Introduction
Fibrosis is an active biosynthetic process characterized by unregulated accumulation of extracellular matrix (ECM) in chronic ischemic lesions as a result of chemical or toxic agents, viral and non-viral infections, physical damage, and immunological reactions. Tumor stiffness is a feature associated with the tumoral microenvironment and recent studies have indicated that it may influence neoplastic progression involving ECM (Thannickal, 2004; Wynn, 2008; Guarino et al., 2009). Tissue stiffness is clinically considered an oncogenic risk factor when correlated to other conditions, as seen in dense mammary tissue and liver cirrhosis. In humans, tumor stiffness is one of the clinical features examined during palpation of the mammary gland (Liu et al., 2016) as the neoplastic tissue is stiffer than normal breast tissue and the stiffening process begins at the early stages of tumoral development (Barr et al., 2015). Chronic fibrosis and inflammation has been associated with mammary tumors, indicating poor prognosis for the patients (Kalliuri and Zeisberg, 2006; Carvalho et al., 2016). Studies have demonstrated that as fibrosis progresses, the signaling of pre-neoplastic cells with pro-inflammatory cytokines gradually increases (Kalliuri and Zeisberg, 2006).
Mammary tumors are the most common neoplasms in entire bitches and approximately 40 to 50% are considered malignant (Soremo, 2003; Cassalli et al, 2014; Visan et al., 2016). Thus, canine mammary tumors can be used as a relevant animal model for breast cancer studies in women (Klopfleisch et al., 2011; Queiroga et al., 2011; Feliciano et al, 2012; Jensen-Jarolim et al. 2015; Visan et al., 2016; Carvalho et al., 2016). In spite of this, the correlation between mammary tumors and the degree of fibrosis in bitches has yet to be evaluated.
Therefore, it is hypothesized that canine malignant mammary tumors with high histopathological grade and short evolution period have more fibrosis than benign tumors. Thus, the aim of this study was to evaluate and quantify fibrosis present in malignant and benign mammary neoplasms in bitches.
Materials and methods
Ethical considerations
The methods used were in agreement with the Ethics Committee on the Use of Animals (CEUA, process N. 023705/12) of UNESP.
Type of study, location, animals and samples
A retrospective study was conducted using mammary tumor samples from animals admitted to the Serviço de Obstetrícia Veterinária da Faculdade de Ciências Agrárias e Veterinárias, Universidade Estadual Paulista (UNESP), Jaboticabal Campus, Brazil, from January 2013 to February 2014.
Eighty-three samples of mammary tumors from bitches of different ages and breeds were used in this study. One sample was selected per animal. In animals that presented two or more histological types, only the sample from the most histologically aggressive type was selected.
At the time of patient admittance, the tumors were examined and tumoral nodule diameter measured using a pachymeter (Lee Tools, Santo André, SP, Brazil). Nodule diameter was classified (T) according to the World Health Organization (Owen, 1980) into T1 (≤3 cm), T2 (between 3 and 5 cm), and T3 (≥5 cm). Nodule size, animal age, and evolution time of tumor were tested for correlation with degree of tumoral fibrosis. Following mastectomy, fragments of mammary tumors were collected and processed for histopathological diagnosis under light microscopy. Samples were fixed in 10% phosphate buffered formalin (pH 7.4) for 24 hours, dehydrated in decreasing alcohol solutions, diaphonized in xylene, and embedded in paraffin. Sections (5 μm) were stained with haematoxylin-eosin (HE) for further identification of main morphological alterations.
Neoplasms were classified under light microscopy, according to criteria by Misdorp et al. (1999). Histopathological grading was performed according to the methodology by Elston and Ellis (1998), and the samples distributed into two experimental groups: Group 1 (G1, benign neoplasms, n= 21) and Group 2 (G2, malignant neoplasms, n= 62).
Histopathological sections stained with Masson’s trichrome (MT) were used to view connective tissue and determine fibrosis degree. A score was generated according to the extent of fibrosis in the samples. Fibrosis degree was determined by the Banff Classification (Solez et al., 1993), as follows: Grade 1 (discrete, 6 - 25% of the sample), Grade 2 (moderate, 25.01 - 50% of the sample), and Grade 3 (severe, ≥50.01% of the sample).
To evaluate the extent of fibrosis, five fields from each slide were randomly photographed (20x objective) and analyzed using the Image-Pro Plus16, version 4.5 (Medica Cybernectics, Inc., Rockville, MD, USA) processing software, which automatically calculates the percentage of staining in the areas marked in the field. The results for each slide were obtained by calculating the mean percent of the areas marked in the five fields (Figure 1).
