Introduction
Embryo transfer (ET) biotechnology in horses is a useful tool for allowing rapid genetic dissemination of valuable animals (Arruda et al., 2001; Azevedo et al., 2015). The ET makes it possible to obtain foals from aged mares, mares with problems to maintain gestation, and mares under training or competition. This biotechnology also allows obtaining foals of young mares (1 to 2 years) without impairing its development and shortens the generation interval of mares with a promising pedigree (Sieme et al., 2018).
The ET technology is less complex than other reproductive biotechnologies, such as in vitro embryo production (Lira et al., 2009; Claes et al., 2016; Sanchez et al., 2016; Rua et al., 2016). Its efficiency depends on proper selection and management of mares (Squires et al., 1999; Vanderwall, 2008), and estrus and ovulation synchrony (Iuliano et al., 1985; Taveiros et al., 2003/2008; Rabelo et al., 2009; Hinrichs, 2012; Azevedo et al., 2014).
Equine embryos are transported from the oviduct to the uterus from the compact morulae to the blastocyst stages, between the 5th and 6th days after ovulation (Oguri and Tsutsumi, 1972). Embryos obtained by post-ovulation artificial insemination (AI) reach the uterus later compared to their counterparts obtained by pre-ovulation AI, possibly due to delayed fertilization (Bertani et al., 1997). Therefore, embryo collections should be performed around 12 hours after day 7 (Day 7.5) and should not exceed day 8 (Cuervo- Arango et al., 2009).
The most appropriate day for embryo collection remains controversial. On one hand, for collections between days 6 and 9 after fertilization, collections made between days 7 and 8 retrieved greater embryo numbers (Cuervo-Arango et al., 2009; Sieme et al., 2018). On the other hand, similar embryo recovery rates could be achieved from days 5 to 8 post- ovulation (Vogelsang et al., 1985). Also, according to Squires and Seidel (1995), day 6 is the most advantageous for embryo cryopreservation, and day 9 -although less used for ET- shows lower results in comparison to days 7 and 8.
The ET into previously synchronized mares can be performed by either surgical or transcervical methods (Fleury et al., 2001). Despite ongoing controversy, the most frequently used period for ET has been from days 5 to 9 after donor ovulation (Iuliano et al., 1985; Vogelsang et al., 1985; Fleury et al., 2001; Hajčić et al., 2008; Sieme et al., 2018).
Given that day for collection and ET in horses plays a crucial role in its overall success, a limited number of reports on the subject under the Brazilian northeast condition are available. The northeast of Brazil is a semi-arid region that in spite of stable luminosity throughout the year, faces substantial variation in forage supply due to low and uneven distribution of rainfall. The breeding season spans from September to March, which is during the dry season. Therefore, the objective of this study was to define the most appropriate days for collection and transfer of embryos in Mangalarga Marchador mares under Brazilian northeast’s conditions.
Material and Methods
Ethical considerations
The study was approved by the Ethics Committee for Animal Research at Universidade Federal Rural de Pernambuco (License: 011/2013).
Location and animals
The experiment was conducted in Limoeiro (Pernambuco state, Brazil). The farm is located at the following geographic coordinates, 7o 52’ 29’’ S latitude and 35o 27’ 01’’ W longitude. Mean annual temperature is 24.6 oC and mean total rainfall is 1,007 mm3.
Mangalarga Machador donor and recipient mares were pluriparous, non-lactating, aged between 5 and 15 years, and body weight ranged from 450 to 550 kg. All mares were raised under semi-extensive conditions, mostly grazing on cultivated pastures (Digitaria decumbes) with access to water and mineralized salt (Suprafós 73, Supranor®, Recife, PE, Brazil) ad libitum. Mares were also supplemented with hay (Cynodon spp), alfalfa (Medicago sativa), and 4 kg/day of a pelletized commercial horse ration containing 16% crude protein, 12.0% fiber, 3,080 kcal/kg fat, 1.3% calcium, 0.75% phosphorus, and 6.0% ether extract (Corcel Reprodução, Presence, São Lourenço da Mata, PE, Brazil).
The body condition score of mares was between 5 and 7, in a 1 to 9 scale, as described by Henneke et al. (1983). Other factors considered for selection were: reproductive performance, clinical condition, and gynecological examination by ultrasonography for identification of uterine fluid and endometrial alterations that could compromise fertility, as suggested by Taveiros et al. (2008). For this examination, an Aquila Pro, Esaote, equipped with a linear multi- frequency transducer (6 and 8 MHz) was used.
Estrus was induced in donors (n= 30) and recipients (n= 76) with IM injection of 5 mg Dinoprost (Lutalyse®, Pfizer, São Paulo, SP, Brazil), and a single estrous cycle was used per mare. This strategy was carried out to obtain an -1 to +3 ovulation synchronies between the recipient and donor ovulations, as proposed by Squires (1993). Ovulation was induced with buserelin acetate (Conceptal®, MSD Saúde Animal, São Paulo, SP, Brazil) after the largest follicle had reached at least 35 mm diameter. At this time, mares were inseminated with fresh semen from a single stallion of proven fertility, using 500 x 106 viable sperm concentration in 48-hour intervals until ovulation occurred, as recommended by Rabelo et al. (2009).
