normal distribution; I is an identity matrix of the appropriate dimension; and
, in which N indicates a normal distribution; I is an identity matrix of the appropriate dimension; and 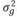 (403 g2/ day2),
(403 g2/ day2),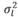 (95 g2/day2), and
(95 g2/day2), and 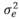 (3,875 g2/day2) are the variances of the corresponding effects. Both direct and social additive genetic effects were fitted, which had the following multivariate normal distribution:
(3,875 g2/day2) are the variances of the corresponding effects. Both direct and social additive genetic effects were fitted, which had the following multivariate normal distribution:
where, 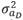 (2,513 g2/day2) is the variance of direct genetic effects,
(2,513 g2/day2) is the variance of direct genetic effects, 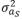 (9 g2/day2) is the variance of social genetic effects,
(9 g2/day2) is the variance of social genetic effects, 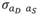 (55 g/day) is the covariance between direct and social genetic effects, and
(55 g/day) is the covariance between direct and social genetic effects, and  denotes the Kronecker product.
denotes the Kronecker product.
Dams and sires with the most extreme high and low SBV (+SBV and -SBV, respectively) were selected from the available population to create the test population. Dams (11 sows) were selected form a total of 161 sows and sires (6 boars) were selected form a total of 129 boars (Table 1). Mating between dams and sires was conducted within the same SBV group and pregnant sows were managed under the same conditions (individual housing). The pregnant sows were reared in separate farrowing crates.
Piglets were reared in farrowing crates with solid plastic flooring and a heat lamp. At 28 days of age, piglets were weaned and reared in eight weaning pens (1.8 × 2.4 m, 8-12 piglets/pen) per SBV group. At 30 kg, 70 pigs were allocated to 10 test pens (3.0 × 3.3 m, 7 pigs/pen) that consisted of five pens for each SBV group and were mixed randomly with the SBV groups from the weaning pens. Therefore, there was mixing within SBV groups; however, there was no mixing between +SBV and -SBV groups. The ratio of females/ males was 35/35. The males were not castrated. The environmental conditions were the same in all pens. The temperature was controlled by ventilation fans and heater, and maintained at 28 ± 1 °C. Each pen was provided with a stainless-steel feeder (125 × 35 × 80 cm) and a nipple drinker that allowed the pigs ad libitum access to feed and water throughout the experiment (Rhim et al., 2015).
Wide-angle video cameras were installed at the corners of the test pens (four cameras per pen), so all areas of the pen could be observed. The behaviors of the pigs were video recorded continuously for 9 h/day. All behavioral data were obtained from video images that were digitally recorded from 09:00 to 18:00 h on days 1, 15, and 30 after mixing. Instantaneous scan sampling was carried out at 10-min intervals with the software Vegas Pro ver. 13.0 (Sony, Tokyo, Japan). All video recordings were viewed by trained observers who were blinded to the treatments to eliminate subjective bias and inter-individual discrepancy (Li and Wang, 2011; Rhim, 2012).
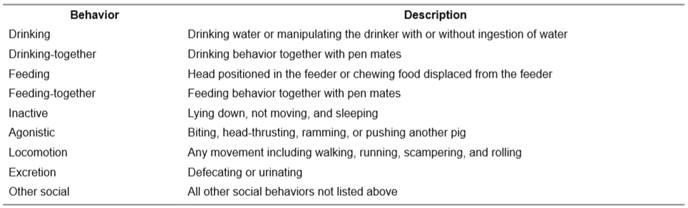
The behaviors recorded were drinking, feeding, agonistic, and activity including drinking-together and feeding-together (Table 2). Duration and frequency of behaviors were recorded and the individual pig performing the behavior, as well as the individual pig receiving the agonistic behavior, was noted. The behavioral time values presented are the means and standard errors of the relative frequencies of each behavior, calculated from the results obtained from each observation of each group (Rhim et al., 2015; Hwang et al., 2016).
Table 1 Social breeding value (SBV) of parents and test pigs with positive (+) or negative (-) SBV in a Landrace nucleus herd.
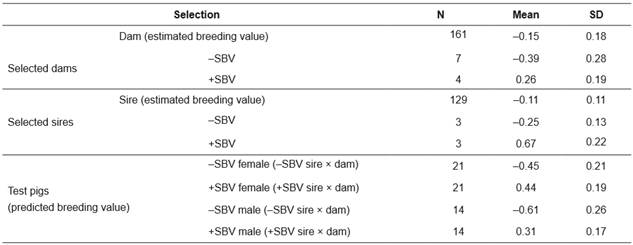
N: number of individuals; SD: standard deviation; -SBV: negative social breeding value; +SBV: positive social breeding value.
Table 2 Ethogram of behavioral categories and their respective definitions (adapted from Statham et al., 2011; Rhim, 2012).