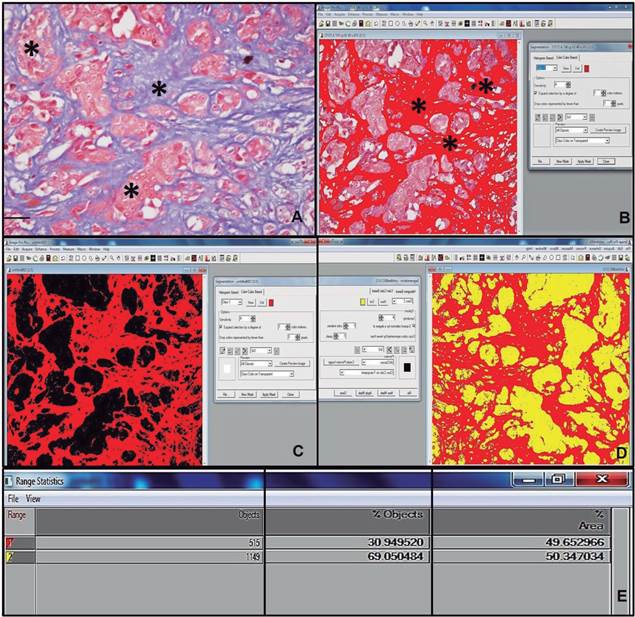
Figure 1 Histopathological photomicrograph of the mammary gland in a bitch. A: Solid carcinoma (*), Masson’s trichrome (MT), bar=100 μm. B: Histopathological photograph preparation of the mammary gland of a bitch through Image-Pro Plus16 software. Fibrosis area marked by Image-Pro Plus 16 image software (*). C: Marked fibrosis area analysis (red), chosen automatically by the image program. D: Calculation of marked fibrosis area (red) and background color (yellow). E: Result of automatic calculation of fibrosis area in solid carcinoma.
Statistical analysis
The percentage of fibrosis was compared between benign and malignant neoplasms regarding histopathological diagnosis, age of the animal, tumor evolution time, and histopathological grade using the non-parametric Kruskal-Wallis test. The correlation between histopathological grade, age, tumor evolution time, and malignancy was performed by Pearson’s test. Once the median of the fibrosis percentage in malignant neoplasms was calculated, the sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy of the diagnostic test for malignancy were determined. The relationship between fibrosis percentage and probability of the diagnosis being malignant was also tested by binary logistic regression. Differences were considered significant at p <0.05.
Results
Out of the eighty-three samples used in this study, 21 (25.30%) were classified as benign and 62 (74.70%) as malignant neoplasms. The benign tumors were further classified into cystic adenomas (1, 1.20%), complex adenoma (4, 4.81%), tubular adenoma (2, 2.40%), benign mixed tumor (4, 4.81%), ductal hyperplasia (4, 4.81%), and lobular hyperplasia (6, 7.22%). The malignant neoplasms were classified into complex carcinoma (7, 8.43%), malignant mixed tumor (21, 25.30%), in situ tubular carcinoma (3, 3.61%), tubulopapillary carcinoma (18, 21.68%), and solid carcinoma (13, 15.66%) (Figure 2). Grade I was the most prevalent histopathological grade in malignant neoplasms (27, 43.55%), followed by grade II (25, 40.32%) and III (10, 16.13%).
Image analysis of the fibrotic tissue area in 45 samples revealed prevalence of severe fibrosis in malignant mammary neoplasms, followed by a lower occurrence of moderate and discrete fibrosis. Moderate fibrosis was also prevalent in benign mammary tumors, followed by severe and discrete fibrosis (Table 1). Fibrosis degree was classified as discrete, moderate or severe. When evaluating fibrosis intensity in canine breast neoplasms (discrete, moderate or severe) between the experimental groups, no statistical difference was found (p=0.174). Mean value (±SD) in group 1 was 3.0 ± 1.0, and group 2 was 2.5 ± 1.0.
Table 1 Frequency and percentage (%) of fibrosis degrees in benign and malignant mammary tumors in bitches.
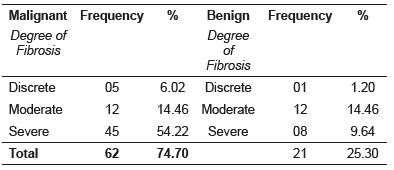
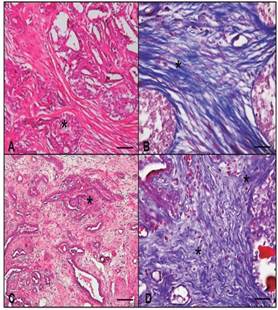
Figure 2 Histopathological photomicrograph of the mammary gland in a bitch. A: Malignant mixed tumor (*), HE, bar 100 μm. B: Area of fibrosis in malignant mixed tumor (*), MT, 100 μm bar. C: Complex carcinoma (*), HE, bar 100 μm. D: Area of fibrosis in complex carcinoma (*), MT, bar 100 μm. MT: Masson’s trichrome, HE: haematoxylin-eosin.