Embryo collections were performed on days 7 (D7), 8 (D8), and 9 (D9) post- ovulation (single embryo collection per mare). The procedure was carried out by the open method, which requires the introduction of a catheter (Bivona, St. Paul, Minnesota, USA) into the uterus body to fixate the balloon to the uterine walls. A total of 3 L of Ringer-Lactate solution was used, divided into three fractions (Fleury and Alvarenga, 1999). After the last uterine flushing, the balloon was emptied, and the catheter was removed. The filtered content was deposited in Petri dishes for embryo recovery using a stereomicroscope (80X), as suggested by Taveiros et al. (2008). Immediately after collection, donor mares received an additional Dinoprost injection to avoid an unwanted pregnancy.
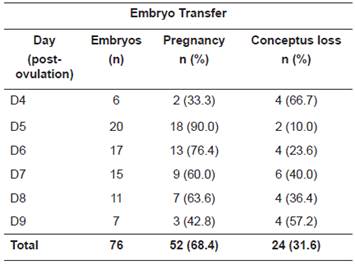
After recovery, embryos were evaluated for the stage of development and graded based on their morphology, as described by McKinnon et al. (1993). Only grades I and II embryos (n= 76) were transferred immediately to recipients at various post-ovulation stages (D4, D5, D6, D7, D8, and D9). Pregnancy diagnosis was performed by ultrasonography on day 25 after ET. All ultrasonographic exams were carried out by the same technician.
Statistical analysis
The statistical analysis of binomial data was performed using a chi-square test with Yates correction (Preacher, 2001). The significance level of 5% was used.
Results
Table 1 shows results relative to embryo collection at different days post-ovulation. Day 8 was more efficient than day 7 for embryo recovery (p <0.05), but it did not differ with day 9 (p >0.05). Moreover, day 9 was not more efficient than day 7 (p >0.05), although showing a trend towards such a difference (p=0.061). Regarding transferable embryos, there was no difference among the days of collection (p <0.05).
Table 1 Influence of the day of the collection on embryo recovery (Positive) and morphological evaluation (Transferable) in Manga Larga Marchador donor mares.
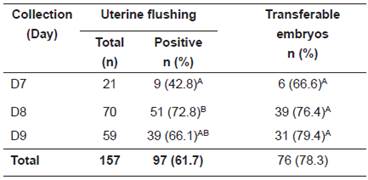
Different superscript letters (A, B) within the same column indicate significant difference (p <0.05).
Table 2 contains the results of pregnancy and conceptus loss about the recipients’ post-ovulatory stage (in days) at the time of ET. There was no effect of recipient’s post-ovulation day at ET on pregnancy rates and conceptus loss (p >0.05).
Discussion
The equine embryo reaches the uterus between the fifth and sixth day after ovulation (Oguri and Tsutsumi, 1972; Webber et al., 1991). That is why the day of collection is described as an essential factor on quantity (Montechiesi, 2015) and quality of retrieved embryos (Betteridge et al., 2000; Allen, 2001). The studies mentioned above tested different days for embryo collection. While day 7 was the least preferable, these reports are in contrast to Vogelsang et al. (1985), Iuliano et al. (1985) and Fleury et al. (2001), who suggested day 7 as the most promising day for embryo collection. The most plausible explanation for this discrepancy is that embryos from Mangalarga Marchador mares at day 7 post-ovulation are smaller in size than other breeds, thus making their recovery more challenging during uterine flushing. Despite the embryo potential for adapting to unsynchronized uterine environments, it is probable that under such conditions, it may have initially faced slower development at gene, cellular, and tissue levels (Gibson et al., 2017).
The results described herein may further support that collection can be preferentially performed at day 8, but can also be carried out at day 9 without diminishing embryo recovery. In a previous report, Iuliano et al. (1985) assumed that collections could be performed at day 8, while Fleury et al. (2001) supported that day 8 is the most promising day for embryo collection. Albeit the possibility that embryos at day 7 post-ovulation can be smaller than their day 9 counterparts, these later embryos are free from the zona pellucida and are more likely to succumb to mechanical damage during uterine flushing (Stout et al., 2005). Nevertheless, their morphological quality was similar, since the percentage of transferable embryos did not differ.
The ET in horses can be performed by surgical or transcervical methods into previously synchronized recipients (Fleury et al., 2001). Despite the controversy among reports on the best day for ET, it has been mostly described during days 5 to 9 after donor ovulation (Iuliano et al., 1985; Vogelsang et al., 1985; Fleury et al., 2001; Stout, 2006; Hajčić et al., 2008; Azevedo et al., 2015).
As described above, donor and recipient mare management, selection, ovulation synchrony, and-particularly- embryo quality for ET could have positively affected the results. This reasoning is based on the fact that pregnancy rates from day four to nine could be similar is ruled out. Thus, the remaining data would be in agreement with those published by several researchers (Wilson et al., 1987; McKinnon and Squires, 1988; Fleury et al., 2001; Taveiros et al., 2008).
Although the absence of statistical difference among pregnancy results, those obtained on days five and six can be outlined from the remaining. In spite of lack of scientific support for these days as the best time-points for ET, it is possible to account for this pregnancy data to the more extended period that embryos have to develop for maternal to fetal crosstalk in the uterus. This crosstalk, among other possibilities, can be in consequence of conceptus migration within uterine horns, thus inhibiting PGF2α production and release (Conley, 2016).
Therefore, embryos transferred more precociously may not have acquired the necessary competence to promote the signaling above, since it is the exact moment of embryonic activation (Stout, 2006). It is possible that this problem overlaps with fluctuating P4 concentration during this time, and P4 available may not be sufficient for establishing uterine conditions to support embryonic development (Irvine et al., 2000).
In conclusion, equine embryos can be collected eight days post-ovulation, and their transfer can be performed in a relatively broad period without any detrimental effect on pregnancy rates.