Pigs were weighed at approximately 90 kg body weight and the data was transformed to the number of days to reach 90 kg using the following calculation (Choi et al., 2013):
Statistical analysis
Data analysis was performed with RStudio version 0.99.892 (http://www.rstudio.com). The behavioral data were analyzed by a Mann-Whitney U test between the +SBV and -SBV groups using R package coin (Hothorn and Zeileis, 2015). The behavioral differences between days after mixing within each group were assessed with the Kruskal-Wallis test. Days to reach 90 kg were analyzed by a t-test between the +SBV and -SBV groups.
Results
The frequency (Table 3) and duration (Table 4) of behaviors were present in positive (+) and negative (-) SBV groups after mixing. For frequency and duration of drinking behavior, there were no significant differences between the +SBV and -SBV groups during the study period. However, the -SBV group had lower frequency compared with the +SBV group (Mann-Whitney U test, Z = -4.36, p<0.001) in drinking-together at day 30 after mixing.
On day 1 after mixing, the feeding frequency (Z= -2.93, p=0.003) and duration (Z= -2.85, p=0.004) were lower in the -SBV group compared with the +SBV group. On day 15 after mixing, the feeding duration was higher (Z=1.88, p=0.06) in the - SBV group than in the +SBV group. However, feeding frequency was not significantly different between the two groups (Z=0.86, p=0.392). Results reported on day 30 were similar to those obtained on day 1 after mixing, i.e., feeding frequency (Z= -2.91, p=0.003) and duration (Z= -2.43, p=0.014) were lower in the -SBV group than in the +SBV group.
For feeding-together behavior, similar results were obtained as for feeding behavior. The feeding-together frequency (Table 3; days 1 and 30 after mixing) and feeding-together duration (Table 4; days 1 and 30 after mixing) were lower in the -SBV group than in the +SBV group (Z= -5.11 to -4.22, p<0.001). On day 15 after mixing, the feeding-together duration was higher in the -SBV group than in the +SBV group (Z=4.31, p<0.001). There were significant differences in frequency (Kruskal-Wallis test, H=20.87, p<0.001) and duration (H=25.14, p<0.001) of feeding behavior over the three observed days in the -SBV group.
On day 1 after mixing, frequency of agonistic behavior was higher in the -SBV group than in the +SBV group (Mann-Whitney U test, Z=2.21, p=0.027, Table 3) and duration of agonistic behavior was significantly different between the -SBV and +SBV groups (Z=1.81, p=0.050, Table 4). The duration of agonistic behavior of the -SBV group was higher than the + SBV group. However, the frequency and duration of agonistic behavior were not significantly different between the two groups on days 15 and 30 after mixing.
In the -SBV group, the frequency (Kruskal-Wallis test, H=26.68, p<0.001) and duration (H=23.76, p<0.001) of agonistic behavior were significantly different over the three observed days. There were higher frequency and duration of agonistic behavior on day 1 compared with days 15 and 30. Moreover, there were significant differences in the frequency (H=11.41, p=0.003) and duration (H=12.10, p=0.002) of agonistic behavior among days in the +SBV group. Agonistic behavior was higher on day 1 than in days 15 and 30 of the +SBV group (Tables 3 and 4 ).
Table 3 Frequencies of different behaviors of pigs after mixing for groups with positive (+) or negative (-) social breeding value (SBV) in a Landrace nucleus herd; comparisons between groups (n = 5 for + SBV, and n = 5 for -SBV) are based on a Mann-Whitney U test.
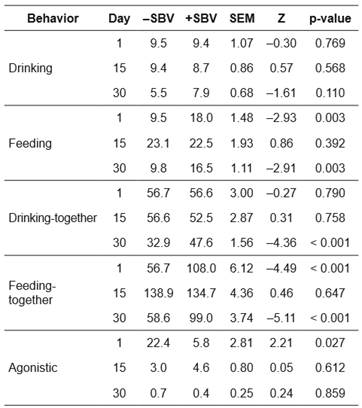
-SBV: negative social breeding value; +SBV: positive social breeding value; SEM: standard error of the mean, Z: value of Mann-Whitney U test.
Table 4 Total duration (sec) spent on different behaviors of pigs after mixing for groups with positive (+) or negative (-) social breeding value (SBV) in a Landrace nucleus herd; comparisons between groups (n = 5 for +SBV, and n = 5 for -SBV) are based on a Mann-Whitney U test.
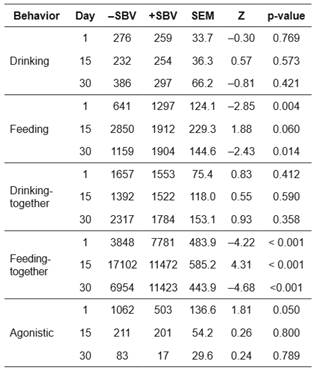
-SBV: negative social breeding value; +SBV: positive social breeding value; SEM: standard error of the mean; Z: value of Mann-Whitney U test.