No significant (p =0.134) difference in the percentage of fibrosis between malignant and benign tumors was observed. Mean (±SD) percentage of fibrosis in malignant and benign neoplasms was 58.2 ± 24.50 and 49.9 ± 19.10, respectively.
Fibrosis percentage was positively correlated with histopathological grade of tumors (p =0.035, r =0.23), which in turn had a positive correlation with age (p =0.003, r =0.32). In malignant tumors, severe fibrosis (≥50.01% of the sample) was present, in decreasing order, in samples of in situ, tubular carcinoma, malignant mixed tumor, solid complex adenoma showed discrete fibrosis (≤ 25% of the sample).
Fibrosis percentage was different (p=0.0284) between histopathological subtypes, as shown in Table 2.
Table 2 Median ± interquartile range (IQR) of tissue fibrosis (%) in the different histopathological subtypes of benign and malignant canine mammary tumors.
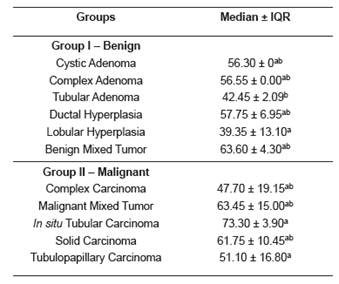
Different superscript letters (a, b) within the same column indicate significant difference between histological types (p <0.05) by the Dunns’ test. IQR: ± interquartile range.
Regarding tumoral evolution time, the nodules appeared between one and 36 months. Mean evolution time in benign and malignant neoplasms was 7.76 and 7.06 months, respectively. There was no significant difference in the percentage of fibrosis between different evolution times (p=0.14). Furthermore, fibrosis percentage was negatively correlated with tumoral evolution time (p=0.008, r=-0.3), suggesting that evolution time does not determine the chronicity of the process.
As the median percentage of fibrosis in malignant neoplasms was 58%, the sensitivity and specificity of the test to estimate tumor malignancy were evaluated. The test showed 75% prevalence, 45% sensitivity, 86% specificity, 90% positive predictive value (PPV), and 34% negative predictive value (NPV). The positive and negative verisimilitude were 94 and 24%, respectively. Test accuracy was 38%.
There was no significant (p=0.528) difference in the percentage of fibrosis between the different nodule sizes, with mean (±SD) percentage of 54.93 ± 21.68, 57.51 ± 22.69, and 60.36 ± 31.63 for T1, T2, and T3 mases, respectively. There was no positive correlation between tumoral size and fibrosis percentage, animal age, histopathological grade, or tumoral evolution time, when they were compared separately. Binary logistic regression was used to test the relationship between fibrosis percentage and probability of a malignant diagnosis. Fibrosis percentage was predictive of malignancy in only 46% of the tumor samples (p=0.074).
Discussion
Histopathological analysis in the present study revealed that malignant neoplasms corresponded to 74.70% of the samples, in agreement with Oliveira et al (2003). Our findings also agreed with Oliveira Filho et al (2010), Sorenmo et al (2009), Feliciano et al (2012), and Soler et al (2016), who reported similar findings (73.4, 79.9, 60, and 65.38%; respectively). The frequency of benign and malignant neoplasms reported in the literature varies markedly due to different classification methods of mammary tumors and lack of standard criteria to differentiate tumor types (Cassali et al., 2003). In other countries, the percentages of malignant neoplasms reported are often lower than 54% (Itoh et al., 2005; Sorenmo et al., 2009; Lana et al., 2013; Goldshimit et al., 2017). On the other hand, some studies in Brazil have reported the prevalence of malignant neoplasms ranging from 60 to 91% (Nardi et al., 2002; Oliveira et al., 2003; Feliciano et al., 2012). This difference is probably due to the high prevalence of malignant neoplasms in Brazilian studies and the extensive time elapsed from tumor appearance to clinical evaluation (Oliveira et al., 2003). Furthermore, there is evidence that prolonged evolution time enables progression of benign into malignant tumors (Sorenmo et al., 2009).
A high prevalence of malignant mixed tumor and tubulopapillary carcinoma amongst malignant mammary neoplasms was observed, in agreement with Cintra et al. (2014) who reported prevalence of malignant mixed tumor when using Moulton’s (1990) classification. However, Soler et al. (2016), using the classification by Goldshimidt et al. (2011), reported higher incidence of complex and simple carcinoma in mammary tumors. In the classification by Cassali et al. (2011), there were some differences to the classification of malignant mixed tumors. This histological subtype (malignant mixed tumor) is separated from the mixed tumor carcinoma subtype due to its reduced invasiveness and better prognosis.