Table 5 Mean days needed for pen mates to reach 90 kg for groups with positive (+) or negative (-) social breeding value (SBV) in a Landrace nucleus herd; comparisons between groups (n = 5 for +SBV, and n = 5 for -SBV) are based on a t-test.
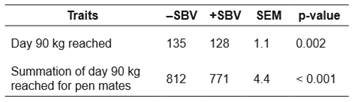
-SBV: negative social breeding value; +SBV: positive social breeding value; SEM: standard error of the mean.
Days to reach 90 kg was significantly different between the -SBV (135 days) and +SBV (128 days) groups (t-test, t=3.20, p=0.002, Table 5). This means growth was faster in the +SBV group than in the -SBV group. Moreover, there were significant differences in the summation of the days to reach 90 kg of pen mates between the -SBV (812 days) and +SBV (771 days) groups (t = 5.75, p<0.001).
Discussion
Commercially-housed pigs are selected for fast growth rates and maintained in competitive and aberrant behavioral conditions (Rodenburg and Turner, 2012). Aberrant behavior is known to harm health and growth of pigs, and is considered an welfare problem in swine husbandry (Schrøder-Petersen and Simonsen, 2001). In addition, social interactions of group-housed pigs are important for their health, productivity, and welfare (Camerlink et al., 2013). Social interactions among pen mates might affect their welfare and performance considerably, in both negative and positive ways (Reimert et al., 2014). In previous studies, positive SBVs for growth were shown to fear-related behavioral traits in suckling piglets (Reimert et al., 2013b). Moreover, pigs selected for SBVs on the growth of their pen mates exhibited less non-reciprocal biting (Camerlink et al., 2015).
Behavior was affected by SBVs for growth in the present study. Pigs in the +SBV group affected the growth of their pen mates more than those in the -SBV group did. SBVs might have profound effects on response to selection and heritable variation in traits (Moore et al., 1997; Camerlink et al., 2013). Behavioral observations revealed differences in agonistic behavior between negative (-) and positive (+) SBV groups. There was higher frequency and duration of agonistic behavior on day 1 in the -SBV than in the +SBV group. Agonistic behavior was not different at later time points once the groups had settled in this study.
However, Canario et al. (2012) found that the more intense aggression was observed at mixing in the +SBV groups with more stable dominance relationship later and faster growing. Moreover, mixing aggression was not different, but on return to a familiar group after 24 hours away while mixed with unfamiliar pigs, the aggression at reunion was less (Camerlink et al., 2015). This may suggest that social memory is improved. Genetic relationships seem to show that high aggression at mixing can subsequently lead to more stable groups (Desire et al., 2015).
A tentative hypothesis for why +SBV pigs showed less agonistic behaviors when they were mixed with unfamiliar pigs than -SBV pigs could be related to dominant relationships (Canario et al., 2012; Camerlink et al., 2013). Therefore, +SBV pigs could establish their dominant relationship with less agonistic behaviors after mixing. In the present study, +SBV pigs showed less biting, head thrusting, ramming, and pushing other pigs especially on day 1 after mixing. In addition, fearfulness is known as an important factor for social interactions. If pigs with reduced aggression and fearfulness of their pen mates are selected, this might have positive consequences in terms of welfare (Reimert et al., 2014).
We hypothesized that SBV could be related to social behavior and growth performance of finishing pigs. The results suggest that pigs with +SBV and -SBV for growth differed in their social behavior response (Camerlink et al., 2015), and +SBV had a positive effect on the growth of their pen mates. In addition, feeding-together and drinking-together behaviors were observed in this study. A real direct effect could be a greater social facilitation of feeding and drinking in the +SBV animals. Decisions to engage in feeding, feeding-together, and agonistic behaviors might be made by pigs based on the relative benefits and costs of behavior, which will vary depending on production efficiency and animal welfare. Moreover, selection for SBVs that have a positive effect on group growth with pen mates could be used as an indirect technique for improving growth performance and animal welfare (Reimert et al., 2014).
Pigs with divergent SBVs showed differences in social behavior. Social interactions, such as feeding- together behavior, among pen mates might affect their growth rate and feed intake. Selection for SBV could be used as an indirect technique for improving growth performance of pigs. In the present study, only a small- scale experiment was applied to behavioral differences in pigs. Knowledge regarding the mechanisms of social genetic effects might assist with optimal breeding and farming of pigs. Further research is needed using +SBV or -SBV pigs to investigate the behavior and growth of their pen mates.