According to McPhail and Robinson (2010), presence of fibrosis in tumor tissue is considered a histological feature associated with poor prognosis in mammary neoplasms. Although there are other histopathological markers for malignant tumors, it has been suggested that the presence of fibrosis in carcinomas could be used as a prognostic factor in mammary tumors, as it originates from the overreaction of the tumoral stroma, which is comprised mainly of fibroblasts and collagen.
A positive correlation has been reported between fibrosis and mammary tumors in rats when using Picrosirius Red stain, which suggests that neoplasms induced by NMU (N-Methyl-N- Nitrosurea) also have high amount of fibrosis, reinforcing the clinical relevance of fibrosis (McPhail and Robinson, 2010). Furthermore, it has been shown that invasive ductal mammary carcinomas containing fibrosis are more aggressive than those without fibrosis (Hasebe et al., 1996).
In agreement with Soler et al. (2016), there were fewer benign tumor samples in the present study than malignant ones, which might explain the lack of significant difference in the parameters between the two types of tumors. Heterogeneity of canine mammary tumors and the limited number of samples make data interpretation challenging; larger samples could result in more significant results. Nevertheless, a positive correlation was observed between histological grade and fibrosis percentage in the tumoral mass, corroborating with the findings by McPhail and Robinson (2010) in rats.
Histologically, fibroblasts and collagen fibers within a fibrotic area are arranged in irregular patterns, associated with increased cellularity and/or fibroblast collagenization. It is still unclear how this dense tissue architecture affects the tumor; however, non-invasive imaging techniques can evaluate collagen in vivo and could identify and delineate a fibrotic focus within the mammary tumor (Brown et al., 2003).
Invasive carcinomas with fibrotic foci are more aggressive than those without fibrosis. Fibrosis has been associated with large tumoral size, great histopathological grade, high proliferative activity of tumoral cells, and necrosis. Smaller studies have shown the independent prognostic significance of fibrosis in patients with invasive breast cancer. Furthermore, it has been shown that the relationship between fibrosis and tumor size could be an important prognostic factor (Van den Eyden et al., 2007).
Thus, it has been demonstrated that presence of fibrosis indicates differences in the malignancy of invasive carcinomas and other mammary tumors (Hasebe et al., 2000). Fibrosis is part of the tumor stroma, indicating that the malignant potential of neoplasms depends not only on the biological features of tumoral cells, but also on the tumoral stroma. Fibrosis consists of fibroblasts or collagen fibers mixed with microvessels and the interaction of these components influences the progression of mammary carcinomas with fibrosis (Hasebe et al., 2000). The presence of atypical fibroblasts in the tumoral stroma, especially in the fibrotic foci, is significantly associated with tumor recurrence and death of patients with invasive ductal mammary carcinoma (Ahn et al., 2012).
The influence of an overactive stroma on neoplastic cells, and vice versa, is still unclear. The way in which in situ carcinomas remain localized within the basement membrane or become invasive is not fully understood (Hanahan and Weinberg, 2000; Kalliuri, 2003). Furthermore, stromal fibroblasts secrete metalloproteinase (MMPs) and other cytokines that are important factors in tumor progression (Hasebe et al., 2000). Therefore, it is presumed that the biological factors of the usual fibroblasts are markedly different from those in the fibrotic focus. This indicates heterogeneity in fibroblast characteristics associated with tumoral stroma, as well as neoplastic cells, which may affect patients with mammary carcinomas.
A study using 140 cases of invasive carcinomas in women was performed to determine the prognostic significance of foci of fibrosis (Hasebe et al., 2000), which was evaluated by the following histological parameters: age, histopathological grade, nodular state (absent or present), size, tumor necrosis (present or absent), and adjuvant chemotherapy (none or administered). The overall disease-free survival time was significantly lower in cases with fibrosis, confirming that its presence and extent are useful histological and prognostic parameters in patients with invasive lobular carcinoma as well as invasive ductal carcinomas (Hasebe et al., 2000).
Several techniques and different histopathological and molecular markers are currently available to evaluate aggressiveness, biological behavior, and prognosis of mammary neoplasms in bitches. Most markers reflect specific aspects of neoplastic cell biology; however, oncogenesis is determined by complex interactions between epithelial and stromal tumor cells. Markers that reflect the behavior of these cells are scarce in Veterinary Medicine; therefore, in the present study, easy and reproducible evaluation of fibrotic areas was proposed as a practical histopathological marker for tumoral aggressiveness.
This study provides evidence that Masson’s trichrome staining could be used for assessing fibrosis in canine mammary tumors. Further in vivo and in vitro studies are necessary to elucidate the complex pathophysiological development of fibrosis in canine mammary tumors, as well as its impact on the interactions between the immune system and chronic diseases. The number of samples used could have limited the evaluation of the correlation between fibrosis and malignancy; therefore, further studies involving a greater number of samples, different breeds, age, groups, malignant and benign histological types, and different histological staining techniques are needed.














